Relationships Creating Functionalities of A Process System and Their Representation by Process Vectors
Diunggah oleh
api-26678889Deskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Relationships Creating Functionalities of A Process System and Their Representation by Process Vectors
Diunggah oleh
api-26678889Hak Cipta:
Format Tersedia
Int.J.
Applied Thermodynamics, ISSN 1301-9724
Vol.5 (No.3), pp.109-117, September-2002
Relationships Creating Functionalities of a
Process System and Their Representation by Process Vectors
Masaru ISHIDA
Chemical Resources Laboratory, Tokyo Institute of Technology,
Nagatsuta-cho 4259, Midori-ku, Yokohama-Japan, 226-8503
Tel: +81-45-924-5254, Fax: +81-45-924-5253
E-mail: ishida@res.titech.ac.jp
Abstract
Understanding of the target functionalities is the origin of creating systems. Those
functionalities can be generated by the system structure. To examine both those
functionalities and the system structure, we introduced the concepts of successional
relationships among processes and mediative relationships between processes. Also the
relation between inputs and outputs of the entire system is demonstrated. Process vectors
were used to represent the above three kinds of relationships. These concepts were
applied for methanol production to verify their usefulness.
Key words: energy & exergy analysis, functionality of a process system, successional
relationship, mediative relationship, input and output relation, process
vector
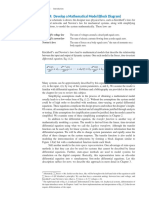
1. Introduction its coupled process 2. A similar relationship can be
When we want to grasp the characteristic seen for change 3 from C to D and its coupled
features of an entire system, we look at its process process 3.
flow diagram (PFD). To examine its energy features,
we construct its energy flow diagram (i.e., Sankey Coupler
diagram). Similarly, to examine its exergy features, 1 Successional relationship
we draw its exergy flow diagram (i.e., Grassmann
diagram).
Substance Substance Substance Substance
Then how can we discuss the functionalities of A B Change C Change D
Change 2 3
a system? The purpose of this paper is to 1
graphically represent the functionalities of an entire
system by use of process vectors. Process synthesis Mediative relationship
is the creation of the system structure that generates Coupler Coupler
the target functionalities. In this paper, attention 2 3
was paid to the relationships among processes to
connect the creation of functionalities and the Figure 1. Relationships among processes in
creation of the structure of the system. a system
We can find two kinds of relationships among
2. Realization of Target Functionalities and the processes in this figure. The relationship
Relationships among Processes between change 1 and its coupled process 1 belongs
Figure 1 shows typical relationships among to the first category. Also the relationships between
processes. Substance A changes to B, then to C, change 2 and its coupled process 2, and between
and finally to D. Change 1 from A to B is a process change 3 and its coupled process 3 are in this
accepting intermediary energy, which is released by category. For these relationships, the two processes
its coupled process (or coupler) 1. This are connected by the intermediary energy denoted
intermediary energy may be heat, work or a mixture by white arrows in Figure 1. Hence we may call them
of the two. ‘relationships through the intermediary energy’ or
‘mediative relationships’ because the intermediary
On the other hand, change 2 from B to C energy plays the role of mediator between the two
releases intermediary energy, which is accepted by processes.
Int.J. Applied Thermodynamics, Vol.5 (No.3) 109
TABLE I. CONNECTION BETWEEN FUNCTIONALITIES AND STRUCTURE OF A SYSTEM
Realization of target functionalities
Relationships among processes
Relations through substances Relations through intermediary energy
(Successional relationships) (Mediative relationships)
Design of system structure
The relationship in the second category can be seen for power generation by a gas turbine, where
seen for the changes of substances from A through change 1 indicates the heating of gas at an elevated
D, namely, the relationships between changes 1 and pressure, change 2 the generation of power by the
2, and changes 2 and 3. For these relationships, the turbine, and change 3 the recovery of heat from the
substance outputted from the previous process or a gas outputted from the turbine.
part of it becomes the substance inputted to the next
process. For these relationships the two processes A Change B Change C Change D
are connected by substances. Hence we may call 1 2 3
them ‘relationships through the substances’ or
(a) Succession of changes of materials
‘successional relationships’, stressing succession of
the changes of substances. ∆
Change 1
In this paper, we want to discuss the ‘creation B
Change 1
of functionalities of a system’ or ‘creation of the
structure of a system generating functionalities.’ ∆H Change 2
Consideration of functionalities of a system seems Change 3 A
C
D
to be quite different from that of structure of a Change 3
system. However, since realization of Change 2
functionalities of a system needs a proper structure, (b) Vectors on thermodynamic compass (c) Translation of vectors
creation of functionalities and creation of a system
Figure 2. Successional relationships among
structure are close to each other. Both subjects are
processes
very difficult, and we need a new methodology.
TABLE I shows that the relationships among Figure 3 shows the case for which the three
processes can make a connection between the processes compose a loop: A is changed to B by
functionalities of a system and the structure of a change 1, to C by change 2, and back to A by
system. change 3. Ignoring the small pump work, the
working fluid, water, in the generation of power by
3. Representation by Process Vectors a steam turbine, follows the above changes. Then
3.1. Successional relationships the tip of process vector 3 reaches the origin of
process vector 1. Hence, for the three processes
A process vector on (∆H, ∆ε) domain can composing a loop, their process vectors compose a
represent the feature of a process (Ishida 1983, loop, too. This means that the process vector
1995, 2002). Principally, a process vector is drawn precisely represent the changes of substances. This
on a thermodynamic compass, as shown in Figure 2 fact can hold because both energy H and exergy ε of
(b). The abscissa, energy change ∆H, represents substances are state properties.
information on the first law, while the ordinate, ∆ε,
information on the second law. B
B
The three vectors in (b) represent the three Change Change
1 2
successive processes in (a). Substance B outputted Change 1
from change 1 is the input for the next change 2. Change 2
A C
Hence it is reasonable to draw the process vector for Change
A C
change 2 from the tip of process vector 1, as shown 3
Change 3
in Figure 2 (c) (Ishida 1999, Ishida and Yamamoto
(a) Successional connection (b) Translated process vectors
1999, Yamamoto and Ishida 1999). Similarly, the
process vector for change 3 can be drawn from the Figure 3. Successional relationship for a
tip of vector 2. Then the origin of the vector for loop
change 1 corresponds to substance A, its tip To be noted is that these successional
substance B, the tip of the vector for change 2 relationships play quite important roles in
substance C, and the tip of the vector for change 3 generating functionalities in a system. Generation
substance D. This connection of processes can be of power by a turbine, which is the functionality of
110 Int.J. Applied Thermodynamics, Vol.5 (No.3)
a power plant, may be realized by a set of processes vector is identical to the vector sum, which
in Figure 2 (c) or Figure 3 (b). Consequently, indicates the exergy loss EXL for this energy
process vectors can be applied to represent the transformation. Although the shape of this triangle
functionalities of a system. This will be discussed for the mediative relationship in (c) is similar to that
by use of other examples in Section 5. for successional relationship in Figure 3 (b), the
meaning is quite different from each other. Hence
3.2. Mediative relationships
we have introduced the special notation for the
Process vectors are useful also to represent the vector sum, as shown in (d). In this mediative
mediative relationship between an energy-donating relationship, this downward vector sum indicating
process and an energy-accepting process shown in the extent of the exergy loss EXL is quite important.
Figure 4 (a). When the intermediary energy
donated from the energy-donating process is 4. Relationship Between Inputs and Outputs in
received by the energy-accepting process, we have a System
−∆Hed = ∆H ed =∆Hea, and the process vectors on a So far we have concentrated our attention on
thermodynamic compass shown in (b) can represent the relationships among processes in a system. By
the relationship between these processes. The examining them, we can find many important
subscripts ed and ea, respectively, refer to the features of a system, as will be shown in a later
energy donor and the energy acceptor. The exergy example system. However, the substances inputted
loss caused by this energy transformation, EXL, is to an entire system or outputted from it have
given as the vector sum, −∑ ∆ εj, of these vectors, important meanings, too. At the start of the system
as shown in (b) (Ishida 1983, 1995, 2002). synthesis, we first consider the resources and the
products of the system. They are inputs or outputs
for the entire system.
Energy-donating process (ed) ∆
Figure 5 (a) represents the relationship
Aed Aea between the inputs and outputs in an entire system.
Intermediary In this figure, a work source, a work sink, a heat
Aea energy ∆H
source, and a heat sink are introduced to supply or
Energy-accepting process (ea)
EXL accept heat or work. When we introduce the
Aed
absolute values of the energy H and the exergy ε of
(a) Energy exchange (b) Process vectors on compass the input and output substances, we can consider a
Self-dependent
substance vector (H, ε) for outputs and (−H, −ε) for
Self-dependent
process EXL process EXL inputs, and examine their relationships in an entire
system, as shown in Figure 5 (b), where we have
Dependent process Dependent process assumed that Win, Qin, and Qout are zero. The sum of
(c) Translation of a vector (d) Special notation for exergy loss these substance vectors represents the exergy loss
Figure 4. Mediative relationships EXL observed in this entire system.
The slope of the process vector is called the 5. Application of These Relational Expressions
energy level A (=∆ε/∆H), which indicates the to Methanol Synthesis
quality of the intermediary energy released or
5.1. A methanol plant as a model system
accepted by the process (Ishida 2002, Ishida and
Kawamura 1982, Ishida and Nakagawa 1985). For Figure 6 shows the PFD for a methanol plant
an energy transformation between two processes, (Ikawa et al. 1983). The resource is natural gas
the slope of the energy donor, Aed, should be greater composed of 90% methane and 10% ethane on a
than or equal to that of the energy acceptor, Aea, molar basis. Natural gas is supplied by 365.1 mol/s
indicating that the quality of the energy donor for resource of reforming and 31.0 mol/s for
should be higher than or equal to that of the energy additional fuel.
acceptor. There are two reactors, R1 and R2. In R1, the
A process with ∆ε<0 can proceed following steam reforming reactions proceed:
spontaneously. Hence it is called a self-dependent CH4+ H2O → CO + 3H2 (1)
process (Ishida 1995, 2002). On the other hand, a
process with ∆ε>0 is called a dependent process C2H6+ 2H2O → 2CO + 5H2 (2)
(Ishida 1995, 2002), because the proceeding of this These reactions are endothermic. Hence
process needs the help of a self-dependent process. supply of a large amount of heat at a high
When we move the origin of the vector of the temperature is a key issue. Also the water-gas shift
dependent process to the tip of the vector of the reaction may take place:
self-dependent process by keeping its direction, we
obtain (c). The vertical downward vector from the CO + H2O → CO2 + H2 (3)
origin of the latter vector to the tip of the former
Int.J. Applied Thermodynamics, Vol.5 (No.3) 111
Work source Heat source
Inputs
Win Qin (−Hin,1, − H
(Hin,1, in,1)
in,1) W'in Q'in (Hout,1, out,1)
EXL
1 1 in system
Inputted Outputted (−Hin,2, − in,2)
substances substances
2 2 (Wout, Wout)
(Hin,2, in,2) W'out Q'out (Hout,2, out,2) (Hout,1, out,1) out,2, out,2)
(H
Wout Qout
Outputs
Work sink Heat sink
(a) Inputs and outputs of an entire system (b) Representation by substance vectors
Figure 5. Representation of inputs and outputs by substance vectors
51 @520,137
@38,137
Water 40 41 42
@38, R1 @130,103
25.3 @339 3 @950
Natural 1 2 @38 11
gas 4 5 6 8 12
34
@38,25.3 7 9 10 43 33
Natural
50 Air R2
gas 13
r
21 Heating 32
Methanol T3
@38,1.0
34 20
Combustion 31 @270
of fuel @96,2.9
3 T1 15 14
Reforming 16
19 @66,1.0
T2
Preheating 18
of reactants @75,3.1 17 ∆
Preheating Ethanol-aq 25MJ/s
@119,3.3
Preheating of air
Water 30
∆H
of fuel @140,3.5 25MJ/s
Figure 6. Process flow diagram with meditative relationships for a methanol plant
The product gas from R1 is cooled in 4 and refinery section. In T1, dimethyl ether (DME), a
excess water vapor is condensed in condenser 5. byproduct in R2, is separated. In T2, ethanol and
In R2, methanol is produced by the following water are separated. Methanol in 99.995% purity is
exothermic reactions: discharged from T3 with its molar flowrate 363
mol/s.
CO + 2 H2 → CH3OH (4)
5.2. Successional relationships in the system
CO2 + 3 H2 → CH3OH + H2O (5)
We can observe various functionalities in this
Since the equilibrium relation limits the system. The main three functions are (1) the
concentration of methanol in the product stream, decomposition of the target reaction into two
the product gas is cooled in 14 and product subtarget reactions, (2) the effect of the loop along
methanol is condensed in 15, recycling the rest gas unit 10 through 15, and (3) separation of impurities
fraction back to compressor 10. Its molar flowrate in T1 through T3. For the separation, the
is 7838 mol/s. This rate is much larger than that of relationship between the function of the separation
the reactant stream from R1, 1606 mol/s. and the structural configuration of the sequence of
In R2, the heat released by the exothermic distillation columns is clear. Hence, we discuss the
reaction is absorbed by the sensible heat of the former two.
introduced stream, giving rise to the increase in its The main target of this system is conversion of
temperature. Hence the cold reactant stream that methane to methanol. Hence the target reaction of
has passed through preheater 11 is divided into five this system, r, may be written as
portions; one is fed from the top of the reactor,
while the others from the side, making the r: 0.9CH4+0.1C2H6+1.1H2O → 1.1CH3OH+H2 (6)
temperature profile in the reactor uniform. At present, this direct reaction cannot be
The liquefied fraction from 15 is sent to a performed commercially and this reaction is
112 Int.J. Applied Thermodynamics, Vol.5 (No.3)
divided into the following two reactions. molecules and recycle molecules. Namely we treat
the reactant-stream molecules that came from
r1: 0.9CH4+0.1C2H6+1.1H2O → 1.1CO+3.2H2 (7) compressor 9 and the recycle molecules that came
r2: 1.1CO+3.2H2 → 1.1CH3OH+H2 (8) from 15 separately. We assume that the
reactant-stream molecules take part in the reactions
The successional relationship between the
in R2 and that the recycle molecules do not
thermal decomposition of natural gas (r1) and the
participate in those reactions. To trace both the
methanol production (r2) constitutes the main
reactant-stream molecules and the recycle
frame of this system, and this relationship can be
molecules separately, we introduce the partial molar
represented by the process vectors, as shown in
Figure 7 (a). The slope of the original vector r for enthalpy h j and partial molar exergy ε j for a mole
direct methanol production is very steep. Since of molecule j in each stream in the loop. Then we
this direct methanol production is unavailable, r is can obtain energy H and exergy ε, for the
decomposed to endothermic reaction r1 and reactant-stream molecules in each stream by
exothermic reaction r2. The slope of a reaction
vector is equal to the energy level of heat at its H = ∑n jh j (9)
j
equilibrium temperature, [1−(T0/Teq)], where T0 is
an ambient environmental temperature. Hence, ε = ∑ n jεj (10)
gentler slopes for r1 and r2 indicate that these j
reactions may proceed at temperatures much lower where nj is the number of moles of reactant-stream
than that required for reaction r. Consequently, molecule j. Similarly we may obtain H and ε for the
process vectors are very suited to represent this recycle molecules, too, by regarding nj as the
important functionality for this system. Although number of moles of recycle molecule j. In this
the above target reaction and the above scheme for manner, we can draw the process vectors for (i) the
its decomposition were selected, there may be other reactant-stream molecules and (ii) the recycle
possibilities. molecules for each change, as shown in Figure 7
(b).
Reaction path adopted
r2 The successional relationships for the reactant
CH3OH
CO, H2 molecules in (i) indicate that both energy H and
Target reaction
r = r1+r2 r1 ∆ exergy ε decrease with the progress of the reactions
25MJ/s and in cooling in 14. Strictly speaking, there are
Natural gas, water ∆H three other vectors: for mixing with colder recycle
25MJ/s
(a) Decomposition of the target reaction stream with ∆H=−4.8 and ∆ε=−1.8 in MJ/s, for
Heating by compression with ∆H=0.4 and ∆ε=0.3, and for
r2 r21 Cooling heat of reaction preheating with ∆H=4.4 and ∆ε=1.1. Hence, the
r25 3 r2 in 14
r24 2 Cooling origin of r21 in (i) is located at (0, −0.4). On the
Cooling Mixing Pre- in 14 other hand, the successional connection of the
in 14 with warmer heating
reactant stream Compression
process vectors for the recycle molecules in (ii)
gives a closed loop. The left-lower end corresponds
(i) Change of reactants (ii) Recycle molecules
to the state after cooling in 14. Then, mixing with
(b) Features of the open loop for methanol production
the warmer reactant stream, compression,
Figure 7. Functionalities represented by preheating and acceptance of heat of reaction follow.
successional relationships The right-upper end corresponds to this state.
In the second reaction, the huge amount of gas Finally the cooling in 14 makes the state back to the
is recycled to the inlet of R2. The flowrate of each starting point. In this presentation, the fraction of
component in the reactant gas supplied from R1 is purge that passes through 32 is neglected, for
1200 for H2, 271 for CO, 106 for CO2 and 4.2 for simplicity.
H2O in mol/s. On the other hand, the flowrate of When we compare (i) and (ii), we find that the
each component in the recycled gas is 7130 for H2, heat released by the reactions is absorbed by the
103 for CO, 96 for CO2, 484 CH4, 20 for CH3OH, abundant amount of the recycle molecules. Hence,
and 5.1 for H2O. Hence, the latter flowrate is much it has become clear that the reactant stream acts as a
larger than the former. heat donor and that the recycle molecules act as a
This recycle forms a loop. Since it is an open heat acceptor. Also we find that the slopes of r21
loop with input and output of substances, we cannot through r25 in (i) are higher than that of the
apply the procedure for analyzing the functionality acceptance of the reaction heat in (ii). When the
of a closed loop represented in Figure 3. However, reaction takes place at the condition close to the
the role of this open loop and its contribution to the equilibrium, the slope for the heat acceptance in (ii)
reaction can be disclosed by dividing the molecules approaches that for reaction in (i). The large
in each stream in the loop into reactant-stream difference in the slopes indicates that the recycle of
Int.J. Applied Thermodynamics, Vol.5 (No.3) 113
EXL
C ombustion EXL EXL
of fue l Work Expansion
1− (T0/T) Compression Work
R e f o rm i n g r 1
( a ) S t e a m r e f o r m in g in R 1 (b) Compression (c) Expansion
E x o t h e r m ic EXL
r ea c t i o n r 2 reaction
1 mixing qreboiler EXL
r22 reaction separation
mixing qcondenser
Temperature
increase
( d) Production of methanol in R2 (e) Separat ion
Figure 8. Representation of various types of meditative relationships
large amount of hydrogen in the recycle molecules for expansion is greater than 45°.
contributes to the increase in the driving forces for In reactor R2, methanol is produced in five
the progress of reactions (4) and (5). Hence the steps. Only the first two steps r21 and r22 are shown
functionalities of an open loop can be disclosed by in Figure 8 (d). The reaction is exothermic and
process vectors based on partial molar energy h j donates heat, which is accepted by the increase in
and exergy ε j. the temperature of the reaction stream. This causes
exergy loss. Then this stream is mixed with the cold
5.3 Mediative relationships reactant introduced from the side. This mixing
Most of the mediative relationships between causes exergy loss, too. Similar relationships are
energy-donating processes and energy-accepting observed from the second through the fifth step. On
ones in this system are shown by use of process PFD in Figure 6, the summed vectors for the
vectors on PFD in Figure 6. The PFD is a suitable exothermic reaction and for the temperature
diagram to represent the structure of the system. It increase and the summed exergy loss by mixing are
can represent the flows of substances and various drawn. In (e), the meditative relationship for a
kinds of apparatuses used in the system. The distillation column is shown. The combination of
temperature and pressure of important streams are supply of heat at a higher temperature in the
presented by @T,P with T in °C and P in bar. reboiler and removal of heat at a lower temperature
However, it has neither quantitative energy in the condenser can make the separation proceed
information nor exergy information. Hence the with the exergy loss.
addition of mediative information by process Since there are many heating and cooling
vectors on PFD gives us quantitative and qualitative processes in a chemical plant, each combination
energy and exergy features of the system and makes between them for heat exchange is not shown on
PFD a very useful diagram. For example, at a PFD. Hence, for heat exchangers, the vectors of all
glance we can observe large exergy loss for the heat donors are linked in the order of the high
reforming reaction in R1. The reforming reaction is values of the slope, while those of all heat acceptors
endothermic and requires large amount of heat at a in the order of low values of the slope, as shown in
high temperature. This causes large exergy loss. Figure 9. Then, the total exergy loss for the heat
The part for the reforming reaction in reactor exchangers is obtained, indicating that this value is
R1 is represented also in Figure 8 (a). The heat also large.
required for this reaction is supplied by combustion
of both the fuel natural gas and the purge gas. The 51 EXL
dash-dotted line represents a thermal vector whose
Heat-donating 42
slope is equal to [1−(T0/T)]. Hence, the exergy loss streams 4 2
is divided into two parts: the lower part caused by 50
the reforming and the upper part by the combustion. 14 41 ∆
Figures 8 (b) and (c), respectively, show the 5 12
8 40 25MJ/s
meditative relationships for a compressor and a 30 1 Heat-accepting ∆H
CW (+ Heat loss) 11 streams 25MJ/s
turbine. The vectors for a work source and a work
sink have the slope of 45°. Hence, the slope of the Figure 9. Mediative relationships for heat
vectors for compression is less than 45°, while that exchange
114 Int.J. Applied Thermodynamics, Vol.5 (No.3)
We can compare the exergy loss in the heat energy of the substance is converted to another
exchange network with other exergy losses in this form of energy, e.g., power and/or heat. For
system by Figures 6 and 9. However, the slope of chemical processes, however, some chemical
the process vector represents the average energy substances are changed to other chemical
level of the released or accepted heat. For a heat substances, and the energy change or exergy change
exchanger, the temperature differences at both the by this reaction is generally much less than the
inlet and the outlet are key values. Hence, T−Q absolute value of H or ε of the chemical substance.
diagram (Hohman 1971) or A−Q diagram (Umeda The third disadvantage is that energy and exergy
et al. 1979) is more appropriate for detailed features are displayed on separate diagrams. Hence,
synthesis of the heat exchange network. the energy-flow and exergy flow diagrams cannot
be recommended for analyses of chemical
5.4. Relationship between inputs and
processes.
outputs of the entire system
On the other hand, the combination of PFD
Figure 10 shows the inputs and outputs for the
and the process vectors has many advantages. For
entire system. Most of the supplied natural gas is
complicated systems, the clear representation of the
the reactant for reforming, but some is introduced
functionalities generated by the system is quite
as additional fuel. On the other hand, methanol is
important. So far these functionalities of a system
the product. This figure can disclose the roles of the
have not been discussed in detail in the field of
resources and the products and the overall view of
process system synthesis, because there was no
the entire system.
appropriate method for representing functionalities.
H
Hence the successional relationships represented by
process vectors that can connect the functionalities
EXL generated by the system and the system structure
Inputs in
system are very important.
Natural gas Moreover, the process vectors can display the
for reforming mediative relationships between processes, too.
They can disclose the locations with large exergy
losses. The PFD is quite appropriate for compactly
Methanol representing the system structure and the flow of
substances in it. The process vectors are powerful to
Outputs represent energy and exergy features
50MJ/s
Natural gas H simultaneously. They can give quantitative energy
for fuel 50MJ/s
[Reduced to half]
and exergy features by their size and qualitative
Heat released
to environment features by their slopes. Further they can display
Figure 10. Relationship between inputs and exergy losses. Consequently, the synergic
outputs for an entire system combination of PFD and process/substance vectors
can give essential views useful for process analyses
The size of this figure is reduced to half. This and syntheses.
indicates that for a chemical reaction, ∆H and ∆ε for
each change are much smaller than the absolute 7. Conclusions
values of H and ε of the stream. Hence we have (1) Process/substance vectors can represent
focused on ∆H and ∆ε for all processes in a system three kinds of relationships: successional
in Figure 6. relationships among processes, mediative
relationships between processes and the
6. Comparison with Other Methods
relationship between inputs and outputs of an entire
The most popular diagram for the discussion system.
on the features of an entire system is the (2) The functionalities generated by a system
energy-flow diagram (i.e., Sankey diagram) or the or a subsystem are represented mainly by
exergy-flow diagram (i.e., Grassmann diagram). successional relationships. Even analyses of
The former is shown in Figure 11, while the latter in successional relationships for an open loop can be
Figure 12. At a glance, we can find that these made by introducing the concepts of partial molar
diagrams become very complex for chemical energy and exergy.
processes. There are disadvantages for these
diagrams. The main disadvantage is the difficulty in (3) The energy utilization can be represented
drawing such a diagram. The second disadvantage by mediative relationships between energy donors
is that each change in energy or exergy is so small and energy acceptors.
as compared with the enthalpy or exergy of the (4) The input and output relations represented
natural gas or the methanol. This disadvantage is by substance vectors can give overall view of the
not so severe in energy systems in which almost all entire system.
Int.J. Applied Thermodynamics, Vol.5 (No.3) 115
Water
Air 48 Steam
Heating at
40&50 11
Heating Power Heating
at 1,32&50 at 1,32,40&50
27 7 7
80 105 Power 11
Heat release
Heat
22 Water
46 5 5 3 0.5
5 4 Ethanol
2 1 0.5 0 2
29 29
1.5
×20
3
Power
66 0 2517 2517 128 0.5
Water ×20 Unit: MJ/s
Steam Recycle in Recycle out Purge gas
Figure 11. Energy-flow diagram for a methanol plant
Air Water
26 Steam
Heating at
40&50 11
10 Heating Power 4 Heating
at 1,32&50 at 1,32,40&50
5.5
76 Power 3 3 0.5 Heat release
24 49 Water
5 5 3 21 4 Ethanol
43 2
1 0.5 0.5 0 2
2 8 4
1 1 2 6
13
0.5
×20
3
Power
25 3 0 2208
2208 111 0.5
Water ×20 Unit: MJ/s
Steam Purge gas
Recycle in Recycle out
Figure 12. Exergy-flow diagram for a methanol plant
References System Synthesis,” Efficiency, Costs, Optimization,
Simulation, and Environmental Aspects of Energy
Hohman, E.C., 1971, Optimum Networks for Heat
Systems, Proc. of ECOS’99, pp. 20-27, Tokyo.
Exchange, Ph.D. Thesis, Chem. Eng. Dept. Univ. of
Southern California, Los Angeles. Ishida, M., 2002, Thermodynamics Made
Comprehensible, Nova Scientific Publishers Inc.,
Ikawa, T. et al., 1983, “Technical Report - Design of
New York.
Methanol Production Process from Natural Gas,”
Japan Petroleum Institute. Ishida, M., and Kawamura, K., 1982, “Energy and
Exergy Analysis of a Chemical Process System
Ishida, M., 1983, “Analysis of Hierarchical
with Distributed Parameters Based on Enthalpy -
Structure of a Process System Based on Energy and
Direction Factor Diagram,” Ind. Eng. Chem.
Exergy Transformation,” Efficiency and Costing,
Process Des. Dev., Vol. 21, 690-695.
ACS Symposium Series 235, R. A. Gaggioli ed.,
Washington, D.C., pp. 179-211. Ishida, M. and Nakagawa, N., 1985, “Exergy
Analysis of Pervaporation System Based on an
Ishida, M., 1995, “Thermodynamics - Its Perfect
Energy-Utilization Diagram,” J. Membrane Sci.,
Comprehension and Application - (in Japanese),”
Vol. 24, pp. 271-283.
Baifukan, Tokyo.
Ishida, M. and Yamamoto, M., 1999, “Comparison
Ishida, M., 1999, “How Can We Jump in Process
116 Int.J. Applied Thermodynamics, Vol.5 (No.3)
of Various Methods to Represent Energy Features Yamamoto, M. and Ishida, M., 1999, “Process
of a Total System,” Proc. of ASME Advanced Vectors Both to Create Functional Structures and to
Energy Division, AES-39, pp. 649-654, Nashville. Represent Characteristics of an Entire System,”
Umeda, T. et al., 1979, “A Thermodynamic Efficiency, Costs, Optimization, Simulation, and
Environmental Aspects of Energy Systems, Proc. of
Approach to the Synthesis of Heat Integration
Systems in Chemical Processes,” Comput. Chem. ECOS’99, pp. 147-152, Tokyo.
Eng. 3, pp. 273-282.
Int.J. Applied Thermodynamics, Vol.5 (No.3) 117
Anda mungkin juga menyukai
- Unidad 2 Sistema Sec Uac I OnesDokumen15 halamanUnidad 2 Sistema Sec Uac I OnesRAUL OSORIO RAMOSBelum ada peringkat
- Hybrid Systems - TutorialDokumen66 halamanHybrid Systems - TutorialKarthik VazhuthiBelum ada peringkat
- Unit 2: Transfer Function: PrefaceDokumen9 halamanUnit 2: Transfer Function: PrefaceNeans PlanterasBelum ada peringkat
- Algebraic Aspects of Linear Control System Stability: (LS) Stabilizability/detectabilityDokumen9 halamanAlgebraic Aspects of Linear Control System Stability: (LS) Stabilizability/detectabilityBilal UsmaniBelum ada peringkat
- Lecture Chapter1 8newDokumen188 halamanLecture Chapter1 8newDinuka RavimalBelum ada peringkat
- Mass EnergyDokumen64 halamanMass EnergyhlvijaykumarBelum ada peringkat
- ManzanoPaule2018 Chapter OpenQuantumSystemsDynamicsDokumen56 halamanManzanoPaule2018 Chapter OpenQuantumSystemsDynamicsفارس الزهريBelum ada peringkat
- 2intro To Physical SystemDokumen4 halaman2intro To Physical SystemMehmet Akif DemirlekBelum ada peringkat
- CH 2Dokumen95 halamanCH 2유지상Belum ada peringkat
- Connecting Transistors and Proteins: Claudio Mattiussi, Dario FloreanoDokumen6 halamanConnecting Transistors and Proteins: Claudio Mattiussi, Dario FloreanozamadaniBelum ada peringkat
- Nonequilibrium Work Relations Foundations and ApplicationsDokumen10 halamanNonequilibrium Work Relations Foundations and ApplicationsMaryalbis A. Patiño S.Belum ada peringkat
- Chapter 2Dokumen38 halamanChapter 2徐郁真Belum ada peringkat
- Compact Heat Exchangers Selection Application Design and EvaluationDokumen18 halamanCompact Heat Exchangers Selection Application Design and EvaluationLailatul QidsiyahBelum ada peringkat
- Hybrid Dynamic Systems Tutorial PDFDokumen66 halamanHybrid Dynamic Systems Tutorial PDFkkkprot100% (1)
- FEM Theory AnswersDokumen45 halamanFEM Theory Answerskhushendrafule14Belum ada peringkat
- 03MathematicalModellingOfChemicalProcesses - Doc 0Dokumen12 halaman03MathematicalModellingOfChemicalProcesses - Doc 0Anonymous P1iMibBelum ada peringkat
- Thermal HydraulicAnalysisofNuclearReactorsChapter2Dokumen49 halamanThermal HydraulicAnalysisofNuclearReactorsChapter2Sai SooryaBelum ada peringkat
- Bond Graph IntroDokumen4 halamanBond Graph IntroPablo SilvaBelum ada peringkat
- Fundamentals of Energy ConversionDokumen39 halamanFundamentals of Energy ConversionJohnBelum ada peringkat
- Jarzynski Statphys23 EpjbDokumen11 halamanJarzynski Statphys23 EpjbMehdi RezaieBelum ada peringkat
- (Ebook) Multivariable Control, An Introduction PDFDokumen14 halaman(Ebook) Multivariable Control, An Introduction PDFCaterine Feria RamirezBelum ada peringkat
- 2016acc 0370 MSDokumen6 halaman2016acc 0370 MSYurdun OrbakBelum ada peringkat
- Fuzzy Control of A Biomass Fired and SolDokumen10 halamanFuzzy Control of A Biomass Fired and SolRoberto ZuñigaBelum ada peringkat
- The Inherent Instability of Disordered Systems: New England Complex Systems Institute 277 Broadway Cambridge, MA 02139Dokumen24 halamanThe Inherent Instability of Disordered Systems: New England Complex Systems Institute 277 Broadway Cambridge, MA 02139poheiBelum ada peringkat
- UML Analysis Using State DiagramsDokumen7 halamanUML Analysis Using State DiagramsronistgdrBelum ada peringkat
- Bond Graph Causality Assignment and Evolutionary Multi-Objective OptimizationDokumen7 halamanBond Graph Causality Assignment and Evolutionary Multi-Objective OptimizationHugo MendesBelum ada peringkat
- Balance de Exerg Ia: Sistemas Cerrados Y Abiertos Exergy Balance: Open and Closeds SystemsDokumen3 halamanBalance de Exerg Ia: Sistemas Cerrados Y Abiertos Exergy Balance: Open and Closeds SystemsLuis CaizaBelum ada peringkat
- MEG 212 Week 3 Lecture 2023 UpdatedDokumen30 halamanMEG 212 Week 3 Lecture 2023 Updatedmazinomaro20Belum ada peringkat
- Analysis of Oscillations With Eigenanalysis andDokumen8 halamanAnalysis of Oscillations With Eigenanalysis andMadhusudhan SrinivasanBelum ada peringkat
- Capitulo 2 y 3Dokumen49 halamanCapitulo 2 y 3Marco GuerreroBelum ada peringkat
- Capitulo 1Dokumen16 halamanCapitulo 1Marco GuerreroBelum ada peringkat
- Control Systems Engineering 4Dokumen5 halamanControl Systems Engineering 4ABelum ada peringkat
- Modal AnalysisDokumen26 halamanModal AnalysisTrinh Duy TanBelum ada peringkat
- Compendium 3Dokumen5 halamanCompendium 3m.jovanoskaBelum ada peringkat
- Investigation of Damping Effects On Statistical Energy Analysis of Coupled StructuresDokumen21 halamanInvestigation of Damping Effects On Statistical Energy Analysis of Coupled StructuresAhmad WaalBelum ada peringkat
- Ijret - Investigation of Behaviour of 3 Degrees of Freedom Systems For Transient LoadsDokumen12 halamanIjret - Investigation of Behaviour of 3 Degrees of Freedom Systems For Transient LoadsInternational Journal of Research in Engineering and TechnologyBelum ada peringkat
- Chapter ThreeDokumen13 halamanChapter ThreeGetahun tuluBelum ada peringkat
- Mathematical Modeling: This Is Expressed As Follows in (5) : "All Models Are Wrong, But Some Are Useful."Dokumen22 halamanMathematical Modeling: This Is Expressed As Follows in (5) : "All Models Are Wrong, But Some Are Useful."Tamer AbdouBelum ada peringkat
- First Page PDFDokumen1 halamanFirst Page PDFmaatheussoouzaaBelum ada peringkat
- Modeling Chemical Reactions Using Bond Graphs.: July 2012Dokumen13 halamanModeling Chemical Reactions Using Bond Graphs.: July 2012Mejbahul SarkerBelum ada peringkat
- ECE11L CRUZ CARLOUI Group2 Lab 2 PDFDokumen8 halamanECE11L CRUZ CARLOUI Group2 Lab 2 PDFCarl Kevin CartijanoBelum ada peringkat
- Geometrical Modeling For Material Flow Management: Reggie DavidrajuhDokumen7 halamanGeometrical Modeling For Material Flow Management: Reggie DavidrajuhZeeshan HaiderBelum ada peringkat
- A Review of The Potts ModelDokumen26 halamanA Review of The Potts ModelBarthélemy HoubenBelum ada peringkat
- Linear Graph Modeling: One-Port ElementsDokumen43 halamanLinear Graph Modeling: One-Port ElementstemkimleangBelum ada peringkat
- H. DeVoe - Particle Model For Work, Heat, and The Energy of A Thermodynamic SystemDokumen9 halamanH. DeVoe - Particle Model For Work, Heat, and The Energy of A Thermodynamic SystemAnonymous RjqSfKmIBelum ada peringkat
- Concept of ThermalDokumen19 halamanConcept of ThermalAamirqadriBelum ada peringkat
- محاضرة مبادئ الهندسة الكيمياوية PDFDokumen121 halamanمحاضرة مبادئ الهندسة الكيمياوية PDFKING of DARKNESSBelum ada peringkat
- Mathematical Modeling of Systems: TextbookDokumen3 halamanMathematical Modeling of Systems: TextbookramBelum ada peringkat
- You'Ve Got A Problem: DV DT C M V V T Time S, G, CDokumen3 halamanYou'Ve Got A Problem: DV DT C M V V T Time S, G, CagusBelum ada peringkat
- Assignment 02: 1 What Is Meant by Mathematical Modelling of A Control SystemDokumen2 halamanAssignment 02: 1 What Is Meant by Mathematical Modelling of A Control SystemSyed AfzalBelum ada peringkat
- Module 004 - Block Diagram of Control Systems: System ModelingDokumen22 halamanModule 004 - Block Diagram of Control Systems: System ModelingDiane GutierrezBelum ada peringkat
- Vorlesung WeberDokumen19 halamanVorlesung Weberdetki007Belum ada peringkat
- Multi-Criteria Assessment of New and Renewable Energy Power PlantsDokumen18 halamanMulti-Criteria Assessment of New and Renewable Energy Power PlantsandresszzBelum ada peringkat
- Flairs04 020Dokumen6 halamanFlairs04 020saleh90Belum ada peringkat
- Linear Graph Modeling: One-Port ElementsDokumen2 halamanLinear Graph Modeling: One-Port ElementszobarogluBelum ada peringkat
- Notes Final Corrd 8-9-11-DistDokumen168 halamanNotes Final Corrd 8-9-11-DistirenerefaatBelum ada peringkat
- Integrated Imaging of the Earth: Theory and ApplicationsDari EverandIntegrated Imaging of the Earth: Theory and ApplicationsMax MoorkampBelum ada peringkat
- Coupled CFD-DEM Modeling: Formulation, Implementation and Application to Multiphase FlowsDari EverandCoupled CFD-DEM Modeling: Formulation, Implementation and Application to Multiphase FlowsBelum ada peringkat
- Reactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsDari EverandReactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFranz-Josef SchmittBelum ada peringkat
- Reviews in Computational ChemistryDari EverandReviews in Computational ChemistryAbby L. ParrillBelum ada peringkat
- Pervaporation PDFDokumen7 halamanPervaporation PDFbai tap hoa vo coBelum ada peringkat
- Phương pháp bay hơi thẩm thấu qua màngDokumen14 halamanPhương pháp bay hơi thẩm thấu qua màngcombo162Belum ada peringkat
- Pervaporation 4 Separating Acetic Acid & Water Mixture Using Hollow Fiber MembranesDokumen254 halamanPervaporation 4 Separating Acetic Acid & Water Mixture Using Hollow Fiber Membranesapi-26678889Belum ada peringkat
- Pervaporation of Hydrazine-Water Through Hollow Fiber ModuleDokumen13 halamanPervaporation of Hydrazine-Water Through Hollow Fiber Moduleapi-26678889Belum ada peringkat
- Pervaporation Membrane Process For Vapour Absorption System: S.B. Riffat, S. Wu, B. BolDokumen8 halamanPervaporation Membrane Process For Vapour Absorption System: S.B. Riffat, S. Wu, B. Bolapi-26678889Belum ada peringkat
- REPORTDokumen76 halamanREPORTapi-26678889Belum ada peringkat
- Chapter 1 Background ( (1-6) : Kober (1995) Binning Et Al. (1961)Dokumen7 halamanChapter 1 Background ( (1-6) : Kober (1995) Binning Et Al. (1961)api-26678889Belum ada peringkat
- Pervaporation 4 Separating Acetic Acid & Water Mixture Using Hollow Fiber MembranesDokumen254 halamanPervaporation 4 Separating Acetic Acid & Water Mixture Using Hollow Fiber Membranesapi-26678889Belum ada peringkat
- Chapter 1 (: 1. (Mulder 1991)Dokumen3 halamanChapter 1 (: 1. (Mulder 1991)api-26678889Belum ada peringkat
- G M Madhu: Department of Chemical EngineeringDokumen30 halamanG M Madhu: Department of Chemical Engineeringapi-26678889Belum ada peringkat
- Pervaporation Separation of Ethylacetate-Water MixturesDokumen6 halamanPervaporation Separation of Ethylacetate-Water Mixturesapi-26678889Belum ada peringkat
- Water Use by Ethanol PlantsDokumen8 halamanWater Use by Ethanol PlantsRCNGBBelum ada peringkat
- Improved Pervaporation Performance of Locally-Produced Chitosan Membranes Mohd Ghazali Mohd Nawawi and Hashim HassanDokumen8 halamanImproved Pervaporation Performance of Locally-Produced Chitosan Membranes Mohd Ghazali Mohd Nawawi and Hashim Hassanapi-26678889Belum ada peringkat
- Agriculture Law: RL33928Dokumen16 halamanAgriculture Law: RL33928AgricultureCaseLawBelum ada peringkat
- E85: Future Gasoline Substitute Fuel TechnologyDokumen45 halamanE85: Future Gasoline Substitute Fuel Technologyapi-26678889Belum ada peringkat
- Index: 1 Introduction To Tobacco 1Dokumen4 halamanIndex: 1 Introduction To Tobacco 1api-26678889Belum ada peringkat
- Ethanol: Impacts On Soil and Water QualityDokumen20 halamanEthanol: Impacts On Soil and Water Qualityapi-26678889Belum ada peringkat
- Very Very ImportantDokumen225 halamanVery Very Importantapi-26678889Belum ada peringkat
- Ethanol 101 For The Gasoline RetailerDokumen31 halamanEthanol 101 For The Gasoline Retailerapi-26678889Belum ada peringkat
- Donal KeaneDokumen18 halamanDonal Keaneapi-26678889Belum ada peringkat
- 3MANOEXMPLEDokumen5 halaman3MANOEXMPLEBlue SkyBelum ada peringkat
- Fresh Water Generator GME 37Dokumen30 halamanFresh Water Generator GME 37srinidhi246100% (2)
- Assessment and Modelling of Dust Concentration in An OpencastDokumen10 halamanAssessment and Modelling of Dust Concentration in An OpencastAsri GaniBelum ada peringkat
- Chap03glencoe PDFDokumen32 halamanChap03glencoe PDFCecilia AugustinBelum ada peringkat
- Home Assignment I PHY 105/PHY 2105 PhysicsDokumen1 halamanHome Assignment I PHY 105/PHY 2105 PhysicsMd. Imran AhmedBelum ada peringkat
- Q2 Summative Test in Science 8Dokumen3 halamanQ2 Summative Test in Science 8MARICEL CANTARABelum ada peringkat
- 1Dokumen22 halaman1Anonymous o29GUBm1TcBelum ada peringkat
- Skema Jawapan PPT Kimia f4 Bahagian C 2021Dokumen10 halamanSkema Jawapan PPT Kimia f4 Bahagian C 2021Fiza MdNgisumBelum ada peringkat
- Geography HSC NotesDokumen47 halamanGeography HSC NotesKaoru SetaBelum ada peringkat
- C W PDV DV V V W PV VDokumen4 halamanC W PDV DV V V W PV VRizwan Ullah BaigBelum ada peringkat
- Pioneering Venus A Planet UnveiledDokumen373 halamanPioneering Venus A Planet UnveiledBob Andrepont100% (2)
- Issues of Pesticide + DDTDokumen10 halamanIssues of Pesticide + DDTTanvi BhanushaliBelum ada peringkat
- Types of Concentrating Solar CollectorsDokumen6 halamanTypes of Concentrating Solar Collectorsshivam agarwalBelum ada peringkat
- Earth Science 8 ReviewerDokumen11 halamanEarth Science 8 ReviewerBraynell Owen ClaroBelum ada peringkat
- CultureDokumen2 halamanCultureRo GomezBelum ada peringkat
- Mathematical Model of Ejector and Experimental Verification PDFDokumen4 halamanMathematical Model of Ejector and Experimental Verification PDFRavi RanjanBelum ada peringkat
- The Perfect Answer Revision Guide To... : Cambridge IgcseDokumen105 halamanThe Perfect Answer Revision Guide To... : Cambridge IgcseOmar AlnaggarBelum ada peringkat
- Lesson 3 Weather PatternsDokumen7 halamanLesson 3 Weather PatternsFernando BogranBelum ada peringkat
- D 8009 (Geography) IIDokumen24 halamanD 8009 (Geography) IIBiki KunduBelum ada peringkat
- UntitledDokumen523 halamanUntitledTanayBelum ada peringkat
- Caustic Soda & Chlorine ManufacturingDokumen43 halamanCaustic Soda & Chlorine ManufacturingahmadrBelum ada peringkat
- Economic 06Dokumen51 halamanEconomic 06ahmed elbatawyBelum ada peringkat
- By NR Commercial GroupDokumen30 halamanBy NR Commercial GroupAmit Kumar GuptaBelum ada peringkat
- Stirling Engine & ApplicationsDokumen32 halamanStirling Engine & ApplicationsLASINBelum ada peringkat
- Modeling and Simulation For A 3.5 KW Grid Connected Photo Voltaic Power SystemDokumen7 halamanModeling and Simulation For A 3.5 KW Grid Connected Photo Voltaic Power SystemEditor IJTSRDBelum ada peringkat
- Genetically Modified OrganismsDokumen3 halamanGenetically Modified OrganismsВладаBelum ada peringkat
- CKLaw Problems Chap 6Dokumen4 halamanCKLaw Problems Chap 6f0% (1)
- Isa PDFDokumen7 halamanIsa PDFairsorBelum ada peringkat
- Corwin IC8 Lectures Ch04Dokumen46 halamanCorwin IC8 Lectures Ch04Agustino MaicahBelum ada peringkat
- How Bangalore Gets ElectricityDokumen1 halamanHow Bangalore Gets ElectricityNabeel AhmedBelum ada peringkat