Calorimeter 04401.00: Operating Instructions
Diunggah oleh
Diego Fernado Avendaño0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
79 tayangan2 halamanThis document provides instructions for using a calorimeter to determine the specific heat of solids and liquids. The calorimeter contains an electric heating element and can measure temperatures up to 100°C. For solids, the mixing method is used which involves heating a sample and mixing it with water in the calorimeter. For liquids, the electric heating method directly heats the liquid sample and measures its temperature increase. Proper use requires precisely measuring temperatures, controlling heating times and voltages, and accounting for heat loss to obtain accurate specific heat measurements.
Deskripsi Asli:
Calorimeter
Judul Asli
Calorimeter
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniThis document provides instructions for using a calorimeter to determine the specific heat of solids and liquids. The calorimeter contains an electric heating element and can measure temperatures up to 100°C. For solids, the mixing method is used which involves heating a sample and mixing it with water in the calorimeter. For liquids, the electric heating method directly heats the liquid sample and measures its temperature increase. Proper use requires precisely measuring temperatures, controlling heating times and voltages, and accounting for heat loss to obtain accurate specific heat measurements.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
79 tayangan2 halamanCalorimeter 04401.00: Operating Instructions
Diunggah oleh
Diego Fernado AvendañoThis document provides instructions for using a calorimeter to determine the specific heat of solids and liquids. The calorimeter contains an electric heating element and can measure temperatures up to 100°C. For solids, the mixing method is used which involves heating a sample and mixing it with water in the calorimeter. For liquids, the electric heating method directly heats the liquid sample and measures its temperature increase. Proper use requires precisely measuring temperatures, controlling heating times and voltages, and accounting for heat loss to obtain accurate specific heat measurements.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 2
Calorimeter
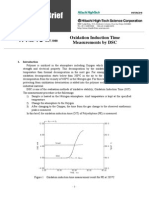
1. PURPOSE AND DESCRIPTION
Calorimeter 04401.00 is a water calorimeter with which the
specific heat of solids or liquids can be determined. Con-
version energies, such as the melting energy of ice, can
also be determined.
The unit is fitted with an electric heating system to heat up
the contents. Power is supplied to the heating resistor over
two 4 mm sockets in the lid. The lid has an orifice (d = 10
mm) to introduce a thermometer or a temperature probe. An
agitator with a yoke handle, which can be lifted, passes
through two smaller orifices in the lid. The calorimeter is de-
signed to stand temperatures up to 100 C.
2. HANDLING
The handling is explained taking the determination of spe-
cific thermal capacities as an example. In the case of solids,
the mixing method is used, for liquids, energy is added by
means of the electric heating element.
In order to accelerate thermal equilibrium in the calorimeter,
the agitator should be continuously moved during the ex-
periment. It may, however, not be lifted to the point where
water is splashed onto the styrofoam lining of the lid.
2.1 Mixing metod
To determine the specific heat of solids according to the
mixing method, a sample body of known temperature and
mass is brought into thermal contact with a quantity of water
of known temperature and thermal capacity inside the
calorimeter. The specific heat of the sample is calculated
from the temperature of the mixture after thermal equilib-
rium is reached.
Carrying out of experiment:
- An adequate quantity of liquid (200 ml or more) is
weighed and filled into the calorimeter.
- The sample body is hanged from a support by means
of a fishing thread and heated in a sufficiently station-
ary water bath, e. g. in boiling water to 100 C. A gauze
bag may be used to carry several small samples.
- The temperature of the heated sample (temperature of
the bath) and the temperature of the water in the
calorimeter are read as precisely as possible from the
corresponding thermometers immediately before im-
mersing the sample in the calorimeter.
- When one is certain the sample body has reached the
temperature of the bath, it is immersed as fast as pos-
sible in the calorimeter. The calorimeter lid is closed at
once and agitation starts.
- When temperature in the calorimeter begins to drop
due to thermal release to the environment, maximum
temperature is read as temperature of the mixture.
2.2 Electric heating method
To determine the specific heat of liquids, a quantity of liquid
of known mass and temperature is filled into the calorime-
ter and heated by the electric heating element. The specific
heat of the liquid is calculated from energy input and tem-
perature increase.
Next to water, only such liquids may be filled into the
calorimeter, which do not attack aluminium, nickel or styro-
foam, e. g. all types of alcohol. Only alternating current
should be used, to avoid corrosion of the heating element.
It is furthermore recommended to use highly purified (dis-
tilled) water.
PHYWE SYSTEME GMBH Robert-Bosch-Breite 10 D-37079 Gttingen Telefon (05 51) 6 04-0 Telefax (05 51) 60 4107
04401.00
Operating Instructions
R
3. EXPERIMENTING REFERENCE LITERATURE
Physik in Schlerversuchen, Ausgabe A/B 01130.01
Physik in Demonstrationsversuchen,
Ausgabe A/B, Elektrik 01141.31
Physik in Demonstrationsversuchen,
Ausgabe A/B, Wrme 01141.51
Physik in Demonstrationsversuchen,
Ausgabe C, Teil 1 01146.01
Physik in Demonstrationsversuchen,
Ausgabe C, Teil 2 01146.11
University laboratory experiments 00067.72
4. TECHNICAL SPECIFICATIONS
Type Water calorimeter
Exterior dimensions d = 134 mm; h = 160 mm
Thermal insulation Styrofoam
Calorimetric capacity approx. 70 J/C
Calorimeter vessel
Material aluminium
Capacity 500 ml
Dimensions d = 88 mm, h = 92 mm
Heating element
Material Canthal
Resistance 2.4 0.2
Max. power
in water 60 W (12 V/5 A)
in air 10 W (5 V/2 A)
Operating power alternating voltage
The heating element can only be operated when it is com-
pletely immersed in the liquid. A filling quantity of 200 ml is
sufficient for this.
The amount of added electric energy is determined mea-
suring current intensity, voltage and heating time. Adequate
current intensities: 3 ... 5 A. Adjustment of the supply volt-
age should be determined in a preliminary experiment, so
that the required heating energy will be immediately avail-
able during the main experiment.
Carrying out the experiment:
- An adequate amount of liquid (200 ml or more) are
weighed and filled into the calorimeter.
- Initial temperature is read; voltage supply and
chronometer are switched on; continuous agitation is
assured.
- After temperature has increased by 5 - 10 C for ex-
ample, voltage supply and chronometer are switched
off simultaneously and the maximum value displayed
by the thermometer, before temperature stops increas-
ing, is read.
To obtain a more precise measurement, thermal energy re-
leased by the calorimeter to the environment must be taken
into account. This is achieved by means of a correction of
the read final temperature:
- a second experiment is carried out with the same
calorimeter contents, during which the drop of temper-
ature is measured at the average heating up temper-
ature during a period of time which corresponds to the
total heating time during the main experiment. Calcula-
tion is now repeated with the final temperature in-
creased by .
2
04401.00
Anda mungkin juga menyukai
- Ian Talks Thermodynamics A-Z: PhysicsAtoZ, #3Dari EverandIan Talks Thermodynamics A-Z: PhysicsAtoZ, #3Penilaian: 5 dari 5 bintang5/5 (1)
- Marcet Boiler Experiment.Dokumen9 halamanMarcet Boiler Experiment.Perez Liber50% (2)
- Sunway Practical Lab Bicarbonate Decomposition 2012Dokumen11 halamanSunway Practical Lab Bicarbonate Decomposition 2012venkieeBelum ada peringkat
- Cooling TowerDokumen22 halamanCooling TowerAsuna100% (2)
- Determination of The Melting Enthalpy of A Pure SubstanceDokumen5 halamanDetermination of The Melting Enthalpy of A Pure SubstanceLuis GuevaraBelum ada peringkat
- 50 Heat and TemperatureDokumen3 halaman50 Heat and TemperatureS'chneider AgudeloBelum ada peringkat
- Cooling Tower ReportDokumen13 halamanCooling Tower Reportjuaxxo100% (1)
- Marcet Boiler Lab ReportDokumen12 halamanMarcet Boiler Lab ReportShameerSamsuriBelum ada peringkat
- E5 - Heat Exchangers - Thermal Sciences LabDokumen8 halamanE5 - Heat Exchangers - Thermal Sciences LabBasel BkBelum ada peringkat
- Amount of Energy in A Fuel Sample by Using A Calorimeter BombDokumen4 halamanAmount of Energy in A Fuel Sample by Using A Calorimeter Bombkokoy123Belum ada peringkat
- Mercet BoilerDokumen7 halamanMercet BoilerDafiMaboBelum ada peringkat
- Marcet BoilerDokumen10 halamanMarcet BoilerMD Atiqur Rahman Faisal100% (14)
- LHV RDRDokumen6 halamanLHV RDRKarl Rodney CerezoBelum ada peringkat
- Experiment 1: Refrigeration and Mechanical Heat Pump ExperimentDokumen12 halamanExperiment 1: Refrigeration and Mechanical Heat Pump ExperimentMohamad FaizBelum ada peringkat
- Refrigerant Unit Lab ReportDokumen19 halamanRefrigerant Unit Lab Reportakmal100% (2)
- Bomb CalorimeterDokumen4 halamanBomb Calorimeteruserh1911100% (1)
- Thermocouple PDFDokumen5 halamanThermocouple PDFjayesh maliBelum ada peringkat
- Merce T Boiler ExperimentDokumen14 halamanMerce T Boiler ExperimentjevaughnBelum ada peringkat
- Marcet Boiler Lab ReportDokumen27 halamanMarcet Boiler Lab ReportamiraaikharahBelum ada peringkat
- Marcet BoilerDokumen18 halamanMarcet BoilerCendolz IssZulBelum ada peringkat
- 41 Heat-TemperatureDokumen7 halaman41 Heat-TemperatureChess ManBelum ada peringkat
- Thermo CalibrationDokumen10 halamanThermo CalibrationAngelo De AsisBelum ada peringkat
- CalorimeterDokumen2 halamanCalorimeterUrvi MaruBelum ada peringkat
- Marcet BoilerDokumen8 halamanMarcet BoilerOjiSofttouchCharlesBelum ada peringkat
- CHEE220 Heat Engine Lab Jan2014Dokumen4 halamanCHEE220 Heat Engine Lab Jan2014mfuzdnldBelum ada peringkat
- Thermal Lab Marcet BoilerDokumen4 halamanThermal Lab Marcet BoilerJack LiewBelum ada peringkat
- Term Odin A MicaDokumen134 halamanTerm Odin A MicaApril WoodsBelum ada peringkat
- P8 IntDokumen6 halamanP8 Intflores 03Belum ada peringkat
- User Manual SPP MUETDokumen18 halamanUser Manual SPP MUETAlyan YousafBelum ada peringkat
- Electrical To Thermal 1 ManualDokumen6 halamanElectrical To Thermal 1 ManualJake SmithBelum ada peringkat
- Pressure SaturationDokumen8 halamanPressure Saturationyumnaalhinai9Belum ada peringkat
- Latent Heat VaporizationDokumen3 halamanLatent Heat VaporizationerorkinBelum ada peringkat
- M.O Heat PumpDokumen11 halamanM.O Heat PumpLawand RaufBelum ada peringkat
- Full ReportDokumen16 halamanFull ReportafiqahanuwarBelum ada peringkat
- Report of Experiment Ex9: Specific Vaporization Heat of WaterDokumen5 halamanReport of Experiment Ex9: Specific Vaporization Heat of WaterTạ HạnhBelum ada peringkat
- Thermodynamic Lab First Class 2017 - 2018: Technical Engineering College Petrochemical DepartmentDokumen3 halamanThermodynamic Lab First Class 2017 - 2018: Technical Engineering College Petrochemical DepartmentMohammed IhsanBelum ada peringkat
- Physics 41 Calorimetry: Determination of Specific Heat Capacity of CopperDokumen2 halamanPhysics 41 Calorimetry: Determination of Specific Heat Capacity of CopperAmeva Ameve Sinangote CañosoBelum ada peringkat
- Thermodynamics Lab ManualDokumen9 halamanThermodynamics Lab ManualEr Shankar Singh Dhami100% (2)
- Specific Thermal Capacity of AluminiumDokumen18 halamanSpecific Thermal Capacity of AluminiumChrise RajBelum ada peringkat
- Calorimetria FULLDokumen6 halamanCalorimetria FULLferney.velasquezBelum ada peringkat
- Electrical Equivalent of Thermal ModelDokumen4 halamanElectrical Equivalent of Thermal ModelXavier Le LargeBelum ada peringkat
- Manual Del Equipamiento Básico de CalorimetríaDokumen14 halamanManual Del Equipamiento Básico de CalorimetríaEzequiel FrimannBelum ada peringkat
- ExperimentDokumen5 halamanExperimentJackson SonBelum ada peringkat
- Marcet BoilerDokumen10 halamanMarcet BoilerDhia EmpayarBelum ada peringkat
- Cooling Tower Lab Report PDFDokumen17 halamanCooling Tower Lab Report PDFHazieqah100% (1)
- Marcet BoilerDokumen7 halamanMarcet BoilerNAJIHABelum ada peringkat
- التجربه tempertur mesurmentDokumen56 halamanالتجربه tempertur mesurmentجلال البركانيBelum ada peringkat
- Boiler Lab ReportDokumen6 halamanBoiler Lab ReportYomal Wijesinghe0% (2)
- Lab Report Heat Transfer in Laminar Flow and Turbulent Flow: Instructor: Prof. Vinod Narayanan Group 5Dokumen11 halamanLab Report Heat Transfer in Laminar Flow and Turbulent Flow: Instructor: Prof. Vinod Narayanan Group 5Pawan KumarBelum ada peringkat
- Marcet Boiler ReportDokumen20 halamanMarcet Boiler Reportgabrielhii1995Belum ada peringkat
- LS2 - Steam Pressure Curve of Saturated Steam (Marcet Boiler)Dokumen4 halamanLS2 - Steam Pressure Curve of Saturated Steam (Marcet Boiler)faezahjalalBelum ada peringkat
- Cooling Tower Heat and MassDokumen18 halamanCooling Tower Heat and MassChris Mark100% (2)
- Energy Experiment II Heat Pump Emir Can Yılmaz 200304052Dokumen11 halamanEnergy Experiment II Heat Pump Emir Can Yılmaz 200304052CANGOBelum ada peringkat
- Lab 8 - Calorimetry Edit June'14Dokumen11 halamanLab 8 - Calorimetry Edit June'14davidrbadkeBelum ada peringkat
- Experiment 4: Cal CalorimeterDokumen4 halamanExperiment 4: Cal CalorimeterKumaraguru RajaBelum ada peringkat
- Experiment 6 (Calibration of Temperature Measuring Devices - A4)Dokumen16 halamanExperiment 6 (Calibration of Temperature Measuring Devices - A4)Jamiel CatapangBelum ada peringkat
- Bomb Calorimeter Principle, Formula ProcedureDokumen8 halamanBomb Calorimeter Principle, Formula ProcedureJosue RamirezBelum ada peringkat
- Module 1 Activity No. 4Dokumen5 halamanModule 1 Activity No. 4MARIANNEANGEL DEVILLENABelum ada peringkat
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesDari EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesBelum ada peringkat
- Galvanizing Note1Dokumen7 halamanGalvanizing Note1pandey008Belum ada peringkat
- Zeus Weathering of PlasticsDokumen7 halamanZeus Weathering of PlasticsDiego Fernado AvendañoBelum ada peringkat
- Top 10 Management Skills You NeedDokumen5 halamanTop 10 Management Skills You NeedDiego Fernado AvendañoBelum ada peringkat
- Cambios Entre Norma Astm d256Dokumen3 halamanCambios Entre Norma Astm d256Diego Fernado AvendañoBelum ada peringkat
- X RayDiffractionDokumen123 halamanX RayDiffractionDiego Fernado AvendañoBelum ada peringkat
- HDT 3-6 Vicat GB-AP911-06Dokumen6 halamanHDT 3-6 Vicat GB-AP911-06Diego Fernado AvendañoBelum ada peringkat
- Tunneling Microscopy and Spectroscopy: L. J. W, Naval Research Laboratory, Washington, DC 20375-5342Dokumen23 halamanTunneling Microscopy and Spectroscopy: L. J. W, Naval Research Laboratory, Washington, DC 20375-5342Diego Fernado AvendañoBelum ada peringkat
- A Comparative Study of Test Methods For Assessment of Fire Safety Performance of Bus Interior MaterialsDokumen12 halamanA Comparative Study of Test Methods For Assessment of Fire Safety Performance of Bus Interior MaterialsDiego Fernado AvendañoBelum ada peringkat
- Positive Material IdentificationDokumen1 halamanPositive Material IdentificationDiego Fernado AvendañoBelum ada peringkat
- Quanto DeskDokumen4 halamanQuanto DeskDiego Fernado AvendañoBelum ada peringkat
- Measuring The Stray Light Performance of UV-visible SpectrophotometersDokumen4 halamanMeasuring The Stray Light Performance of UV-visible SpectrophotometersDiego Fernado AvendañoBelum ada peringkat
- Application TA 049eDokumen3 halamanApplication TA 049eDiego Fernado AvendañoBelum ada peringkat
- HFT Sensors: MovementDokumen2 halamanHFT Sensors: MovementDiego Fernado AvendañoBelum ada peringkat
- Emissivity Measurements of Common Construction MaterialsDokumen9 halamanEmissivity Measurements of Common Construction MaterialsDiego Fernado AvendañoBelum ada peringkat
- Topic: Chemical Combination: Na 2, 8, 1 CL 2, 8, 7 Na 2, 8 CL 2, 8, 8 Na + CL NaclDokumen2 halamanTopic: Chemical Combination: Na 2, 8, 1 CL 2, 8, 7 Na 2, 8 CL 2, 8, 8 Na + CL NaclVictor OkosunBelum ada peringkat
- Physics Q PaperDokumen2 halamanPhysics Q Papermaulik2323Belum ada peringkat
- Gas SolubilityDokumen59 halamanGas SolubilityAustine Ameh0% (1)
- Aluminium NitrateDokumen3 halamanAluminium NitrategumtammBelum ada peringkat
- Elements Mixtures and Compound Plan Grade 9 APSEDokumen9 halamanElements Mixtures and Compound Plan Grade 9 APSETrudy- Ann CaineBelum ada peringkat
- OkDokumen24 halamanOkSamsulBelum ada peringkat
- C3642 eDokumen3 halamanC3642 eErwin CabangalBelum ada peringkat
- Last Name, First CHE426:: F 33.54 GPMDokumen5 halamanLast Name, First CHE426:: F 33.54 GPMGiancarlo HuertaBelum ada peringkat
- Heterogeneous Catalysis and Solid Catalysts 1Dokumen117 halamanHeterogeneous Catalysis and Solid Catalysts 1lotannaBelum ada peringkat
- Pele de CaçãoDokumen8 halamanPele de CaçãoJuliano SouzaBelum ada peringkat
- January 2020 Chemistry Mark Scheme 1CDokumen6 halamanJanuary 2020 Chemistry Mark Scheme 1CMeenakshie ChaudrieBelum ada peringkat
- Bulletin 23 PDFDokumen20 halamanBulletin 23 PDFmanuelavfBelum ada peringkat
- Lecture-III Basics of Pinch Analysis - 2Dokumen35 halamanLecture-III Basics of Pinch Analysis - 2Chalachew NigussieBelum ada peringkat
- 1problems THERMODYNAMICSDokumen4 halaman1problems THERMODYNAMICSKim AquilingBelum ada peringkat
- G 17Dokumen18 halamanG 17Serious ExamBelum ada peringkat
- The Gibbs Phase Rule RevisitedDokumen3 halamanThe Gibbs Phase Rule Revisitedleizar_death640% (1)
- Fabry Perot PDFDokumen2 halamanFabry Perot PDFAnitaBelum ada peringkat
- Comparison of Adsorption Equilibrium of Fructose, Glucose and Sucrose On PDFDokumen6 halamanComparison of Adsorption Equilibrium of Fructose, Glucose and Sucrose On PDFDUVAN FELIPE ABRIL BLANCOBelum ada peringkat
- Lab Questions AnalatycalDokumen9 halamanLab Questions AnalatycalMayson BaliBelum ada peringkat
- Reverse Osmosis ReportDokumen25 halamanReverse Osmosis ReportMuhammad Ishfaq100% (1)
- MKKKLDokumen21 halamanMKKKLdaney67299Belum ada peringkat
- Assignment2 SolDokumen4 halamanAssignment2 SolSrea11Belum ada peringkat
- CBSE XII Chemistry Project Spectroscopy and Its ApplicationsDokumen21 halamanCBSE XII Chemistry Project Spectroscopy and Its ApplicationsRichie SinghBelum ada peringkat
- JurnalDokumen7 halamanJurnalReno SaktianBelum ada peringkat
- Chapter 8: Thermal Conductivity and The Mechanism of Heat TransportDokumen33 halamanChapter 8: Thermal Conductivity and The Mechanism of Heat TransportConrad MonterolaBelum ada peringkat
- Technip Document No. 068107C001-702-PDS-0610-003-0Dokumen10 halamanTechnip Document No. 068107C001-702-PDS-0610-003-0Ryan Goh Chuang HongBelum ada peringkat
- Activity7 Cabbage ChemistryDokumen3 halamanActivity7 Cabbage ChemistryJohn Hayden Dela CruzBelum ada peringkat
- MCQ 1Dokumen94 halamanMCQ 1AkshayBelum ada peringkat
- 2018 Nanodiamonds Synthesis Argon EthanolDokumen11 halaman2018 Nanodiamonds Synthesis Argon EthanolYoucef FermiBelum ada peringkat
- Chapter 4electric Fields in MatterDokumen51 halamanChapter 4electric Fields in MatterAxel Coronado PopperBelum ada peringkat