Isek. Men. Keb. Sultan Ismail, Johor Bahru. Physical Chemistry/ Upper Six/ 2013 Topic: Chemical Energetics
Diunggah oleh
Ph'ng Jiun YanJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Isek. Men. Keb. Sultan Ismail, Johor Bahru. Physical Chemistry/ Upper Six/ 2013 Topic: Chemical Energetics
Diunggah oleh
Ph'ng Jiun YanHak Cipta:
Format Tersedia
1 | G S S
iSEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
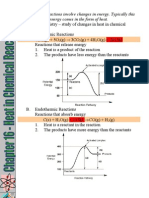
EXOTHERMIC AND ENDOTHERMIC REACTIONS
1. In all chemical reactions, both bond formations and bond cleavages occur.
2. Bond formation releases heat energy to the reaction mixture or environment. This
is manifested with a temperature rise of the reaction mixture.
3. Bond cleavage absorbs heat energy from the reaction mixture or environment. As a
result of this, the temperature of the reaction mixture falls.
4. If in a chemical reaction the total heat energy released due to bond formations is
larger than the total heat energy absorbed from the reaction mixture due to bond
cleavages, then the overall chemical reaction is a heat energy loss reaction. Such a
chemical reaction is termed as an exothermic reaction.
5. On the other hand, if the total heat energy absorbed due to bond cleavages is larger
than the heat energy released due to bond formations, then the overall chemical
reaction is a heat energy absorbing reaction. Such a chemical reaction is known as
the endothermic reaction.
6. Exothermic reaction causes temperature rise of the reaction mixture while
endothermic reaction causes temperature fall of the reaction mixture.
7. Hence, this change in temperature of the reaction mixture can be used as a parameter
to determine the amount of heat energy change (E) or enthalpy change (H) that
occurs in a chemical reaction.
ENTHALPY AND ENTHALPY CHANGE
1. All chemical and physical reactions are accompanied by changes in energy content.
The study of these energy changes is called energetic.
2. The enrgy stored in compound is called the energy content or the enthalpy of the
compound. It is not possible to measure enthalpies directly, but it is possible to
measure both enthalpy change associated with a chemical change. The enthalpy
change represents the difference between the enthalpies of the reactants and products.
H = H
p
- H
r
where H = change in enthalpy
H
p
= enthalpy of the products
H
r
= enthalpy of the reactants
3. Enthalpy is the energy content of a substance and is symbolized as H. The energy
content is in the form of kinetic energy and potential energy.
4. The enthalpy of a substance depends on :
(a) the temperature under which the experiment is carried out
(b) the external pressure
(c) the physical state of the substances involved
(d) the concentration of solutions
(e) the type of allotrope of the reactants.
2 | G S S
5. When a chemical reaction occurs, reactants and products are formed. The enthalpy of
the reactants and the products are not the same. Hence, an enthalpy change, H
occurs during a chemical reaction.
A + B C + D
reactants products
H = H
products
H
reactants
If H
products
> H
reactants
, then H > 0 (positive)
This means the reacting species draw heat energy from the surroundings and
increase the enthalpy, causing them to change to products. This causes the
temperature of the reaction mixture and its surroundings to fall. This is called
the endothermic reaction.
If H
products
< H
reactants
, then H < 0 (negative)
This means the reacting species release heat energy to the surroundings and
decrease the enthalpy, causing them to change to products. This causes the
temperature of the reaction mixture and its surroundings to rise. This is called
the exothermic reaction.
6. Enthalpy change, H is referred to as the heat energy change that occurs in a
chemical reaction at constant pressure. The enthalpy change only depends on the
initial and final energy states of the reaction mixture or the system. The normal unit
of H is kJmol
-1
.
Question
Why does an enthalpy change occur during a chemical reaction ?
Answer
This is because during a chemical reaction, old bonds in reactants are broken and new
bonds in products are formed. Hence, the state of the reactants and products are altered
resulting in an enthalpy change.
3 | G S S
The Standard Conditions for Thermochemical Measurements
1. The standard conditions of temperature and pressure for thermochemical
measurements are 298K (25
0
C) and 1 atm (or 101 kPa) respectively.
2. Any enthalpy change measured under these conditions is described as a standard heat
of reaction and given the symbol H
.
3. The value of an enthalpy change depends on :
(a) the temperature under which the experiment is carried out
(b) the external pressure
(c) the physical state of the substances involved
(d) the concentration of solutions
(e) the type of allotrope of the reactants.
4. The standard heat of reaction is defined as the amount of heat absorbed or evolved
when the amounts of reactants as stated in the reaction equation react together under
standard conditions (i.e. at a pressure of 1 atm, at a temperature of 298k, with
substances in their normal physical states under these conditions and solutions having
concentration of 1.0moldm
-3
) to give products in their standard states.
5. The following are two important rules for manipulating thermochemical equations :
(a) When a thermochemical equation is multiplied by any factor, the value of H
for the new equation is obtained by multiplying the value of H in the original
equation by that same factor.
(b) When a chemical equation is reversed, the value of H is reversed in sign.
(c) For example, consider the thermochemical equation for the synthesis of
ammonia :
N
2(g)
+ 3H
2(g)
2NH
3(g)
H = -92 kJ
(i) Doubling the reactants, the H would be 2 (-92kJ) = -184 kJ
2N
2(g)
+ 6H
2(g)
4NH
3(g)
H = -184kJ
(ii) Reserving the equation gives the dissociation of two moles of ammonia
into its elements :
2NH
3(g)
N
2(g)
+ 3H
2(g)
H = +92 kJ
4 | G S S
Calorimetry
1. Calorimetry is the experimental technique to determine the enthalpy
change of a reaction.
2. It consists of a well insulated container called a calorimeter inside which
the reaction occurs.
3. The energy evolved during the reaction is transferred to water or to the
reaction mixture itself, and the change in temperature is noted.
4. The heat change (Q) can then be calculated using the following equation :
Q = mct
Where m = mass of the solution
c = specific heat of the solution
t = change in temperature.
termometer
stirer
insulation
Reacting
system
5 | G S S
Standard Enthalpy Of Combustion
1. The standard enthalpy of combustion of an element or a compound is the enthalpy
change when 1.0 mole of the substance is completely burnt in oxygen under the
standard conditions at 298 K and 1.0 atm.
2. The symbol for the standard enthalpy of combustion is H
c
. All H
c
values are
negative. For example,
C
2
H
2(g)
+
O
2(g)
2CO
2(g)
+ H
2
O
(l)
H
c
= -1310 kJmol
-1
Standard Enthalpy of Formation
1. The standard enthalpy of formation of a compound is the heat evolved or absorbed
when 1.0 mole of the compound is formed from its elements in their normal physical
states under the standard conditions of 298 K and 1.0 atm.
2. The symbol for standard enthalpy of formation is H
f
.
C
(s)
+ O
2(g)
CO
2(g)
H
f
=
-393.5 kJmol
-1
C
(s)
+ 2H
2(g)
CH
4 (g)
H
f
=
-75 kJmol
-1
3. In many cases, the standard enthalpy of formation cannot be measured directly
because the elements will not combine directly under experimental conditions. For
example, carbon and hydrogen do combine directly to form methane. Hence, the
standard enthalpy of formation cannot be measured directly.
4. When the standard enthalpy of formation of a compound cannot be found directly, it
is often possible to find its value indirectly by the application of Hesss law.
Calculating enthalpies of reaction from enthalpies of formation
The enthalpy change of a chemical reaction equals the sum of the enthalpies of formation
of all the products minus the sum of the enthalpies of formation of all the reactants.
) (reactants - (products) reaction
f
AH AH =
Example :
The enthalpies of formation of carbon dioxide, ethane and water are -393 kJmol
-1
,
-85kJmol
-1
and -286 kJmol
-1
respectively. Calculate the enthalpy of combustion ethane.
6 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
Hess Law
1. The total enthalpy change of a chemical reaction depends on the
difference between the enthalpy of the products and that of the reactants.
It does not depend on how the reaction is completed.
H = [ H
(products)
] [ H
(reactants)
]
2. This concept is given in Hess Law which states that the overall enthalpy
change of a reaction is dependent of its pathway.
3. Consider the thermochemical cycle in the diagram :
The cycle above shows three different ways to convert substance W to Z.
By Hess Law :
H
1
+ H
3
= H
2
+ H
4
= H
5
4. Hess law is especially useful to calculate enthalpy changes that cannot
measured directly from experiments.
W X
Y Z
H
1
H
4
H
2
H
3
H
5
7 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
Hess Law
1. Many reactions have enthalpy changes that cannot be found directly
from a single experiment. Hess law is a method for the indirect
determination of enthalpy changes.
2. Hess law states that the overall enthalpy change in a chemical reaction
is constant, and independent of the path taken, provided the initial and
final stages remain unchanged.
3. For example, for the reaction A B, according to Hess law, the
enthalpy change (H
r
) will be the same, whether by a one-step reaction A
B; or by a series of steps A X Y B
A H
r
B or
H
1
H
3
X H
2
Y
H
r
= H
1
+ H
2
+ H
3
4. Different methods based on Hess law can be used to calculate enthalpy
changes, depending on the information provided, as shown in the
following example:
(a) Method 1 (Constructing an enthalpy diagram)
Question : Calculate the standard enthalpy change of combustion of
methane from the following data :
Standard enthalpy change of formation (in KJ mol
-1
);
CH
4
= -74.4 ; CO
2
= -393.5 ; H
2
O = -285.8
enthalpy
Y
X
A
B
H
r
H
1
H
2
H
3
8 | G S S
Method 2 (Combining equations)
Question : The standard enthalpy changes of formation of SnO
2
, CO and
CO
2
are -581 kJ mol
-1
; -111 kJ mol
-1
; and -394 kJ mol
-1
respectively.
Calculate the enthalpy change of the reaction :
SnO
2(s)
+ 2CO
(g)
Sn
(s)
+ 2CO
2(g)
Method 3 (Using standard enthalpies of formation)
The enthalpy change of a reaction can be calculated using the formula :
H
reaction
= H
f
(products) - H
f
(reactants)
Question : Use the data below to calculate the standard enthalpy change for
the reaction : N
2
O
4(g)
+ 3CO
(g)
N
2
O
(g)
+ 3CO
2(g)
compound H
f
(kJ mol
-1
)
CO
(g)
-110
CO
2(g)
-393
N
2
O
(g)
+81
N
2
O
4(g)
+9.7
9 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
Bond Enthalpy
1. Bond enthalpy is the heat change required to break one mole of bonds
in the molecules of a gaseous element or compound so that the resulting
gaseous species exert no forces on each other.
(a) this energy refers to a specific bond in a molecule, but if a molecule
has more of the same type of bond (e.g. the four C-H bonds in CH
4
),
then different bond enthalpies can occur.
For example :
CH
4(g)
CH
3(g)
+ H
(g)
H = + 427 kJ mol
-1
CH
3(g)
CH
2(g)
+ H
(g)
H = + 371 kJ mol
-1
So, it is much more useful to know the average amount of energy needed
to break a particular bond. In this case, the process of breaking all the
bonds in methane ending up with gaseous atoms. This process could be
written as : CH
4(g)
C
(g)
+ 4H
(g)
H = + 1648 kJ mol
-1
The enthalpy change for this reaction is +1646 kJ mol
-1
, so the mean (or
average) bond enthalpy is + 1648/4 = =412 kJ mol
-1
(b) Bond enthalpy is always endothermic as energy must be put in to
break any chemical bond. (Making a bond is always exothermic as it
is the opposite of breaking a bond)
(c) The smaller the bond energy, the weaker the bond and the easier it is
to break.
10 | G S S
2. Using bond enthalpies to calculate the enthalpy change of a reaction.
(a) Example 1
Use the bond enthalpies given to calculate the enthalpy change for the
hydrogenation of ethene.
C
2
H
4(g)
+ H
2(g)
C
2
H
6(g)
Bond Bond enthalpy (kJ mol
-1
)
H H 436
C H 413
C C 346
C = C 611
(i) Construct an enthalpy diagram:
(ii) Calculate the total energy required to break bonds of reactants,
H
2
(iii) Calculate the total energy required to break bonds of products,
H
3
(iv) Applying Hess law : H
1
=
11 | G S S
(b) Example 2
Use the data given to find the enthalpy change for each of the
following reaction.
(i) C
2
H
4(g)
+ 3O
2(g)
2CO
2(g)
+ 2H
2
O
(g)
(ii) N
2(g)
+ 3H
2(g)
2NH
3(g)
Bond Bond enthalpy
(kJ mol
-1
)
C H +413
C = O +740
O = O +497
O H +463
C = C +612
C C +347
N N +945
H H +436
N H +391
12 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
Standard Enthalpy Change of Formation and Stability
1. Generally, substances with large negative standard enthalpy change of formation are
more stable than those with large positive standard enthalpy change of formation.
2. The standard enthalpy change of formation of water is -286 kJ mol
-1
H
2(g)
+
f
= -286 kJ mol
-1
This indicates that water is energetically more stable than its elements hydrogen and
oxygen.
3. On the other hand, the standard enthalpy change of formation of nitrogen trichloride,
NaCl
3
, is + 230 kJ mol
-1
f
= +230 kJ mol
-1
Hence, nitrogen trichloride is energetically less stable than its elements nitrogen and
chlorine.
4. The table below lists the standard enthalpy change of formation of the hydrides of
chlorine, bromine and iodine.
Compound HCl HBr HI
H
f
/ kJ mol
-1
-92.0 -36.0 +26.5
5. From the standard enthalpy change of formation above we can predict that HI is least
stable of the three hydrides followed by HBr and HCl. This indeed is the case if we
look
at the percentage dissociation of the hydrides into their constituent elements at
different
temperatures.
Temperature /
0
C Percentage dissociation
HCl HBr HI
200 - - 13
1000 0.01 0.4 25
2000 0.4 4.2 30
13 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
Standard Enthalpy of Neutralisation Between Strong Acids and Strong Bases
1. From the table given, the standard enthalpy of neutralization between strong acids
and strong bases is a constant (-57.3 kJmol
-1
).
2. This is because all strong acids and strong bases dissociate completely in water to
form aqueous ions :
NaOH
(aq)
Na
+
(aq)
+ OH
-
(aq)
(aq)
-
(aq)
(aq)
OH K KOH +
+
(aq)
-
(aq)
(aq)
Cl H HCl +
+
(aq)
-
3
(aq)
(aq) 3
NO H HNO +
+
3. Neutralisation between strong acids and strong bases involves the same reaction,
which is the combination of H
+
(aq)
and OH
-
(aq)
to form H
2
O. The other ions present are
just spectator ions (in bold face) that do not take part in the reactions.
Na
+
(aq)
+ OH
-
(aq)
+ H
+
(aq)
+ Cl
-
(aq)
Na
+
(aq)
+ Cl
-
(aq)
+ H
2
O
(l)
K
+
(aq)
+ OH
-
(aq)
+ H
+
(aq)
+ NO
3
-
(aq)
K
+
(aq)
+ NO
3
-
(aq)
+ H
2
O
(l)
The overall reaction is :
H
+
(aq)
+ OH
-
(aq)
H
2
O
(l)
Hence, the heat released is the same.
4. However, the standard enthalpy of neutralization between sulphuric acid and sodium
hydroxide is more exothermic than expected.
(l) 2
(aq)
2
4
(aq) (aq) (aq) (aq) (aq)
2
4
O H SO
2
1
Na Na OH H SO
2
1
+ + + + +
+ + +
= -66.8 kJmol
-1
5. However, the heat released when 1 mole of water is formed from the above reaction
is only 57.3 kJmol
-1
. So, where the extra 9.5 kJmol
-1
comes from ?
6. This extra heat released is the enthalpy change of dilution of sulphuric acid.
7. When aqueous sodium hydroxide is added to sulphuric acid, the acid is diluted in the
process.
8. The heat released due to dilution is significant. This make the standard enthalpy
change of dilution more exothermic.
[The H
for the reaction = heat of neutralization + heat of dilution]
9. Heat of dilution of other acids is insignificant and is ignored.
14 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2011
TOPIC : THERMOCHEMISTRY
Standard Enthalpy of Neutralisation Between Weak Acids or Weak Bases
1. The standard enthalpy of neutralization involving weak acids or weak bases is less
than 57.3 kJmol
-1
.
Example :
-1
(l) (aq) 3 (aq) (aq) 3
kJmol -56.1 O H COONa CH NaOH COOH CH
2
= AH + +
-1
(l) 2 (aq) 4 (aq) (aq) 2 3
kJmol 53.4 - O H Cl NH HCl O .H NH = AH + +
-1
(l) 2 (aq) (aq) (aq)
kJmol 11.7 - O H NaCN HCN NaOH = AH + +
2. This is because weak acids are only partial dissociated in water.
H COO CH COOH CH (aq) (aq)
-
3 (aq) 3
+
+
On addition of a strong base such as NaOH, the OH
-
from NaOH will react with the
H
+
from the dissociation of COOH CH
3
.
negative O H OH H
(l) 2
(aq)
-
(aq) = AH +
+
3. Removal of H
+
will cause the above equilibrium to shift to the right where more
COOH CH
3
molecules would dissociate. However, the dissociation process requires
absorption of heat energy.
positive H COO CH COOH CH
(aq) (aq)
-
3 (aq) 3
= AH +
+
As a result, the total heat released is less than expected.
4. Similarly, for the case of ammonia, a weak base :
(aq)
-
(aq) 4 (l) 2 3(aq)
OH NH O H NH + +
+
On addition of a strong acid such as HCl, the H
+
from HCl will react with
-
OH from
ammonia to form water.
negative O H OH H
(l) 2
(aq)
-
(aq) = AH +
+
5. Removal of
-
OH causes the above equilibrium to shift to the right, and more ammonia
molecules dissociate. However, this dissociation requires absorption of energy.
positive OH NH O H NH
(aq)
-
(aq) 4 (l) 2 3(aq)
= AH + +
+
As a result, the total heat released is less than expected.
15 | G S S
Standard Enthalpy of Neutralisation Involving Hydrofluoric Acid, HF
1. Hydrofluoric acid, HF, is a weak acid that dissociates partially in water.
HF
(aq)
H
+
(aq)
+ F
-
(aq)
2. Hence, we would expect the standard enthalpy of neutralization between HF and a
strong base such as NaOH to be less than 57.3
-1
kJmol .
3. However, the experimental standard enthalpy of neutralization between NaOH and
HF is more negative than expected.
-1
(l)
2
(aq) (aq) (aq)
kJmol -68.6 O H NaF NaOH HF = AH + +
This is in contradiction to our discussions above.
4. This is because the dissociation of hydrogen fluoride molecule is an exothermic
process.
5. Dissociation of HF involves the breaking of the H-F bond and energy needs to be
absorbed. However, the F
-
ions, due to its small size, has high hydration energy.
F
-
(g)
+ aq F
-
(aq)
-1
kJmol -506 = AH
This makes the whole process of dissociation exothermic.
HF
(aq)
H
+
(aq)
+ F
-
(aq)
negative
= AH
As a result, the standard enthalpy change of neutralization is more negative than
expected.
Standard Enthalpy Of Solution
1. The heat evolved or absorbed when 1.0 mole of solute dissolves in water to form an
infinitely dilute solution is known as the standard enthalpy of solution. For example,
NaCl
(s)
+ water NaCl
(aq)
H
= standard enthalpy of solution
2. A solution is said to be at infinite dilution if the volume of water used is sufficient so
that addition of more water produces no further heat change.
Question :
1. When 10.6 g of anhydrous sodium carbonate are dissolved in 100cm
3
of water, the
maximum temperature rise recorded is 5
0
C. Calculate the enthalpy of solution.
(The specific heat capacity of water is 4.2 Jg
-1
k
-1
)
H
= mc = 100 x 4.2 x 5 = 2100 J
Number of moles of Na
2
CO
3
(M
r
= 106) used =
Therefore standard enthalpy of solution = 2100 x
J
Na
2
CO
3(s)
+ water (infinite volume) = Na
2
CO
3(aq)
H
neut
= - 21.0 kJ mol
-1
16 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2011
TOPIC : THERMOCHEMISTRY
Standard Enthalpy Change of Hydration (H
h
)
1. The standard enthalpy change of hydration is defined as the enthalpy change or heat
energy at constant pressure when 1 mol of gaseous ions dissolves in infinite amount
of water (that is, an infinitely dilute solution is formed with no further enthalpy
change) at standard conditions.
2. For a cation
X
+
(g)
+ aq X
+
(aq)
;
H
h
= standard enthalpy change of hydration
For a anion
Y
-
(g)
+ aq Y
-
(aq)
;
H
h
= standard enthalpy change of hydration
Note : aq represents water. Hence, the reaction equation of hydration can also be
written as :
X
+
(g)
X
+
(aq)
and Y
-
(g)
Y
-
(aq)
Water molecules evelope a
cation through ion-dipole
bonds or attractions. Hence,
the cation is hydrated . This
denoted as X
+
(aq)
hydrated
aqueous
Water molecules evelope an
anion through ion-dipole
bonds or attractions. Hence,
the cation is hydrated . This
denoted as Y
-
(aq)
hydrated
aqueous
water water
17 | G S S
3. Since hydration occurs through the formation of ion-dipole bonds or attractions
between the polar water molecules and charged ions, the standard enthalpy change of
hydration is always exothermic.
4. The magnitude of the enthalpy change of hydration depends on the charge density of
the ion.
5. A larger charge density will exert a stronger ion-dipole attraction and hence,
produce a larger magnitude of enthalpy change of hydration.
6. Charge density of an ion depends on the magnitude of the charge carried as well as on
its ionic radius.
7. Charge density is directly proportional to the charge carried by the ion but inversely
proportional to the ionic radius. Hence, mathematically,
radius ionic
charge ionic of magnitude
density charge
8. An ion with a large magnitude of charge and a small ionic radius has a very high
charge density. Hence, has a large magnitude of enthalpy change of hydration.
Example :
Al
3+
ions have a high charge density due to a very large magnitude of charge (+3) and
a small ionic radius. Hence, the magnitude of its standard enthalpy change of
hydration is large.
H
+
ions have a small magnitude of charge but at the same time has a very small ionic
radius. Hence, its charge density is still considered to be large. It standard enthalpy
change of hydration is expected to be large. In fact, the standard enthalpy change of
hydration of H
+
ions is -1090
-1
kJmol .
Question :
The magnitude of the standard enthalpy change of hydration of magnesium ions is
1920
-1
kJmol .
(a) Write a thermochemical equation depicting the standard enthalpy change of
hydration.
(b) Predict the standard enthalpy change of hydration of calcium ions.
Solution
(a) Standard enthalpy change of hydration is always exothermic with H
h
being
negative.
Mg
2+
(g)
+ aq Mg
2+
(aq)
H
h
= -1920 kJ mol
-1
(b) - the charge on both calcium and magnesium ions is the same.
- the ionic radius of the Ca
2+
ion is bigger than Mg
2+
ion.
- hence, the charge density of Ca
2+
ion < Mg
2+
ion
- consequently, the magnitude of the standard enthalpy change of hydration of
Ca
2+
ion is expected to be smaller than the magnesium ions.
18 | G S S
SEK. MEN. KEB. SULTAN ISMAIL, JOHOR BAHRU.
PHYSICAL CHEMISTRY/ UPPER SIX/ 2013
TOPIC : CHEMICAL ENERGETICS
Standard Enthalpy Change of Atomisation , H
atm
1. The standard enthalpy change of atomisation of an element is the enthalpy change
when one mole of free gaseous atom is formed from the element under standard
conditions.
2. The standard enthalpy change of atomisation of sodium is +107 kJmol
-1
. This refers
to the following process :
Na
(s)
Na
(g)
H
= +107 kJmol
-1
3. The standard enthalpy change of atomisation of chlorine is +121kJmol
-1
. This refers
to:
(g) (g) 2
Cl Cl
2
1
H
= +121 kJmol
-1
4. The standard enthalpy change of atomisation of the Noble gases (He, Ne, Ar, Kr, Xe
and Rn) is zero, because all of them exist as monatomic gases at standard conditions.
5. The standard enthalpy change of atomisation of diatomic gases (e.g. O
2
, Cl
2
andN
2
,)
is equal to half the value of their bond energies.
-1
(g)
kJmol 242 2Cl Cl - Cl + = AH
-1
(g)
kJmol 6 . 45 9 2N N N + = AH
-1
(g)
kJmol 0 . 94 4 2O O O + = AH =
6. The processes that are involved in the atomisation of a solid metal comprise the
following steps (sodium is used as an example) :
(a) Heat is absorbed by one mole of the solid to increase its temperature from 298 K
to its melting point (371 K).
(b) The enthalpy of fusion is absorbed (+2.6kJmol
-1
) to change one mole of the solid
to liquid at its melting point.
(c) Heat is absorbed to increase the temperature of one mole of the liquid sodium to
its boiling point (1156 K).
(d) The enthalpy of vaporisation is absorbed (+96.8kJmol
-1
) to change one mole of
liquid sodium to vapour at its boiling point.
(e) This is summarized in the diagram below :
19 | G S S
Na
(g)
enthalpy of vaporisation (+96.8 kJmol
-1
)
Na
(l)
at boiling point
(1156 K)
Heat absorbed
Na
(l)
at 371 K
enthalpy of fusion (+2.6 kJmol
-1
)
Na
(s)
at melting point(371 K)
Heat absorbed
Na
(s)
at 298 K
Lattice Energies For Simple Ionic Crystals
1. The lattice energy (H
L
)of a given ionic compound is the enthalpy change on
forming one mole of the compound from its isolated gaseous ion. For example,
Na
+
(g)
+ Cl
-
(g)
NaCl
(s)
H
L
= -781 kJmol
-1
2. All lattice energies are negative, that is, heat energy is released when oppositely
charged ions are brought together.
3. Lattice energy is a measure of the strength of interior attractions. An ionic compound
with small and highly charged ions has the strongest electrostatic attraction and thus
has the highest lattice energy.
4. Lattice energy is proportional to the product of the charges on the ions and inversely
proportional to the sum of the ionic radii of the cation and anion.
Lattice energy
radius) anionic radius cationic (
anion) on the (charge x cation) on the (charge
+
This means that lattice energy
- Increases as the ionic charge increases
- Increases as the sum of the ionic radii of the cation and anion decreases
The sum of the ionic radii of the cation and anion is also known as the interior
distance.
5. The energy required to separate one mole of the ionic compound into its constituent
ions in the gas phase has the same magnitude but opposite in sign to the lattice energy.
Thus, Na
+
(g)
+ Cl
-
(g)
NaCl
(s)
H
L
= -781 kJmol
-1
and the reverse reaction is endothermic
NaCl
(s)
Na
+
(g)
+ Cl
-
(g)
H
= +781 kJmol
-1
Enthalpy change of atomisation
20 | G S S
6. Applications of lattice energies
Lattice energy can be used to explain :
- The melting points of ionic compounds
- The presence of covalent character in some ionic compounds.
Melting Points and Lattice Energies
Lattice energy is a measure of the strength of interionic attractions. The greater the lattice
energy, the higher the melting point.
Determining The Lattice Energy of Ionic Compounds
1. The lattice energy of an ionic compound cannot be determined directly from
experiment because, it would involve bringing one mole of Na
+
gaseous ions and one
mole of Cl
-
gaseous ions together and combining them to form NaCl solid.
2. Instead, the lattice energy has to be calculated from other known enthalpy changes
(that can be determined from experiments) and by using Hess Law.
3. The thermochemical cycle used to determine the lattice energy is called the Born-
Haber cycle.
4. Example : sodium chloride
The standard enthalpy change of formation of sodium chloride = -411kJmol
-1
. This
refers to following process :
Na
(s)
+
(s) (g) 2
NaCl Cl
2
1
H
= -411 kJmol
-1
This reaction can be assume as comprising the following hypothetical steps. The
actual reaction does not follow these individual steps. However, it makes our
calculation easier to follow.
(a) Atomisation of sodium
Na
(s)
Na
(g)
H
= +108 kJmol
-1
(b) Ionisation of sodium :
Na
(gs)
Na
+
(g)
+ e
H
= +494 kJmol
-1
(c) Atomisation of chlorine
(g) (g) 2
Cl Cl
2
1
H
= +121 kJmol
-1
(d) Formation of Cl
-
ion : first electron affinity.
Cl
(g)
+ e
(g)
-
Cl H
= -364 kJmol
-1
(e) Combination of Na
+
(g)
to (g)
-
Cl form
(s)
NaCl
Na
+
(g)
+ Cl
-
(g)
NaCl
(s)
H
lattice
21 | G S S
5. The Born Haber cycle is then constructed as follows :
Na
+
(g)
+ Cl
(g)
-364
+121 Na
+
(g)
+ Cl
-
(g)
Na
+
(g)
+
(g) 2
Cl
2
1
+494
Na
(g)
+
(g) 2
Cl
2
1
+108
Na
(s)
+
(g) 2
Cl
2
1
Na
+
Cl
-
(s)
Base on Hess law :
(+108) + (+494) + (+121) + (-364) + H
(lattice)
= -411
H
(lattice)
= -770 kJmol
-1
6. Sometimes, the Born-Haber can be drawn as shown below :
Na
+
(g)
Cl
-
(g)
+494 -364
Na
(g)
Cl
(g)
H
(lattice)
+108 +121
-411
Na
(s)
(g) 2
Cl
2
1
Na
+
Cl
-
(s)
H
(lattice)
22 | G S S
Lattice energy of sodium chloride from the Born-Haber cycle.
H
l
Na
+
(g)
+ Cl
-
(g)
NaCl
(s)
H
ea
Na
+
(g)
+ Cl
(g)
H
ie
Na
(g)
+ Cl
(g)
H
f
H
a
[Cl
2(g)
]
Na
(g)
+
(g) 2
Cl
2
1
H
a
[Na
(s)
]
Na
(s)
+
(g) 2
Cl
2
1
Using Hess law
H
l
= -H
ea
- H
ie
- H
a
[Cl
2(g)
]- H
a
[Na
(s)
] + H
f
H
l
= lattice energy of crystalline sodium chloride
H
ea
= first electron affinity of chlorine
H
ie
= first ionization energy of sodium
H
a
[Cl
2(g)
] = standard enthalpy change of atomisation of chlorine
H
a
[Na
(s)
] = standard enthalpy change of atomisation of sodium
H
f
= standard enthalpy change of formation of sodium chloride
Question :
The following enthalpies are given :
Standard enthalpy of atomisation of magnesium, H
a
= +146 kJmol
-1
Standard enthalpy of atomisation of chlorine, H
a
= +122 kJmol
-1
First ionization energy of magnesium, H
ie
= +740kJmol
-1
Second ionization energy of magnesium, H
ie
= +1450kJmol
-1
First electron affinity of chlorine, H
ea
= -348kJmol
-1
Standard enthalpy change of formation of magnesium chloride, H
f
= -566kJmol
-1
Construct a Born- Haber cycle and determine the lattice energy of magnesium chloride
Anda mungkin juga menyukai
- Enthalpy Changes: 2 1 2 C D 1 A BDokumen21 halamanEnthalpy Changes: 2 1 2 C D 1 A BJue MayaBelum ada peringkat
- Lesson 8 ThermochemistryDokumen38 halamanLesson 8 ThermochemistryLyndy PantaoBelum ada peringkat
- 1 2 3 Literature Review 4 Experiment Objective 5 Methodology 6 Results 7 Discussions 8 Conclusion & Recommendations 9 References 10 AppendicesDokumen16 halaman1 2 3 Literature Review 4 Experiment Objective 5 Methodology 6 Results 7 Discussions 8 Conclusion & Recommendations 9 References 10 Appendicesmonkeystar12100% (3)
- Johnmar S. Deligero: Chemistry/Biology 12 (Nova Scotia Curriculum)Dokumen36 halamanJohnmar S. Deligero: Chemistry/Biology 12 (Nova Scotia Curriculum)Sahid SantosBelum ada peringkat
- Thermodynamics & Kinetics: Oakland Schools Chemistry Resource UnitDokumen49 halamanThermodynamics & Kinetics: Oakland Schools Chemistry Resource UnitAntz JabonetaBelum ada peringkat
- Chapter 05 Energetics TextbookDokumen26 halamanChapter 05 Energetics TextbookMirei IidaBelum ada peringkat
- Energetics by Abhishek JaguessarDokumen10 halamanEnergetics by Abhishek Jaguessarreedoye21Belum ada peringkat
- CHAPTER 8 (References)Dokumen10 halamanCHAPTER 8 (References)JeromeBelum ada peringkat
- Topic 4 Notes (New)Dokumen12 halamanTopic 4 Notes (New)amenaBelum ada peringkat
- Thermodynamics 2Dokumen28 halamanThermodynamics 2Edd VillamorBelum ada peringkat
- ENERGETICS AND THERMOCHEMISTRYDokumen60 halamanENERGETICS AND THERMOCHEMISTRYIsadora ThibauBelum ada peringkat
- Thermodynamics: MR Edd VillamorDokumen25 halamanThermodynamics: MR Edd VillamorEdd VillamorBelum ada peringkat
- Lesson4 - Unit3 - Heat of ReactionDokumen16 halamanLesson4 - Unit3 - Heat of ReactionLelouchBelum ada peringkat
- Enthalpy AsDokumen9 halamanEnthalpy AsA LEVEL TOPBelum ada peringkat
- AS Chem CH 1.2 Hess LawDokumen21 halamanAS Chem CH 1.2 Hess LawRaymond Chan100% (1)
- Chapter 6 Chemical EnergeticsDokumen12 halamanChapter 6 Chemical EnergeticsJood ObeidatBelum ada peringkat
- Chem 211-212 Hess's LawDokumen17 halamanChem 211-212 Hess's LawRiff ShahBelum ada peringkat
- Chem Module Heat of ReactionDokumen5 halamanChem Module Heat of ReactionSIR Arjay PerezBelum ada peringkat
- Heat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherDokumen12 halamanHeat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherAeyyjayyBelum ada peringkat
- Topic 5 Energetics-ThermochemistryDokumen45 halamanTopic 5 Energetics-ThermochemistryLucia PesentiBelum ada peringkat
- Experiment 7 INtro, OBjective TazihanDokumen6 halamanExperiment 7 INtro, OBjective TazihanFredo AreroBelum ada peringkat
- IB HL Chemistry Assessment Statements Topic 5Dokumen4 halamanIB HL Chemistry Assessment Statements Topic 5AndrewBelum ada peringkat
- CHM 101 Lecture Note On Thermo Chemical KineticsDokumen24 halamanCHM 101 Lecture Note On Thermo Chemical Kineticsekanadefestus007100% (1)
- THERMOCHEMISTRY Hand Outs 2023Dokumen6 halamanTHERMOCHEMISTRY Hand Outs 2023Paul Willard GumapacBelum ada peringkat
- Thermochemistry PowerPointDokumen43 halamanThermochemistry PowerPointMagno CostaBelum ada peringkat
- Final ExamDokumen8 halamanFinal ExamermiasBelum ada peringkat
- Chapter 11: Thermochemistry - Heat and Chemical Changes Part 1 - Notes: Enthalpy and Bond EnergiesDokumen11 halamanChapter 11: Thermochemistry - Heat and Chemical Changes Part 1 - Notes: Enthalpy and Bond EnergiesSarthakBelum ada peringkat
- Chapter 16 - ThermochemistryDokumen4 halamanChapter 16 - ThermochemistrycaffeinewriterBelum ada peringkat
- Chemistry For EngineersDokumen34 halamanChemistry For EngineersObianuju Ezuka100% (1)
- Enthalpy of Formation and Combustion ExplainedDokumen10 halamanEnthalpy of Formation and Combustion ExplainedatulsemiloBelum ada peringkat
- Thermodynamics of Chemical ReactionsDokumen9 halamanThermodynamics of Chemical ReactionsJohnson EaBelum ada peringkat
- Hess's Law: Enthalpy Level DiagramsDokumen5 halamanHess's Law: Enthalpy Level DiagramsEnica RichardBelum ada peringkat
- Chemistry Notes 2.1 NotesDokumen10 halamanChemistry Notes 2.1 NotesOsama Bin AmerBelum ada peringkat
- Chemical EnergeticsDokumen34 halamanChemical EnergeticsNisidini JasingheBelum ada peringkat
- Sheet - 01 - ThermochemistryDokumen78 halamanSheet - 01 - ThermochemistrySushant VermaBelum ada peringkat
- ENERGY AND HEAT CHANGESDokumen72 halamanENERGY AND HEAT CHANGESRonna IturaldeBelum ada peringkat
- Chem Project DharanishDokumen9 halamanChem Project Dharanishnathinsp8mvmBelum ada peringkat
- ThermochemistryDokumen25 halamanThermochemistrydanielmahsaBelum ada peringkat
- The correct statement is d. Since ΔH is negative (-430 kJ), the reaction is exothermic. This means the heat content of the reactants (A and B) is greater than the heat content of the products (C and DDokumen34 halamanThe correct statement is d. Since ΔH is negative (-430 kJ), the reaction is exothermic. This means the heat content of the reactants (A and B) is greater than the heat content of the products (C and DMyra Lee Camarista EsmayaBelum ada peringkat
- Enthalpy of Formation MgODokumen8 halamanEnthalpy of Formation MgOJessica Ashley HaynesBelum ada peringkat
- ChemicalDokumen34 halamanChemicalVia Ysabel ManacopBelum ada peringkat
- Thermodynamics Fundamentals ExplainedDokumen3 halamanThermodynamics Fundamentals ExplainedAngelene Nova MondaresBelum ada peringkat
- ThermochemistryDokumen5 halamanThermochemistryjoelsantos1981Belum ada peringkat
- Thermochemistry Y11Dokumen23 halamanThermochemistry Y11ZlureBelum ada peringkat
- ThermochemistryDokumen44 halamanThermochemistryAmi NatBelum ada peringkat
- Hukum HessDokumen4 halamanHukum HessLys HopeBelum ada peringkat
- Plan 8 Hess's Law ChemDokumen9 halamanPlan 8 Hess's Law ChemBernardo AblenBelum ada peringkat
- 3 1 EnthalpyDokumen30 halaman3 1 EnthalpymahmoudBelum ada peringkat
- EXERCISE 5 Enthalpies of ReactionDokumen18 halamanEXERCISE 5 Enthalpies of ReactionJustine GuerreroBelum ada peringkat
- Chemistry ThermochemistryDokumen6 halamanChemistry ThermochemistryWiktoria KaczmarzykBelum ada peringkat
- VCE Chemistry Unit 4Dokumen311 halamanVCE Chemistry Unit 4Danny GoldstoneBelum ada peringkat
- Prac 7 ThermochemistryDokumen16 halamanPrac 7 ThermochemistryErmanno RoncoBelum ada peringkat
- Endothermic and Exothermic ReactionDokumen70 halamanEndothermic and Exothermic Reactionactive learning educationBelum ada peringkat
- 04 EnergeticsDokumen14 halaman04 EnergeticsafshinBelum ada peringkat
- Laporan Kimia TermokimiaDokumen17 halamanLaporan Kimia Termokimiashlynnn dyahBelum ada peringkat
- 03 - Energetics PDFDokumen33 halaman03 - Energetics PDFAbu ZafarBelum ada peringkat
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterDari EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterPenilaian: 5 dari 5 bintang5/5 (1)
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4Dari Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4Belum ada peringkat
- Practice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersDari EverandPractice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersBelum ada peringkat
- ES PresentationDokumen35 halamanES PresentationPh'ng Jiun YanBelum ada peringkat
- MSEC PresentationDokumen33 halamanMSEC PresentationPh'ng Jiun YanBelum ada peringkat
- Schiffman CB10e IM 06Dokumen20 halamanSchiffman CB10e IM 06Ph'ng Jiun YanBelum ada peringkat
- Mathematics TDokumen97 halamanMathematics TPh'ng Jiun YanBelum ada peringkat
- Mathematics TDokumen97 halamanMathematics TPh'ng Jiun YanBelum ada peringkat
- Isek. Men. Keb. Sultan Ismail, Johor Bahru. Physical Chemistry/ Upper Six/ 2013 Topic: Chemical EnergeticsDokumen22 halamanIsek. Men. Keb. Sultan Ismail, Johor Bahru. Physical Chemistry/ Upper Six/ 2013 Topic: Chemical EnergeticsPh'ng Jiun YanBelum ada peringkat
- Surgical Nursing Calculation PracticesDokumen2 halamanSurgical Nursing Calculation PracticesJéssica OinonenBelum ada peringkat
- Big Batch Soap MakingDokumen24 halamanBig Batch Soap MakingAnonymous Vu1R35s4WZ100% (2)
- ME6008 WELDING TECHNOLOGY Part B IQDokumen1 halamanME6008 WELDING TECHNOLOGY Part B IQVikram mBelum ada peringkat
- Is 10585 2002 PDFDokumen9 halamanIs 10585 2002 PDFmsreerajvarmaBelum ada peringkat
- Omeprazole: by Jennica Mae V. CuicoDokumen7 halamanOmeprazole: by Jennica Mae V. Cuicoジェンニカ メイBelum ada peringkat
- Agoo Montessori Learning Center and High School Inc.: Fruit Waste As Biodegradable PlasticDokumen6 halamanAgoo Montessori Learning Center and High School Inc.: Fruit Waste As Biodegradable PlasticAlejandro De la GarzaBelum ada peringkat
- Unit 1 - Part A-Water Supply IDokumen24 halamanUnit 1 - Part A-Water Supply IIsha SinghBelum ada peringkat
- ESSO Shaft Sealing Systems SpecificationDokumen13 halamanESSO Shaft Sealing Systems SpecificationFlorin Daniel AnghelBelum ada peringkat
- CHEMISTRY LAB VIVA QuestionsDokumen3 halamanCHEMISTRY LAB VIVA QuestionsUjjWal MahAjan55% (20)
- Hele 6Dokumen16 halamanHele 6Ella Mae Berro0% (1)
- Lubricant Properties CalculatorDokumen14 halamanLubricant Properties CalculatorzamijakaBelum ada peringkat
- Hodson G - Methanol in WineDokumen5 halamanHodson G - Methanol in WinePhaimBelum ada peringkat
- 1 Auxilliary Equipment - US PricingDokumen132 halaman1 Auxilliary Equipment - US PricingOscar EspitiaBelum ada peringkat
- Amarex KRT8041eDokumen36 halamanAmarex KRT8041eRui Alves da Silva100% (1)
- Cosmetics 09 00063 v2Dokumen44 halamanCosmetics 09 00063 v2maizhafiraBelum ada peringkat
- Integrated Ferroelectrics: An International JournalDokumen10 halamanIntegrated Ferroelectrics: An International JournalBhabani Sankar SwainBelum ada peringkat
- Solution Manual Chemical Reaction Engineering, 3rd EditionDokumen137 halamanSolution Manual Chemical Reaction Engineering, 3rd Editionboni_briantoni75% (8)
- Lesson 2 Dna Structure and Dna ExtractionDokumen8 halamanLesson 2 Dna Structure and Dna ExtractionGreatel Elijah TorregosaBelum ada peringkat
- Biopolymers - Sustainability For The Automotive Value-Added ChainDokumen4 halamanBiopolymers - Sustainability For The Automotive Value-Added ChainDiana LondoñoBelum ada peringkat
- Method Statement FOR Waste Polymer Slurry Disposal: PT Bauer Pratama IndonesiaDokumen4 halamanMethod Statement FOR Waste Polymer Slurry Disposal: PT Bauer Pratama IndonesiaFendi Rang TigorBelum ada peringkat
- Final - Report Hydroelectric Power PlantDokumen39 halamanFinal - Report Hydroelectric Power PlantnisarBelum ada peringkat
- Introduction To R.T.P.P: 1.1 GeneralDokumen12 halamanIntroduction To R.T.P.P: 1.1 GeneralSairam Kumar ChowdaryBelum ada peringkat
- Urea MsdsDokumen5 halamanUrea MsdsVinnyVidichiBelum ada peringkat
- Chemistry SBA7 ReportDokumen6 halamanChemistry SBA7 ReportSam ChanBelum ada peringkat
- Recall Machine Design Past Board Cebu Mar 2011 11 PDFDokumen12 halamanRecall Machine Design Past Board Cebu Mar 2011 11 PDFCMD100% (1)
- Catalysis Norskov 05 15 02Dokumen36 halamanCatalysis Norskov 05 15 02Rafael Ricardo Celin ManceraBelum ada peringkat
- JHP 8 69Dokumen9 halamanJHP 8 69Khalil El BayadBelum ada peringkat
- Assignment 2Dokumen1 halamanAssignment 2Varun PahujaBelum ada peringkat
- 02 Heubach No 00181 ZPA RZ Epoxy Dispersion WDokumen2 halaman02 Heubach No 00181 ZPA RZ Epoxy Dispersion WnanoBelum ada peringkat
- SeminarDokumen15 halamanSeminarAditi ChandraBelum ada peringkat