Sulphur Burning and The Formation of So3
Diunggah oleh
rubcarvDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Sulphur Burning and The Formation of So3
Diunggah oleh
rubcarvHak Cipta:
Format Tersedia
87
A BRIEF REVIEW OF A, PORTION OF THE LITERATURE
DEALING WITH SULPHUR BURNING AND THE FORMATION
AND DISSOCIATION OF SULPHUR TRIOXIDE
R. j.HOGARTH and C. T. JENKIN
, '
In this paper an attempt will be made to sum
up the facts and figures contained in a host of
papers and books already published on the subject
of sulphur burning. For the sake of clarity the
two sections :--
I. Theory.
II. Practice.
have been chosen to enable the reader to be in a
better position to differentiate between theoretical
hypotheses and conjectures and actual observations
recorded from plant practice. In the theoretical
section, especially, all calculations have been made
with a view to simulating those conditions pre-
vailing in the sugar industry in this country.
It sho.l.1ld be explained that the sequence of
operations.vwhich may be generally referred to as
sulpliur burning, has' been dealt with mainly from
the point of view of standard sulphite pulp and
paper mill practice. This angle of approach was
chosen because of the quantity and quality of
literature that is readily available from this source.
However, this should not cloud the issue at all
from the sugar technologist's point of view as,
basically, the problem of the production of sulphur
dioxide is the same in both cases.' Like the sugar
chemist, the pulp and paper technician is concerned
with the production of a burner gas which is rich
in SO2 and free from all but the smallest possible
amounts of SO3, under the operating conditions
prevailing. Only after the sulphur dioxide is
produced does the application differ for the two
industries. We shall therefore concern ourselves
only with those operations that are common to
either industry.
The source of information. has been indicated
when a particular work is referred to, and, where
possible, the more modern methods of plant practice
or theory have been chosen, although in some cases
personal preference or dislikes may have predom-
inated and influenced the choice of certain facts and
theories.
PART I. THEORY.
When 'sulphur is heated hi the presence, ofexcess
air it will, under favourable conditions, ignite and
continue burning to form, in the main," sulphur
dioxide.
If the reaction:-
S + O2-+ S02
is considered, it will be seen that one molecule
of sulphur and one of oxygen combine to form a
molecule of sulphur dioxide. However, it is not
necessary to use pure oxygen for the purposes of
combustion: air will meet the requirement equally
well, and, furthermore, its use is more economical.
Basically, air may be considered to consist of
21 per cent. oxygen and 79 per' cent, nitrogen by
volume. Thus, when one volume is
used to produce one volume of SO2' there will be
or 3,76 volumes of nitrogen involved. The
final burner gas would then consist of:-
1.00 volume SO2
3.76 volumes N 2
4:. 76 total volume of burner gas.
H is obvious, theri, that the theoretical maximum
sulphur dioxide content of the burner gas would be
4.\6 X 100 = 21 per cent. by volume. This
calculation assumes that dry air is used and no
S03 is formed during the actual burning of the
sulphur. .
Continuing, it is apparent from the above equation
that in order to completely oxidise lib, of sulphur,
it is necessary to have 1 lb. of oxygen present when
2 lbs. of SO2 will be formed. This amount of
oxygen is present in 53.4 cubic feet of air at S.T.P.
Practical considerations, however, demand that the
air supplied to the burneris in excess of the theoretical
minimum in order to ensure that all the sulphur
is completely burned off in the space of time allowed.
Because of this, the maximum S02 content of a
burner gas in practice would be less than the
theoretical maximum. In fact, with the con-
ventional rotary burner the maximum SO2' content
is approximately 18 per cent. .
Quantity' of Burner Gas.
Having made these preliminary calculations,
the next point of interest is the variation of the
quantity of burner gas produced when the SO2
content is raised from a minimumto a maximumval ue.
88
On top of this it would be of assistance to know
how moisture in 'the atmosphere would effect these
values.
Table I and II, Graphs A and B, supply the
answers here" and all calculations were made
according to the methods outlined in Lundberg's
book."
Where moist air was assumed in the theory in
the calculations, this was taken as air 74 per cent
saturated with water vapour at 66. 6F. This
represents roughly the average humidity and
temperature taken over a number of years at Mount
Edgecombe during the months of May to December.'
"'Graphs A and B show how the volume of the
burrier gas: produced decreases with increasing
SO2 concentration. A comparison of the results
withdry and moist air indicates that the effect of
moisture is '. not very marked upon the final gas
coming from the burner.
...
. Theoretical Flame Temperature.
.: When sulphur is burned in air, the reaction is
exothermic and therefore the products are at a higher
temperature than that of the reactants.' For every
particular concentration of SO2 in a gas, there will
be a corresponding flame temperature to be reached
in the burner.
the deduction' of these flame
.temperatures ,from basic principles is a fairly long
and' tedious procedure: A method suggested by
Lundberg" was usedhere and it is reasonably rapid
and convenient, although comparison with flame
temperatures calculated from basic thermodynamic
principles indicates that this rapid method may be
slightly inaccurate and especially so at the lower-
concentrations of, SO2' It must be stressed that
the, temperatures listed in Tables III and IV are
'approximate only! and should be taken more as an
indication of the order of magnitude of temperature
and not as accurate flame temperatures.. Anyone
wishing to derive theoretical flame temperatures
;may.do so by consulting the technical literature
with this. subject 5 8 _9 10
'.. Once again, results have been caleulated for
dry and 'moist air, and in order to show how the
temperature of the flame may vary due to heat
losses by radiation, the two cases:
(a) No Heat Losses
, (b) Radiation of 15 per cent. of the total heat input
have been considered. ' /
, .
and Graphs show how dilution Of the
burner.gas with air causes the flametemperature to
-drop.' ;;Moi;;tiire'again appears to have a negligible
effect.' -;
.Formation.
Most of the points of theoretical importance have
been listed, and all that remains to completejhe
picture is a consideration of the factors influencing
the formation of SO3' It is known in practice that
a small amount of this compound is always formed
along with the SO2 in the burner, and therefore
a knowledge of 'the conditions which favour SO3
formation should be useful in that it would then
be possible to arrange an atmosphere and conditions
in the burner which would, keep the amount of SO3 '
formed at an. absolute minimum.
When SO3 is formed in the presence of excess
air, the following reaction occurs: . .
250
2
+ 02 2S0
3
This reaction is reversihle and therefore it will
proceed from left to right until equilibrium is
reached. At this point, as much SO3 as is being
formed will be dissociating once more into' its
components., The equilibrium can only be dis-
turbed by removing one of the reactants or product
of the. reaction, .or changing the steady state of
conditions prevailing, otherwise, if temperature,
concentration, etc. are kept steady, then 'the
equilibrium will be stable and constant for those
sets of conditions. .
Fortunately, this reaction has been studied. in
some detail by many observers,and it is possible
to calculate the amount of SO3 that will be formed
under almost any given set of 'conditions: The
subject of catalysis and the effects of catalysts have
no direct bearing on the theory of SO3 formation
as it is conceded that catalysts do not disturb the
equilibrium' point, but merely serve to speed up
the rate Of reaction, thereby ensuring that equili-
brium will be reached in a shorter space of time. They
also allow lower temperatures to be used when S03
is being formed. Catalysts are therefore of im-
portance in' the Contact Process for theproductiori
of sulphuric acid: " .
The theoretical conversion percentages which
have been calculated here do not take into account
the time factor, that is to say; no allowance has
been made forthe period that is necessary to ensure
that the reaction reaches equilibrium before the
gases pass from the burning apparatus. Often
it will be found in plant practice that the conversion
of SO2 to SO3 as measured, is considerably less
than that predicted in theory under the conditions
specified. Thus, the conversion figures listed may
be taken as the maximum possible, which might
never be attained in the plant. .' " ....' . ,
In orderto be in a position to predictthe amount
of conversion of SO2 to SO3 under a given set of
89
conditions, it is necessary to know the equilibrium
constant, K, for the reaction:
S02 + i
0
2 S03
which occurs at various temperatures. This re-
action, and not the previous one having double the
quantities, has been chosen purely for convenience,
The equilibrium constant, K, differs for the two
reactions, the former being the square of the latter,
but the calculated conversions would' be the same
in each case.
For the purpose of this paper, the equilibrium
constant has been calculated from the average of
three authorities, viz.
Fairlie
4
-
T 4760
10glOI<.. = T - 4.474
Lewis and Randall--
4936
10glOK = T - 4.665
5186.5
10glOK = ~ + O. 611log
10T
- 6.7497
where K is the equilibrium constant and T is the
absolute temperature in degrees Kelvin.
The above equations showthat K is dependent
upon temperature only. Naturally, the equili-
brium constant can be calculated from thermo-
dynamic first principles along these lines:
K ["
S03J
h . th tivit
. ["
S02J
["OJt' were a IS re ac IVI y co-
efficient for each respective reactant or product,
and this equation may be re-written as
K
() (PSO 3) h . h . I
~ (PSG 2) (PO 2)f were pIS t e partia pressure
of each substance as. indicated. However, the
calculation of K from first principles is somewhat
lengthy, but should this expedient be necessary
or desired, reference should be made to textbooks
on thermodynamics." B 9 10
Knowing K, it is then possible to deduce the
expected conversion of SO2 to SO'3 tinder various
conditions. However, the conversions calculated
in this paper were carried out on the lines suggested
by Browning andKress.? . The equilibrium constant,
. K, was calculated from an equahonwhere the
variables were in terms of concentrations of the
various products as follows:
K=_x_ 1
l-'Xjb'1..
- 2
ax
, 100 -'-. lax
where a = initial concentration of SO2in %
where b = initial concentration of 2 in %
where x = Fraction (%cOnverSion) of S03
100
K is calculated from assumed values of conversion
for a gas of specific SO2 content, and then the
equilibrium temperature for that value of K cal-
culated from the Lewis and Randall equation given
previously. . ,. ~
Table V and Graph Edetail the variation of
equilibriumconstant, K, with temperature, Tables VI
VII and Graphs F and G, record the equilibrium
temperature for any assumed degree of conversion
with a gas of known composition. '
With a gas of known strength, the amount of
SO3likely to be formed can be reduced by increasing
the temperature. Generally, in order to reduce
the possibility of trioxide formation, the SO2
content of the gas should be increased, .i.e. the
amount of excess air used, in burning should be
kept at a minimum. On top of this, high corn-
bustion chamber temperatures help to reduce the
incidence of SO:I formation. However, theory
indicates that it is literally impossible to prevent
SO2 undergoing a certain amount. of oxidation,
albeit a very small amount at temperatures over
1,000C. .
The sugar chemist is attempting to produce a
burner gas containing S02 only, and none of the
higher oxide, and in the light of the foregoing .theory
it would appear that this could best be accomplished
by the following:-
(a) Maintaining the quantity of excess air used
at an absolute minimum for the plant in
question. .
(b) Ensuring that the flame temperature. in the
burner is kept as high as possible and heat
losses due to radiation are minimised.
(c) Arranging for the hot burner gases to be
cooled as rapidly as possible in order that they
need not be held at those temperatures for any
length of time, where maximum conversion
of SO2 to SO:i is likely to occur.
(d) On top of this it would be helpful to draw
the gases through the burner as rapidly. as
possible, with the apparatus in use, to n ~
deavour to reach a state 'of affairs where the
reaction 250
2
+ 0 2 ~ 2'50
3
, does riot have
time to reach equilibrium.' However,' this
rate of flow of the gases in the burner will
naturally be dictated largely by the rate at
which sulphur can be burned without sublim-
ation taking place.
This completes the theoretical study of sulphur
burning, and all that remains is to observe what
occurs in practice. The theory is in fair agreement
with what is experienced in actual pant operation
andit is hoped that the connections between theory
and practice will be obvious.
PART II. PRACTICE.
The most convenient raw material for the pro-
duction of SO2 is sulphur, and although there are
many alternative sources available, sulphur still
proves the popular choice for a variety of obvious
reasons. The sulphur used in the sugar industry
in this country is obtained almost solely from the
U.S.A., this source being chosen with consideration
to price, quality and availability.
In 1949 the production of crude sulphur in
America amounted to approximately 41 million
long tons.v mined by the Frasch-process and it is
interesting to note that the Texas Gulf Sulphur
Company was the major producer. During the
same period the Union of South Africa is credited
with the importation of just over 65,000 long tons
of crude sulphur.P most of which was used in the
manufacture of sulphuric acid. It can be seen,
therefore, that the sugar industry's requirements of
between 4,500 and 5,500 long tons per annum, are
quite small when compared with the total imports
into the country.
Sulphur is abundantly distributed in nature,
and on the Gulf Coast it is usually associated with
salt dome intrusions. The element is found to
occur in the limestone, gypsum and anhydrite cap
rock and a few of these domes contain commercial
quantities of sulphur.i! The occurrence of these
domes appears to be limited mainly to the Gulf
Coast region of the States of Texas and Louisiana.
Details of the mining and production of sulphur
are contained in a highly informative circular en-
titled "Sulphur-General Information" issued by
the United States Bureau of Mines-! and anyone
interested would be well-advised to procure a copy
of this publication.
The element sulphur is non-metallic and exists
in a variety of allotropic forms and therefore may
be said to have a series of properties depending on
the form in which it is found. Natural sulphur
usually occurs as the more stable rhombic or
a-sulphur form. However, it can exist in many
forms," but they all tend to revert to the rhombic
form. A study of the various properties and
allotropic forms of sulphur is outside the scope
of this paper and a brief account of those properties
of relative importance to sulphur burning only,
will be given. Sulphur, S8' is a brittle, yellow ele-
ment which is solid at room temperature, is in-
soluble in water, but will dissolve in carbon bisul-
90
phide. The rhombic form melts at 112. 8C. and the
melt forms a mobile liquid which becomes more
viscous as the temperature is increased, until at
200C. it becomes so thick it will not flow. However,
at 350C., the melt again becomes mobile. It ignites
at about 248C., finally boiling at 444.6C.2 The
above temperatures should be taken as approximate
only as Mellor" gives specific figures for the known
allotropic forms and any particular allotropic
mixture would probably have its own characteristic
physical and chemical constants.
A typical analysis of the sulphur supplied to the
sugar industry is roughly as follows:-
16
Moisture at 105C. 0.10%
Total Residue on Burning 0.13%
Organic residue 0.03%
Mineral residue 0.10%
Arsenic as As
20 3
... 2 p.p.m.
Selenium as Se Negligible
Acidity as H 2S04 <0.1%
Sulphur (by difference) 99.6%
Sulphur as supplied contains very little sulphuric
acid, but storing in the presence of air and moisture
may cause sulphuric acid to be formed." It has
been found that the oxidation of sulphur is negligible
if moisture is absent." As sulphur contains a small
amount of hydrocarbons which form water on
combustion, this causing mists in the burner, and
furthermore, as a finite quantity of moisture may be
present originally, it is often considered good
practice to melt and strain all sulphur going to the
burner. This process ensures that moisture and
hydrocarbons are removed before combustion takes
place.
From these brief observations, it is fairly safe
to conclude that sulphur should be stored in a
cool, dry place under cover to prevent contamination
by moisture, carbonaceous matter, etc. When
stored in the open,a certain amount of moisture
will be absorbed from the air or rains, as will be
carbon and silica which are present in the particles
of dust in the air. The first component, moisture,
helps the oxidation of sulphur to H 2S04' while
the second, carbon, may form a carbonaceous scum
on the surface of the molten sulphur in the burner,
or, alternatively, ignition of the carbon will occur,
the CO2 formed causing dilution of the burner gas.
The silica increases the ash content of the sulphur
and this in itself is a nuisance in that it would
involve frequent ash removal from the burner.
Obviously, these three constituents, i.e. H
20,
C and SiO2' would, if present in sufficient quantity,
affect the rate of burning of the sulphur in addition
to altering the final composition of the burner gas.
It is not suggested here that storing sulphur in the
open would involve the above contaminants being
present in quantities sufficient to affect the efficiency
c.
of the burner, but it is possible that in many cases
they may reach a proportion where they cause
trouble.
Types of Burners.
(a) Rotary Burner.
This burner is proably the most commonly used
and is known as the Glens Falls Rotary Burner.
The makers, Glens Falls Machine Works of America,
claim that maximum efficiency can be attained with
unskilled labour and that installation and operating
costs are low. Furthermore, they emphasise that
losses due to sulphur sublimation or formation of
SO3 can be eliminated almost entirely.'! With
this type of burner it is possible to operate con-
tinuously at SO2 concentrations ranging between
5 per cent. and about 18 per cent. In order to
prevent fluctuations in gas strength it is desirable
to feed the sulphur by mechnical means and not
by hand.
The sulphur in the lower part of the horizontal
rotating drum forms a molten pool as well as a thin
film around the circumference of the drum. This
film, in effect, increases the surface area of the
sulphur exposed to the incoming air and a further
increase in surface area is brought about by the
sulphur that drips down from the top of the drum.
These factors help to increase the capacity of the
burner and ensure that combustion is complete.
On top of this, it is claimed that the sulphur film
protects the drum metal from deterioration due to
hot SO2 gases as the heat conductivity of sulphur
is less than that of cork.
A combustion chamber is fitted at the back of
the horizontal drum and this serves to complete
the combustion of any sublimed sulphur as well as
to mix the gases and dilute them to the desired
strength. This chamber is therefore constructed
so as to have air ports and one or more baffles.
Impurities in the sulphur do not readily affect
the operation of these burners because the rotation
of the drum agitates the molten sulphur sufficiently
to prevent any blanketing films forming on the
surface. A further refinement that can be added
is a sulphur melter and the heat of radiation from
the burner may be utilised to keep the sulphur
molten. Steam heating coils in the Melting Tank
are only required for starting up the burner after
a long shut-down. In order to further improve
the efficiency of this machine, the molten sulphur
should be strained prior to passing it to the burner.
The Teeding of molten sulphur ensures that the
burner is handling moisture-free material.
Ideal results are obtained with this type of burner
by using sulphur of a minimum purity of 99.6
per cent. .The molten sulphur in the horizontal
drum should be at the highest possible level without
allowing any overflow to take place.
91
The makers suggest the following capacities for
a burner 30 in. in diameter and 8 ft. long.
Capacity with l in. water draught is 1351bs. Sulphur
per hour.
Capacity with ~ in. water draught is 200 lbs.
Sulphur per hour.
Capacity with 2 in. water draught is 270 lbs. Sulphur
per hour.
A burner of identical diameter, but only 6 ft. long
would have corresponding capacities of about 75
per cent. of those given above. As a rough guide,
it may be taken that:- .
For a 1in. draught, the burner consumes approxi-
mately 2 lbs. Sulphur/sq. ft.yhr.
For a -1 in. draught, the burner consumes approxi-
mately 3 lbs. Sulphur/sq. ft./hr.
For a 2 in. draught, the burner consumes approxi-
mately 4:.2 lbs. Sulphur/sq. ft.yhr.
Another authoritv" considers that a burner 36 in.
in diameter and '8 ft. long is capable of handling
nearly 8 lbs. of Sulphur/sq. ft./hour. Fairlie!
finds it practical, without resorting to high draught,
to run a burner 4: ft. in diameter at a rate of 1 ton-
of Sulphur per day per foot of length.
The size of the combustion chamber is variable
between fairly wide limits and it should be borne
in mind that a large chamber permits a higher
concentration of SO2 in the burner gas without
danger of sulphur sublimation. It is suggested that
a chamber spa e of 60 cubic feet per ton of sulphur
per 24 hours- is adequate, while the makers of this
type of burner indicate that a minimum space of
(it cubic feet per pound of sulphur per hour per
square foot of burner area should suffice for burners
up to 30 in. in diameter. These figures should be
taken as approximate indications only and no hard
and fast rules seem to apply. The size of the
combustion chamber should be dictated by practical
considerations and previous experience.
Sutermeister'" states that rotary sulphur burners
produce a gas of varying composition owing to
sudden rushes of cold air through the apparatus,
but Fairlie" claims that with a continuous feed, it is
possible to maintain the gas at a uniform con-
centration of SO2 with only minor fluctuations.
He stipulates, however, that this is dependent on
the depth of the molten sulphur in the rotating-
cylinder being kept uniform. Any change in this
depth will alter the area of the unsubmerged film-
covered surface and this in tum will alter the rate
of combustion of sulphur.
92
(b) The Acme Burner.
This type is deigned to give a constant rate of
burning for any given concentration of SO2 in the
gas. The makers, Acme Coppersmithing and
Machine Company, point out that the rate of com-
bustion of sulphur bears a definite relation to the
amount of air supplied. The amount of air re-
quired for a given concentration of SO2 is given by
the expression:-
o/SO . C b ti G 90.65
/0 2 In om us IOn ases = ---
A + 0.026
where A = lbs. of air at 70F. per lb. of sulphur
burned when the burner is operating ata rate of
2t lbs. of sulphur per square foot of burning surface.
Should the sulphur burning rate be changed, then
the above formula has to be modified slightly.
It is claimed that a tray or rotary burner does not
satisfy the requirements of a definite constant
burning rate because of the following factors:-
(1) Ordinary sulphur contains organic matter
and this accumulates as a carbonaceous film
on the surface of the burning sulphur which
may eventually extinguish the flame.
(2) Additions of fresh charges of sulphur to the
burning surface disrupts the burning rate and
normal conditions are only returned to after
s v r ~ hours have elapsed.
With this burner, the makers have overcome the
first drawback by operating the burner at a rate
greater than 2 lbs.of Sulphur per Square foot of
burning surface per hour, finding that this prevents
the formation of carbonaceous scum by burning it
off with the rest _of the gases. The drawbacks of
factor (2) were prevented by arranging a special
feeding device that fed molten sulphur in a manner
that did not disturb the burning surface, but, at
the same time, ensured that a constant level of
molten sulphur was maintained in the burner.
This burner is operated on compressed air and
a special sulphur melter is supplied with the
apparatus. Sulphur is fed to the bottom of the
burner via a main feed pipe, and a side feeder arm
on this main feed allows for positive control of
sulphur feed, thereby guaranteeing a constant
level of molten sulphur in the burner.
All the data." given here and the brief description
of the principles behind this burner were taken from
a pamphlet issued by. the manufacturers, viz.
Acme Coppersmithing and Machine Company of
Oreland, Pennsylvania. A description of this burner
may be found in the technical literature. 19
(c) Spray Type Burner.
The Research Department of the Texas Gulf
Sulphur Company is accredited with the invention
of this burner. The development of apparatus
was described in a technical paper issued in 1934
20
and it was therein explained how the burner was
operated. Briefly, molten sulphur is fed to a spray
nozzle which injects a fine spray of this material
into the burner. At the same time the desired
amount of air is forced into the burner chamber
and ignition of the sulphur takes place. The
burner chamber is fitted with baffies to mix the
resultant gases and prevent any unburned sulphur
passing through to the absorption apparatus.
The burning and combustion chambers are in one
unit. Normally, the burner chamber is lined with
fire-brick to reduce heat losses and minimise the
formation of SO3'
Certain advantages are claimed for this burner
and among those listed are:-
(1) Operation is simple and the burner requires
little attention once the rate of combustion
has been set. The combustion rate can be
changed simply by altering the stroke of the
sulphur pump and adjusting the compressed
air supply accordingly.
(2) Starting up and shutting down operations are
comparatively easy, and if the burner is started
up when cold, maximum gas concentration can
be reached within 2;} hours. In order to shut
down it is merely necessary to stop the sulphur
metering pump and shut off the compressed
air supply.
(3) The burner is extremely flexible insofar
as rate of combustion is concerned and there-
fore it is possible to vary the capacity of the
burner within fairly wide limits.
(4) It will produce a gas with an SO2 content of
anything up to 20 per cent. when run con-
tinuously.
(5) Sublimation of sulphur should not occur
provided proper care is taken, and since there
is no burning-down period, as with conventional
equipment, the hazards of possible sublim-
ation are reduced to an absolute minimum.
(6) Provided the gas concentration is kept high
and the burner is lined with refractory brick,
little SO3 should be formed at the elevated
temperatures (2,400 to 2,700F.) of operation.
During tests'" it was found that the SO3
content of the gas passing to the cooler was
only 0.14 per cent. of the sulphur burned.
(7) The maintenance costs are not likely to be
high and the initial cost of installation should
compare favourably with that of any of the
more usual types of burner.
(8) Kress" found that power costs could be re-
duced by 75 per cent. over that of conven-
tional burners.
"
93
Anyone wishing' to study in detail the design of
a spray type burner is well-advised, to read the
paper by Conroy and johnstone" on this subject.
The authors indicate that the internal volume of a
rotary burner should be 13/14 cubic feet per ton of
Sulphur per day and on top of this a combustion
chamber space of 60 cubic feet per ton of Sulphur
per day is required, whereas the total volume of a
spray type burner is 24 cubic feet per ton of Sulphur
per day.
It is felt that the three burners listed cover the
types of particular and possible interest to the sugar
industry. In order to complete our practical con-
siderations of sulphur burning, it is only necessary
to study how SO3 formation. occurs and what can
be done to minimise its formation as this information
should then make it possible to successfully produce
a gas that is rich in SO2and, at the same time, free
of all but traces of SO3'
The Formation and Dissociation of SO3'
The conditions favouring the formation of SOa
are:-
(i) Decomposition of any sulphuric acid that
may be present in the commercial sulphur.
(ii) Formation of trioxide during the actual
burning of the sulphur.
(iii) Oxidation of the SO2 present in the burner
gas.
The first source accounts for only a relatively
small amount of the SO3 normally found in burner
gas. Naturally, sulphur will oxidise. to a certain
extent in the presence of moist air, but the amount
of sulphuric acid formed is bound to be extremely
small.
Undoubtedly, a certain amount of trioxide will
be' formed during the burning of the sulphur, but
this is difficult to determine accurately. It will
depend, to a large extent, upon the proportion of
excess air present and upon the temperature reached
in the burner. It can be seen therefore that the
SO content of the gas should be as high as possible
for the type of burner in use.
The third source, i.e. the oxidation of SO2.
accounts for the major portion of trioxide found in
a burner gas and consequently this aspect of the
problem will have to be examined in fairly minute
detail. In practice it has been found that oxidation
will take place only until a certain ratio of dioxide
to trioxide is reached.. At this point equilibrium
is established between the reactants and products
oof the reaction and as fast as SO3 is being formed,
it"is again decomposed into SO2 and oxygen.. This
equilibrium may be distrubed or changed by altering
the concentration of the reactants on either side of
the equation:
O
2
+ 2S0
2
2S0
3
A rotary steel or cast-iron burner operating
continuously has been found to convert between
1 per cent. and 2 per cent. of the S02 into S032,
while a similar burner lined with refractory brick
will produce a gas of much lower trioxide content.
Obviously, then, the materials of construction have
an effect upon the oxidation of SO2and an investig-
ation led to the discovery that certain metals or
their oxides could increase the rate at which trioxide
was formed. These metallic substances are known
as catalysts and while they do not take part in the
reaction in a quantitative way, that is to say, they
are not used up or depleted during the course of
oxidation, they most certainly speed up the rate of
reaction. These substances, therefore, warrant
study as well. .
It will probably be advisable to study the various
effects of physical and chemical conditions tlpon
trioxide formation separately to ensure that the
picture be complete.
Effects of Excess Air,
Comog'" and co-workers found that when sulphur
vapour was burned in the presence of 250 per cent.
excess air at 4:60C., approximately 3.4 per cent.
to 3.8 per cent of the sulphur appeared as the
trioxide. However, Browning and Kress'" observed
less than 0.02 per cent. conversion under similar
conditions in the absence of a flame. The obvious
inference is that atomic oxygen in a flame has a
considerable influence upon the reaction. The
results of their experiinents indicated that trioxide
formation could be reduced by decreasing the
equilibrium conversion of SO2 to SO3 by keeping
the quantity of excess air at a minimum in con-
junction with high combustion chamber temper-
atures. A perusal of the theory will show agreement
with this statement.
Obviously, then, the amount of air supplied to
the burner should be carefully metered, or, failing
that, regular analyses of the gases from the com-
bustion chamber should be carried out to ensure
that the SO2 content is being maintained at a
maximum.
A rough guide to the correct amount of air re-
quired is based on observation of the flame in the
burner. If the sulphur burns with a blue flame,
conditions are just right. A flame with a brown
tinge indicates insufficient air and sublimation of
sulphur. However, these observations do not
prevent one from operating the burner with' an
excess quantity of air and one is well-advised to
rely more on chemical tests and mechanical aids
than on rule-of-thumb methods such as those men-
tioned above.
A study of Table VII will show how the quantity
of excess air can influence the formation of SO3'
94
Should the burner be operated with insufficient
air to complete the combustion of sulphur, then
sublimation takes place and slight mists of unburned
sulphur may form.
Influence of Temperature.
Theory has already indicated to us that temper-
ature exerts a considerable influence upon the
amount of trioxide formed. In practice it has been
found that the optimum temperature range favour-
able to the formation of SO3 lies between MOC
and 980C22. Frohberg-" found that maximum
oxidation occurred at 400
0-500e.,
while at an
approximate temperature of 900/1,000C., dissoci-
ation took place. It has been shown that at
1,000C. nearly 50 per cent. of the SO3 formed is
decomposed, this decomposition starting at 700e.
at which temperature the conversion of SO2 to
S03 is about 60 per cent." Above 1,000e. the
dissociation of the trioxide is more rapid than its
formation. It may be generally stated that below
400e. the rate of formation of SO3 is too slow to
be regarded as a nuisance, while above 1,000e.,
even though SO3 is formed rapidly, the rate of
decomposition predominates. However, even at
305e., oxidation of S02 has been found to occur,
albeit at a very slow rate." Thus we have two con-
flicting states, viz. where the rate of conversion of
SO2 to SO3 increases rapidly with increasing
temperature, but where the dissociation of SO3
overhauls the rate of conversion, and at the higher
temperature (above 1,000e.), therefore, the per-
centage conversion, as measured, tends to decrease.
The reaction:
2S0
2
+ O
2
2S0
3
takes place nearly to completion at 450C. in the
presence of a catalyst, and. it has been shown that,
'while the rate of the forward reaction is only
moderate at 400e., it increases to 40 times this
value at 500e.,the decomposition reaction only
becoming perceptible at 550e. and upwards.
It may therefore be said that up to a temperature
of 450C., reaction of formation prevails, and only
well above this temperature does dissociation come
into play.25
Sulphur trioxide is very stable in the absence
of contact substances. and once formed is difficult
to dissociate. Generally, decomposition will not
take place completely at temperatures as high as
1,100/1,200e. Decomposition of trioxide already
formed cannot be relied upon to keep the loss from
this source at a reasonable value at temperatures
below 1,00e., the amonnt of dissociation at this
temperature only reducing the loss by an amount
of less than 0.5 per cent.
It ' is well worth .remembering 'that the final
equilibrium condition of the reaction depends only
upon the temperature and the composition of the
gas mixture, and oxidation of SO2 will take place
until a certain ratio of trioxide to dioxide is reached.
High temperatures favour a low ratio and low
temperatures a high ratio of SO3 to SO2.
7
Presence of Moisture.
The air drawn into a burner is not normally
dried, but to prevent the formation of corrosive
sulphuric acid mists, the water content of the air
used should not be more than 5 mgm. per cubic
foot of air at S.T.P.24 Under conditions prevailing
in Natal, this would involve drying the air with
either concentrated sulphuric acid or P 205 or :a
similar efficient drying agent.
If the air is thoroughly dried with P 205' there
is little oxidation of SO2 up to a temperature of
450e. and in general it may be stated that dry
air helps to retard oxidation. In the presence of
water vapour, oxygen does not combine with SO2
at 100e. but oxidation does occur, even at this
low temperature, if particles of liquid water are
present. 6 Mellor" found that moisture does not
appear to affect the oxidation of SO2' but the
presence of CO2 and nitrogen causes more SO3
to be formed.
Moisture has a poisoning effect on some catalysts
and Tolley" has shown this to be true for iron
oxide. Upon continued exposure, however, cata-
lysis slowly increases until after 40 hours the
catalysis with wet gases is half that of dry gases.
He found that water vapour has its greatest in-
hibiting action at a temperature of 475e., put
even at 635e., the catalytic activity of iron oxide
is reduced to ! of that with dry gases.
Browning and Kress? investigated the dew-point
of burner-gas mixtures and showed that the cor-
rosion to be expected would be greatest at the dew-
point of the particular gas because of the combined
action of scale and condensed liquid upon the metal
base. Above the dew-point, only gases are present
and corrosion is much reduced, while at temper-
atures below the dew-point, the rate of corrosion
is considerably less on account of the slowing up
of the chemical reactions at these lower temperatures.
Their experiments revealed that the dew-point
increased as the SO2 content of the gas was raised
and for safe operating practice, using moist air,
the temperature of the gas in iron should be kept-
above 200C. to prevent undue corrosion of the
metal. When the gas is required to be cooled
below this temperature, it should be transferred to
a lead pipe.
As previously stated,. moisture in the air can
cause mists to form in the burner gas and these are
most difficult to, remove. A method which helps
in the removal of mists consists of cooling the mixed
gas to below 46C., when the S03 will condense.
The gas should now be passed through a filter-box
packed with charcoal or iron borings and then
through a water-spray .scrubbing tower. This
method, however, is not always very successful
and it is easier to prevent mists than, having once
formed, to remove them.
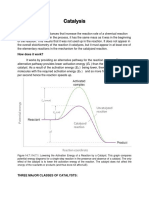
Action of Catalysts.
Catalysts do not affect the equilibrium point of
a gas, mixture, but merely alter the rate at which
equilibrium is approached. The ratio of the original
and final substances present in the gas will not be
changed upon contact with a catalyst. The contact
substance will only ensure that this equilibrium
ratio, is reached in less time.
A list of various contact substances normally
encountered follows, and these, with their effects
upon SO3 formation, have been listed separately
for the sake of clarity. Anyone wishing to know
more about the effects of various catalysts on SO3
formation, should read the monumental work of
Browning and Kress," as this covers the subject
most comprehensively.
A. Iron Compounds.
Iron or its oxides have long been known to exhibit
catalytic properties as far as the oxidation of SO2
is concerned and the Mannheim Process for the
manufacture of suphuric acid utilised iron oxide
as a catalyst. For reasons of efficiency, this oxide
has been replaced by either platinum or vanadium
in the Contact Process.
Tolley" predicted that the first reaction to occur
when steel was in contact with sulphur dioxide
and oxygen at reasonably high temperatures, would
be a combination of oxide and sulphide formation,
represented as:-
2Fe + S02-+ 2FeO + S
Fe + S-+ FeS
or alternatively:-
5Fe + 2S0
2
-+ 2FeS' + Fe 304
He pointed out that as FeO cannot exist below
570C., the first reaction shown above could only
occur above this temperature. The experiments
showed that the catalytic activitiy of mild steel
increased rapidly during the first few hours of
exposure to S02' but after about 10 hours the
activity became reasonably constant. It was felt
that this initial rapid increase in the rate of oxid-
ation of SO2was probably due to the formation of
iron oxide.
95
The catalytic effect of iron compounds depends to
a large extent upon their physical and chemical
state. Experiments have shown that iron oxides,
as such, are not good catalysts, but their activity
may be increased after a certain period by the
formation of sulphates and other compounds.
At elevated temperatures both the dioxide and
trioxide react with iron, and this would explain
the short life of iron under these conditions of
service.
In a rotary sulphur burner, there will be surges
of SO3 formed when starting up or burning down
as more iron will be exposed during these periods.
Furthermore, the temperature range at which -
maximum SO3 formation occurs will be passed
through during these stages."
Reverting to the influence of physical state upon
catalytic activity, it is worth noting that ferric
oxides may exhibit contact properties, and the
surface of the material will exert the least influence
when freshly precipitated oxides, which are not yet
dried, are used. The activity increases if the oxide
has been moderately heated or kept for a long time
so as to become dry. Oxides obtained by heating
ferric- or ferrosulphate give a much lower contact
action than that obtained with an oxide prepared
by igniting a precipitated hydroxide or pyrites-
cinders." An oxide containing 21 per cent. Arsenic
as As shows considerably more contact reaction
than that of a pure oxide at 700C., while the
addition of copper oxide to an oxide of iron is
favourable to the formation of S03.
26
Observations have shown that when a burner
gas is in contact with an iron pipe, maximum
conversion occurs at 750C., there being a 13 per cent.
maximum conversion for a gas with a 10.5 per cent.
S02 concentration. This conversion drops to 10
per cent. when the SO2content is raised to 14 per
cent. and a further decrease in conversion to 3 per
cent. for a gas of 19.5 per cent. content is observed. 23
At 1,000C., the conversion at all concentrations
IS zero.
Ferric oxide begins to exhibit catalytic activity
at 550C., and this activity increases to a maximum
in the temperature range 600
0-620C.
6
Increasing
the S02 content of the gas from 2-12 per cent.
does not affect the conversion to any appreciable
extent, although with higher concentrations, the
yield of SO3 is lower. As a contact substance,
this oxide only becomes really effective at a temper-
ature of 600C. when a percentage conversion of
SO2 to SO3 of 40 to 50 per. cent.! is attainable.
In practice, however, the conversion never exceeds
60-66 per cent.!"
Iron oxide formed from pyrites-cinders shows
little activity, and this only begins above 650C., .
increasing with temperature and reachirig a con-
version of 1 percent. at 1,000C. with a gas con-
taining 15 per cent. SO2' However, it should be
considered worthy of note that if 3 per cent. of SO3
is formed in a burner gas during normal burning
operations, this figure may, under favourable
conditions, be raised to three or four times this
value if the gases are passed through red-hot
pyrites-cinders. 26
Pure iron oxide exhibits maximum contact action
at 650C., the respective conversion figures being
15 and 5t per cent. for gases of 10.5 and 19.5 per
cent. SO2 content. These conversion figures drop
to 4i and Ii per cent. respectively when the temper-
ature is raised to 1,000C., the activity in this
case being only above 500C.7
Ferrous sulphate has a maximum contact action
at 650C. when the conversion of a gas containing
15 per cent. S02 is 12t per cent., this conversion
decreasing to t per cent. at 1,000C. Similar con-
version figures for ferric sulphate are 8 per cent. at
650C. and t per cent. at 1,000C.; indicating that
it is less active than the ferrous form.
All the above observations indicate that iron in
its many forms exhibits varying degrees of catalytic
activity and if the formation of. SO3 is to be mini-
mised then obviously gases of high S02 concen-
tration must be produced at the highest possible
combustion chamber temperature that can be
attained in practice. It is of further benefit to
note that in many instances, the amount of SO3
that will be formed according to theory will not be
reached in practice as the gas passes through the
combustion apparatus before equilibrium is reached.
Silica and Silicates.
It is generally conceded that vitreous fused
silica exerts no catalytic effect on the oxidation of
S02.7 The same applies to Dialite brick, and
therefore these materials are considered satisfactory
for the lining of furnaces, combustion chambers,
etc.
The Efficient Operation of a Burner.
In America it is a generally accepted fact that
the air to the burner should be carefully metered
by mechanical means and furthermore, allowance
is made for fluctuations in the burner. These
changes in burning rate are catered for by the
installation of an automatic recording device which
continuously analyses the SO2 content of the
gases from the combustion chamber. A diaphragm
operated valve then automatically operates the
air inlet and sulphur feed and regulates these,
thereby keeping the SO2 content of the burner gas
constant within fairly narrow limits.
96
Furthermore, the sulphur feed should be
mechnical to enable a constant rate of feed to be
maintained. With. all these refinements it is
possible to arrange for high combustion temper-
atures and a burner gas containing the highest
concentration of SO2 possible with the type of
apparatus in use. Even better results will be
obtained if the sulphur. is melted and strained
before it is fed to the burner, for reasons already
gIven.
The combustion chamber should be fitted with
one or more baffles to ensure thorough mixture of
the gases and prevent any possibility of unburned
sulphur passing to the absorption plant. Coolers
are normally fitted after the combustion chamber,
and it is imperative that cooling of the gas be as
rapid as possible to minimise SO3 formation.'
Initially the gases would be air- or. water-cooled
and then passed to either a direct or indirect cooler
to bring the temperature down to 200/300C.
At this stage, if further cooling is attempted, the
pipes conducting the gases should be lead-lined.
The subject of cooling is outside the scope of this
paper, but is well-worth : pursuing. by reading
Lundberg- and others.
Summary.
The practice of sulphur burning, the production
of SO2 and the formation and dissociation of SO3
has been outlined and the effects of:
Excess Air,
Temperature, and
Catalysts
upon SO3 formation detailed. Where possible a
comparison with the theory has been made and it
has been shown that the following points all help
to increase the efficiency of this type of plant:-
(a) It is preferable to feed the sulphur con-
tinuously by mechnical feeder. The feeding
of strained molten sulphur is preferable as
this eliminates moisture and deleterious hydro-
carbons.
(b) The quantity of excess air should be carefully
controlled and the SO2content of the burner-
gas kept at a maximum.
(c) The temperature of the burner and com-
bustion chamber must be maintained above
1,000C. if at all possible.
(d) Automatic recording and operating apparatus
. to maintain an even feed of sulphur and
constant SO2 content of the gas is helpful.
(e) The gas from the combustion chamber should
be filtered and cooled as rapidly as possible
to prevent formation of SO3'
Conclusions.
The authors have made everv endeavour and
taken all possible precaution's to ensure that the
information given is accurate. However, mistakes
may have cropped up and no responsibility can be
taken for such errata.
Many of the improvements listed may possibly
make the production of SO2 by these methods rather
costly and their inclusion should not be taken as
a recommendation, but rather as a guide or example
of how efficiency may be improved. . These refine-
ments are in practice in America in many of the
larger paper mills, so obviously they are economical
for the production of large quantities of SO2'
It is admitted that it is literally impossible to
prevent the formation of SO3 entirely, but if pre-
cautions, along the lines of those mentioned in this
paper, are taken, the quantity of SO3 can be reduced
to such a low level that it no longer constitutes
a nuisance.
The types of burners listed were limited to those
generally used in the. Sugar Industry and those
which may be of interest. The spray type burner
is proving very popular in America and it produces
a gas of low SO3 content and furthermore it is very
flexible in that it may be started or shut down in
a very short space of time. Control is easier than
with the conventional rotary burner and paper
mills in America have found that installation costs
are not excessive. Maintenance costs are low
and power consumption compares most favourably
with other types.
For the sake of those who would like to know
more about sulphur burning and its applications,
a reading list has been added.
Acknowledgments.
Our sincere appreciation and thanks are duly
made to those firms and Institutions in America
who corresponded with us, supplied technical
articles and generally went out of their way to be
helpful.' In particular we would like to mention:-
The Paper Institute, Texas Gulf Sulphur Company,
Acme Coppersmithing and Machine Co., and the
Glens Falls Machine Works-all American organis-
ations that were extremely helpful and co-operative.
REFERENCES.
1 Beater: The Distribution of Temperature in the Sugar Belt
of Natal and Zululand-S.A.S.T.A. 1949.
2Lundberg: Acid Making in the Sulphite Pulp Industry.
3Chemical Control Plant Data: Booklet issued by Chemical
Construction Company.
4 Fairlie: Sulphuric Acid Manufacture.
5Lewis and Randall: Thermodynamics and the Free Energy
of Chemical Substances.
6 Mellor: A Comprehensive Treatise on Inorganic and
Theoretical Chemistry-Vol. X..
97
7Browning and Kress: A Study of Some Factors Influencing
the Formation and Dissociation of 50
3
in Burner Gases-Paper
Trade Journal, 100 No. 19, 31-43, 1935.
8Glasstone : Thermodynamics for Chemists.
o Dodge: Chemical Engineering Thermodynamics.
10 Whitney, Elias and May: Chemical Reaction Equilibria-
TAPPI; 34, No.9, 11.)51. .
11 The Glens Galls Rotary Sulphur Burner: Pamphlet from
Glens Falls Machine Works.
12 Darrah: The Preparation of S02-Paper Trade Journal,
pp. 132, Nov. 30, 11.)50.
13 Sutermeister: Chemistry of Pulp and Paper Making.
14 Josephson and Downey: Sulphur and Pyrites-U.S. Bureau
of Mines Yearbook, lU49.
15Ridgway: Sulphur-General Information-U.S. Bureau
of Mines I.C. 6329. . .
16 Sulphur: Technical Service Note No. 32-African Explosives
and Chemical Industries, Ltd.
17 Furniss: Rogers Manual of Industrial Chemistry.
18 General Description of Acme Burner-Pamphlet from
Acme Coppersmithlng and Machine Co.
10 Cain and Chatelain: New Low-Capacity Sulphur Burner-
Chemical and Metallurgical Engineering, 46, Oct. 1939.
20 Kress and Others: Spray Type. Sulphur Burner-The Paper
Mill, Oct. 11.)34.
21 Conroy and Johnstone: Combustion of Sulphur in a Venturi
Spray Burner-Industrial and Engineering Chemistry 41, pp.
2741, Dec. 194U.
22 Barker: Sulphite Acid Preparation-Paper Trade Journal,
pp. 136, Nov. 30, lU50.
23 Newell. Stephenson: Pulp and Paper Manufacture, Vols. I
II and III.
2< Modern Chemical Processes.
25 Riegel: Industrial Chemistry.
26 Lunge: Manufacture of Sulphuric Acid and. Alkali, 3rd
Edition.
27 Tolley: The Catalytic Oxidation of SO2 on Metal Surfaces
Journal Society for Chemical Industry, 67, pp. 369, Oct. 1948.
READING LIST.
Chemical Engineering-53, 225 (1946).
54, 221 (1947).
Chemical Engineering Process-46,614 (1950).
Chemical Markets-30, 261 (1932).
Chemical and Metallurgical Engineering 42, No. 7,374-7 (1935)
Chemical Process Principles-Hougen and Watson.
Industrial and Engineering Chemistry-17, 593 (1925).
34, No.9, 1017 (1942).
35, 522, 541-5 (1943).
41, 2741 (1949).
42, No.4, 713-8 (1950).
42, 2215-7 (1950)
Industrial Chemistry-Riegel.
Journal Am. Chern. Soc.-48, No. 11 (1926).
Chern, Soc.-123, 3203 (1923).
Paper Trade Journal-94, No. 15, 39-42 (1932).
" " " 99, No. 17,48-51 (1934).
Proc. Roy. Soc. London-Series A, 138, 635 (1932).
Pulp and Paper Mag. Canada-31, No. 52, 1390-3 (1931).
33, No.2, 78-80 (1932).
Conv. 37, No.3, 140-8 (1938).
44, No.4, 320 (1943).
52, 108-111 115 (1951).
"" "" Conv. 243-8 (1951).
Soc. Chern. Ind. Journal-67, 369-73 401-4 (1948).
Textbook of Physical Chemistry-Glasstone.
Trans. Am. Inst. Chern. Engs.-27, 264 (1931).
U.S. Bureau of Mines-Bulletin 406 (1\)37),.
98,
TABLE V.
Variation of Equilibrium Constant, K, with Temperature for the
Reaction SO. + !O. = SOa
APPENDIX OF TABLES.
TABLE I.
Weight and Volume of 'Burner Gas at S.T.P. hased on One Pound
of Sulphur-Dry Air.
Temperature.
C.
Equilibrium Constant J{,
Fairlie Lewisand Miles
Randall.
Average
K.
Average.
Log: 10K.
Weight and Volume'of Burner Gas at S.T.P. based on One Pound
of Sulphur-Air Saturated with Water Vapour.
Compositionof Burner Gas. Quantity of Quantity of Air.
% by Volume.. %50, Burner Gas.
Oxygen. SO, Nitrogen. Water Weight CubicFeet, Lbs. CubicFeet. Lbs.
15 6 79 12.48 187.0 16.02 187.0 15. I
13 8 79 16.31 140.3 12.26 140.3 1l.3
II 10 79 19.98 112.2 10.01 112.2 9.1
9 12
79 '
23.50 93.5 8.51 93.5 7.5
5 16 79 30.1.5 70.1 6.63 70.1 5.7
3 18 79 33.30 62.3 6.01 62.3 5.0
1 20 79 36.33 56.1 5.51 56.1 4.5
Variation of Theoretical 'Flame Temperature and Molecular
Weight with Burner Gas Composition.
A.-Dry Air-No Radiation Losses.
B.-Dry Air-Assuming 15 per cent. of Total Heat Lost by
Radiation.
TABLE VII.
Variation of Equilibrium Temperature with Burner Gas Com-
position Assuming Degree of Conversion of SO. to SO,,-Dry Air.
500 48.2 52.5 48.7 49.8 1.697
600
0
9.53 9.75 9.74 9.67 0.985
700
0
2.62 2.55 2.56 2.58 0.412
800
0
0.915 0.859 0.864 0.879 1. 944
,900 0.384 0.349 0.353 0.362 1.559
1000 0.1845 0.163 0.167 o. in 1.236
1200 0.0573 0.0485 0.O511 0.0523 2.719
1400
0
0.0235 0.0193 0.0209 0.0212 2.326
1600
0
0.01l7 0.0093 0.0104 0.0105 2.021
1800 0.0066 0.0052 0.0060 0.0059 3.771
2000 0.0041 0.0032 0.0038 0.0037 3.568
TABLE VI.
V!'riation of Equilibrium Temperature with Burner Gas Com-
position Assuming Degree of Conversion of S02 to SOa-Dry Air.
Equilibrium Temperature
DegreesCentigrade.
Gas SO, 6% 8% 10% 12% 16% 18%
%Conversion. Composition 0, 15% 13% 11% 9% 5% 3%
99 421 415 407 394
95 499 492 482 467
90 540 533 523
0
507
0
80 591
0
583 573 557
70 629
0
621 610
0
596
0
60 662 654 644 630 526
50 695
0
687
0
677
0
664 600
0
40 731 723
0
713 700 649
30 773
0
765
0
755
0
742 698 600
25 798 789 779 767
0
725
0
668
0
20 828
0
819 809 797 757
0
710
10 923
0
913
0
902 889
0
849
Quantity of Air.
CubicFee!. Lbs.
Quantity of Burner
Gas.
CubicFeet. Lbs.
TABLE II.
%05,
By
Weight
Composition of Burner Gas. Molecular Theortetical Flame
% by Volume. Weight of Temperature-e-X'.
Oxygen. SO, Nitrogen. Burner Gas. A. B.
15 6 79 30.8 593 508
13 8 79 31.4 766 659
II 10 79
. 32.0
928 795
9 12 79 32.7 1095
0
\J44
5 16 79 34.0 1384 1224
3 18 79 34.6 1534 1328
1 20 79 35.3 1667 1411
14.8 5.9 77.7 1.6 12.36 190.2 16.18 190.2 15.3
12.8 7.9 77.7 1. 6. 16.21 142.0 12.34 142.0 1l.4
10.9 9.8 77.7 1.6 19.71 114.5 10.15 114.5 9.2
8.9 1l.8 77.7 1. 6, 23.26 95.1 8.60 95.1 7.6
4.9 15.8 77.7 1.6 29.97 71. 0 6.67 71. 0 5.7
3.0 17.7 77.7 1.6 32.99 63.4 6.06 63.4 5.1
1.0 19.7 77.7 1:6 36.05 57.0 5.55 57.0 4.6
TABLE III.
Composition of Burner
Gas.
% by Volume.
Oxygen. SO, Nit.
TABLE IV.
Equilibrium Temperature-Degrees Centigrade.
Gas SO, 6% 8% 10% 12% 16% 18% 20%
Composition Os 150/0 130/0 110/0 50/0 3,fo 1%
0;0 Conversion.
14.8 5.9 77.7 1.6 30.5 582 496
12.8 7.9 77.7 1.6 31. 2 757 646
10.9 9.9 77.7 1.6 31.8 914 783
0
8.9 1l.8 77.7 1.6 32.5 1080 927
4.9 15.8 77.7 1.6 33.7 1368 1182
3.0 17.7 77.7 1.6 34.3 1496 1292
1.0 19.7 77.7 1.6 35.0 1624 1403
Variation of Theoretical Flame Temperature and Molecular
Weight with Gas Composition.
A.-Air 74per cent. Saturated with Water Vapour-No Radiation
Losses.
B.-Air 74 per cent. Saturated with Water Vapour-I:) per cent.
of Total Heat Lost by Radiation.
Composition of Burner Gas.
%by Volume. ,
Oxygen. SO, Nitrogen.
Molecular
Weight of
Water. Burner Gas.
Theoretical Flame
Temperature-e-v'C.
A. B.
5 1025 1015 1002 988 945 815
4 1061 1050 1037 1021" 977 938 850
3 1l10 1098
0
1084 1068 1020 965 893
0
2 1184 1170 1155 1137 1086
0
1044 954
0
1.5 1241 1227 1210 1190 1138 1091" 998
1.0 1328 1312 1295
0
1276 1212 1173 1064
0.9 1354 1336
0
1317 1295 1233 1183 1082
0.8 1381
0
1364 1345 1321
0
1257
0
1207 1l02
0.7 1414 1397 1376 1352 1286
0
1232
0.6 1454 1436 1414 1389 1320
0
1264
0
1154
0.5 1503 1484 1461 1435 1362
0
1303 1188
0.4 1568 1547 1522
0
1495 1417 1355 1233
0.3 1659 1635 1608 1578
0
1493
0
1425
0
1294
0.2 1801
0
1775 1745 1709
0
1612 1535 1388
0.1 2103 2068 2028 1982 1858 1760 1577
0.05 2505
0
2458 2405 2343 2176 2048 1814
0.02 3308 3230 3142 3041
0
2780 2584 2237
0.01 4308 4182 4041
0
3881 3481" 3188 2691
19
17
IS
GRAPH "A"
VARIATION OF QUANTITY OF BURNER GAS
WITH SO, CONCENTRATION.
VOLUME OF BURNER GAS-DRY AIR.
WEIGHT OF BURNER GAS DRY AIR
SCALE A: UNITS = LBS.
UNITS x 10 "CUBIC FEET.
13
Z
:J
Cl:
III
":"1
oJ
"
;
III
9
6 10
-e-
6 18 2
17
IS
13
"
9
7
6
% SO, BY VOLUME.
GRAPH "B"
VARIATION OF QUANTITY OF BURNER GAS
WITH SO, CONCENTRATION.
VOLUME OF BURNER GAS MOIST AIR.
WEIGHT OF BURNER GAS-MOIST AIR.
SCALE A: UNITS LBS.
UNITS x 10co CUBIC FEET.
0;.50, BY VOLUME.
20
17
GRAPH ..C"
FLAME TMPEAATURES yo. '50, CONC:ENTRATION.
ORY AIR.
100
. GRAPH "0"
FLAME TEMPERATURES \'I. SO CONCENTRATION.,
MOIST AIR.
1750
1500
U
0
III
1250 a:
36
36
:J
...
,{J""
U
8'Pi
i'
:r
<t': ..
III
:r
III o ' q,1i ...
\ 0 a:
..
$\0 ..
r-
S
. ,
0
J:
m
34
r:
III ..I
n
m
...
C
n
s
III
otP\V C
..
s
:JD
J:
III
:JD
m
... -750
32
Ci
m
:I
s
:-4
:I
:-4
30 500 30
6
6 10 104 18 22
%50, BY VOLUME. %50, BY VOLUME.
GRAPH "E"
Log" K. \'I. TEMPERATURE
FOR REACTION: S0, + SO,.
+
+1
0
il
500 700 900 1100 1,3pO h
l.7oo 1900 2100
I
TEMPERATURE C.
'-1
100
80
Z
0
-60
iii
II:
'" >
Z
0
--40
U
~
20
101
GRAPH "F"
EQUILIBRIUM TEMPERATURE VI. % CONVERSION.
5
4
Z ~
0
0ll'
~
< ~
0ll'
'" 9
>
z
0
U
2
~
5 1,100
TEMPERATURE C.
GRAPH "G"
VARIATION OF EQUILIBRIUM TEMPERATURE WITM
SO, CONCENTRATION AT LOW CONVERSION PERCENTAGES.
BOO 1000 12 20
TEMPERATURE C.
2
102
Mr. Dymond said that they were indebted to the
authors for this very important paper: . He expressed
the view that the standard of papers at the Congress
was very high. This paper provided data that had
been wanted for many years.
Mr. Main agreed and said that the paper was a
most valuable addition to the technical knowledge.
He referred to Maxwell's book on sulphur burning
which was now out of print. Since then he had not
found anything as valuable as the paper presented.
He hoped that it might be possible to provide
illustrations.
Mr. Hogarth said the point had been discussed
and photostat copies of a paper dealing more
especially with one type of sulphur burner could be
made. He hoped that these could be fairly generally
distributed.
Mr. Rohloff said he thought the three types of
sulphur burners could be classed as three separate
stations in the development of sulphur burners.
Much the same could besaid about the development
of boilers. He drew a parallel between the different
types of sulphur burners and the different types of
boilers. This development seemed to suggest that
the trend was towards greater flexibility and he
thought it was fit to mention this, as it indicated
a desire to make sulphur burners for the Sugar
Industry more flexible and more applicable to sugar
production. Mr. Rohloff said that they had asked
the S.M.R.I. about the Acme burner and were told
that this had been investigated, but the type of
sulphur used in the Industry was not applicable to
this burner.
Mr. Hogarth .said the sulphur supplied to the
Sugar Industry did not vary in composition froin the
sulphur used overseas and presumably used in
Acme burners.
Mr. Perk said that speaking from memory, he
had told Mr. Rohloff that the method of storing
sulphur in Natal precluded the sulphur being used
in the closed type of burner used in J ava. In order
to operate these burners for months without loss
of capacity or interruption for cleaning, the sulphur
had to be kept free from dust and moisture. It
was therefore routine at the Java factories to store
the sulphur in a separate compartment, and
particularly not in the store-room where lime was
kept.
Mr. Main expressed the view that sulphur storage
conditions did not materially affect the sulphur
used in burners in Natal.
Mr. Hogarth said he was inclined to agree with
Mr. Main, as the contaminants in the sulphur
supplied to the Sugar Industry were very small
indeed.
Mr. Rault said the problem in the Sugar Industry
was not only to burn the sulphur but also to absorb
it. He asked whether there was a more modern,
compact' and controllable absorbtion system than
the "eye sore" and dirty plant called the sulphur
tower, commonly used in our factories.
Mr. Hogarth said that the absorption of SO2 was
a most complicated question, but there was equip-
ment available which would assist in the absorption
more efficiently than was done at the moment in
the Industry. He offered to, supply this inform-
ation to Mr. Rault.
Mr. Dymond agreed that the problem of hot
gases going into the juices required further in-
vestigation and he hoped this would be done by the
S.M.R.I.
Mr. Barnes referred to the possibility of using
S02 instead of raw sulphur in the Sugar Industry, as
increasing quantities of SO2 were being generated
by industry.. He thought the possibility of using
cold gases should be investigated more thoroughly.
He referred to the use of liquid ammonia as a
fertiliser and said it was not many yeCjlrs since this
had been thought impossible, but today most of the
nitrogen used in Louisiana was applied in the form
of gas or liquid. He felt the same might apply to
gaseous S02 in the Sugar Industry.
Mr. Dymond concluded by calling for a hearty
vote of thanks to Mr. Hogarth for hisexcellent paper.
Anda mungkin juga menyukai
- The Mechanism of Gold Cyanide of Elution From Activated CarbonDokumen2 halamanThe Mechanism of Gold Cyanide of Elution From Activated CarbonwandadwilestariBelum ada peringkat
- Industrial _ Engineering Chemistry Process Design and Development Volume 19 issue 4 1980 [doi 10.1021_i260076a001] Garside, John; Shah, Mukund B. -- Crystallization Kinetics from MSMPR Crystallizers.pdfDokumen6 halamanIndustrial _ Engineering Chemistry Process Design and Development Volume 19 issue 4 1980 [doi 10.1021_i260076a001] Garside, John; Shah, Mukund B. -- Crystallization Kinetics from MSMPR Crystallizers.pdfIka SulistyaningtiyasBelum ada peringkat
- Escaid110 PDFDokumen1 halamanEscaid110 PDFPablo Valenzuela ArredondoBelum ada peringkat
- Silicones and Structural AdhesivesDokumen12 halamanSilicones and Structural AdhesivesDries Seuntjens100% (1)
- Basic Principles of Unit Processes and Unit OperationsDokumen19 halamanBasic Principles of Unit Processes and Unit OperationsAisyah Murti Condro100% (2)
- Glycerol Dehydration To Acrolein in The Context of New Uses of GlycerolDokumen20 halamanGlycerol Dehydration To Acrolein in The Context of New Uses of GlycerolBernyke ContracyBelum ada peringkat
- On Paratacamite and Some Related Copper ChloridesDokumen12 halamanOn Paratacamite and Some Related Copper ChloridesHJKB1975Belum ada peringkat
- ASTM D5705-20 Measurement of Hydrogen Sulfide in The Vapor PhaseDokumen4 halamanASTM D5705-20 Measurement of Hydrogen Sulfide in The Vapor PhaseSergey KichenkoBelum ada peringkat
- Refining Gas Processing Petrochemicals: Petroleum Technology QuarterlyDokumen108 halamanRefining Gas Processing Petrochemicals: Petroleum Technology QuarterlyTruth SeekerBelum ada peringkat
- Hydrometallurgy 2019Dokumen96 halamanHydrometallurgy 2019Farhan SuhermanBelum ada peringkat
- Sugar Industry Technologists Meeting 2000 Paper Optimizes Water UsageDokumen12 halamanSugar Industry Technologists Meeting 2000 Paper Optimizes Water Usageroy@daesBelum ada peringkat
- Homogeneous Catalysis - Wikipedia, The Free EncyclopediaDokumen3 halamanHomogeneous Catalysis - Wikipedia, The Free EncyclopediaBHAAJI0001Belum ada peringkat
- Novel coker naphtha hydrotreatingDokumen13 halamanNovel coker naphtha hydrotreatingGarry DavidBelum ada peringkat
- Graduation-Project - Sulfuric AcidDokumen195 halamanGraduation-Project - Sulfuric AcidMuntazer QasimBelum ada peringkat
- Coal OxidationDokumen11 halamanCoal Oxidationmukesh vikramBelum ada peringkat
- Vacuum Leaf FilterDokumen2 halamanVacuum Leaf FilterGangadharan NagappanBelum ada peringkat
- Solvent extraction of Palladium from chloride media using TBPDokumen6 halamanSolvent extraction of Palladium from chloride media using TBPMoreno MarcatiBelum ada peringkat
- Advanced Recycle Paraffin Isomersation TechnologyDokumen8 halamanAdvanced Recycle Paraffin Isomersation TechnologytungksnbBelum ada peringkat
- Mercury Treatment Options For LNGDokumen5 halamanMercury Treatment Options For LNGamitBelum ada peringkat
- Journal Hydrometallurgy Solvent ExtractionDokumen13 halamanJournal Hydrometallurgy Solvent ExtractionLeochemical ChemicalBelum ada peringkat
- DOWEX Ion Exchange ResinDokumen12 halamanDOWEX Ion Exchange ResinsaphyranicoletaBelum ada peringkat
- Exp - ZN (NO3) 2.6H2O - KOHDokumen4 halamanExp - ZN (NO3) 2.6H2O - KOHManal AwadBelum ada peringkat
- Loss On Ignition Test Method and Procedures For Limestone SampleDokumen4 halamanLoss On Ignition Test Method and Procedures For Limestone SampleJa Phe TiBelum ada peringkat
- (Hydrazine) LectDokumen25 halaman(Hydrazine) Lectabdo mahmoudBelum ada peringkat
- CopperGlycine PDFDokumen5 halamanCopperGlycine PDFJaume HernandezBelum ada peringkat
- HydratesDokumen21 halamanHydratesAkande AyodejiBelum ada peringkat
- Jacobi Tds Colorsorb Hp120a A4 Eng E0116Dokumen2 halamanJacobi Tds Colorsorb Hp120a A4 Eng E0116Alfonso García100% (1)
- 1996 - Walford - Composition of Cane JuiceDokumen2 halaman1996 - Walford - Composition of Cane JuiceMartha GamalBelum ada peringkat
- Oxygen Enrichment FundamentalsDokumen15 halamanOxygen Enrichment FundamentalsahmadBelum ada peringkat
- O3Dokumen15 halamanO3ECRDBelum ada peringkat
- GDN 211Dokumen23 halamanGDN 211Vasant Kumar Varma50% (2)
- A Green Chemistry Approach To Mercury ControlDokumen7 halamanA Green Chemistry Approach To Mercury ControlDennis Daniel Condori EspilcoBelum ada peringkat
- Chemical Species in Marine Fuel Oil: ASTM D7845-13Dokumen4 halamanChemical Species in Marine Fuel Oil: ASTM D7845-13Ian RidzuanBelum ada peringkat
- Extraction of ZincDokumen15 halamanExtraction of ZincAmit MishraBelum ada peringkat
- SeminarDokumen17 halamanSeminarMohammad RagibBelum ada peringkat
- 18 - Kesetimbangan Fasa Dalam Kimia Fisika - Ch.4Dokumen13 halaman18 - Kesetimbangan Fasa Dalam Kimia Fisika - Ch.4SholèhNurUdinBelum ada peringkat
- Activated Alumina (Al2O3)Dokumen10 halamanActivated Alumina (Al2O3)Salman RasheedBelum ada peringkat
- Chapter 3 Separation of Oil and Gas - 1987 - Developments in Petroleum ScienceDokumen50 halamanChapter 3 Separation of Oil and Gas - 1987 - Developments in Petroleum ScienceFalokid RaboBelum ada peringkat
- Properties of Petroleum ProductsDokumen30 halamanProperties of Petroleum ProductsBharath KumarBelum ada peringkat
- 4500 SulfiteDokumen3 halaman4500 SulfiteTaniaCarpioBelum ada peringkat
- Optimization of Sugar Cane Bagasse Pretreatment Process Using RSMDokumen10 halamanOptimization of Sugar Cane Bagasse Pretreatment Process Using RSMInternational Journal of Application or Innovation in Engineering & ManagementBelum ada peringkat
- Plant Design Process SimulationDokumen20 halamanPlant Design Process SimulationABRAR ABDULLAHBelum ada peringkat
- Sodium Chlorate - Properties and Reactions PDFDokumen5 halamanSodium Chlorate - Properties and Reactions PDFangelofglory100% (1)
- Jntuworld: R07 Set No. 2Dokumen6 halamanJntuworld: R07 Set No. 2Dolly PriyaBelum ada peringkat
- Grading For Vacuum Pan (Plantation White and Refined) Sugar: Draft Indian StandardDokumen16 halamanGrading For Vacuum Pan (Plantation White and Refined) Sugar: Draft Indian StandardAkhilesh Dhar DiwediBelum ada peringkat
- Carbon-Coal Proximate Analysis ISO-17246-2010Dokumen9 halamanCarbon-Coal Proximate Analysis ISO-17246-2010engr kazamBelum ada peringkat
- Fydp Proposal by Group b4Dokumen17 halamanFydp Proposal by Group b4nokhaizBelum ada peringkat
- When Nameplate Is Not EnoughDokumen9 halamanWhen Nameplate Is Not Enoughgreenisin100% (1)
- PonaDokumen6 halamanPonaMahjabin Nadia100% (1)
- IS-12437 Zirconium PowderDokumen8 halamanIS-12437 Zirconium PowderAnuradhaPatraBelum ada peringkat
- Coal Gasification PDFDokumen8 halamanCoal Gasification PDFmrizalygani99Belum ada peringkat
- Tanzania Audits Final Report (Workstations GEITA) PDFDokumen22 halamanTanzania Audits Final Report (Workstations GEITA) PDFRegina Efraim100% (1)
- D 4607 - 94 R99 RDQ2MDCDokumen5 halamanD 4607 - 94 R99 RDQ2MDCMoch Arief AlbachronyBelum ada peringkat
- Industrial Report On Carew and CompanyDokumen60 halamanIndustrial Report On Carew and CompanyShariful IslamBelum ada peringkat
- UREADokumen5 halamanUREANitin HansaliaBelum ada peringkat
- Nalco CGMax A Tool For Alumina Yield Improvement and Product Quality ControlDokumen9 halamanNalco CGMax A Tool For Alumina Yield Improvement and Product Quality ControlAndreyBelum ada peringkat
- Synthesis of Furfural From Bagasse: Submitted To UIT-RGPVDokumen14 halamanSynthesis of Furfural From Bagasse: Submitted To UIT-RGPVvivekBelum ada peringkat
- High Pressure Fluids Visualization CellsDokumen4 halamanHigh Pressure Fluids Visualization Cellsapi-3703671Belum ada peringkat
- 7 tools statistical process control SPCDokumen3 halaman7 tools statistical process control SPCSachin MethreeBelum ada peringkat
- Meyer Coal 2014Dokumen23 halamanMeyer Coal 2014Molote Erwin MalieheBelum ada peringkat
- 〈92〉 Growth Factors and Cytokines Used in Cell Therapy ManufacturingDokumen4 halaman〈92〉 Growth Factors and Cytokines Used in Cell Therapy Manufacturingmehrdarou.qaBelum ada peringkat
- Chlorine: International Thermodynamic Tables of the Fluid StateDari EverandChlorine: International Thermodynamic Tables of the Fluid StateBelum ada peringkat
- Physical Chemistry: SO H O H SODokumen11 halamanPhysical Chemistry: SO H O H SOavmurugan87Belum ada peringkat
- Work PlanDokumen1 halamanWork PlanrexxmanuBelum ada peringkat
- Basics of Catalysts - Chemistry LibreTextsDokumen6 halamanBasics of Catalysts - Chemistry LibreTextsStatus loverBelum ada peringkat
- Thesis On Photocatalytic Degradation of Methylene BlueDokumen49 halamanThesis On Photocatalytic Degradation of Methylene BlueChirag Deepanshu Beck100% (1)
- Four Factors That Affect Chemical Reaction Rates (39Dokumen5 halamanFour Factors That Affect Chemical Reaction Rates (39Feliciano Tristan E.Belum ada peringkat
- Book of AbstractsDokumen158 halamanBook of AbstractsJaime Tiburcio CortésBelum ada peringkat
- Journal: AicheDokumen1 halamanJournal: AicheJohn AnthoniBelum ada peringkat
- Suicidal Inhibition or Reaction Based Inhibition PDFDokumen7 halamanSuicidal Inhibition or Reaction Based Inhibition PDFMuhammad JameelBelum ada peringkat
- Aniline by ReductionDokumen2 halamanAniline by Reductionraja.mtBelum ada peringkat
- 7D Sulphuric Acid: Milesh Kothari's Chemistry TutorialsDokumen12 halaman7D Sulphuric Acid: Milesh Kothari's Chemistry TutorialsarinnBelum ada peringkat
- Food Iii To Viii PDFDokumen54 halamanFood Iii To Viii PDFRaja PrabhuBelum ada peringkat
- FACT FertilizersDokumen40 halamanFACT FertilizersaswinBelum ada peringkat
- Sr. Chemistry Important Questions - 2023Dokumen4 halamanSr. Chemistry Important Questions - 2023lohithsoujan4569Belum ada peringkat
- Synthesis of Isoamyl AcetateDokumen3 halamanSynthesis of Isoamyl AcetateClaire TiongsonBelum ada peringkat
- Allenation of Terminal Alkynes With Aldehydes and Ketones (Accounts of Chemical Research) (2019)Dokumen12 halamanAllenation of Terminal Alkynes With Aldehydes and Ketones (Accounts of Chemical Research) (2019)RamaBelum ada peringkat
- Acs Iecr 0c05041rtyDokumen25 halamanAcs Iecr 0c05041rtyAmir HassanBelum ada peringkat
- Defect-Enriched Iron Uoride-Oxide Nanoporous Thin Films Bifunctional Catalyst For Water SplittingDokumen11 halamanDefect-Enriched Iron Uoride-Oxide Nanoporous Thin Films Bifunctional Catalyst For Water SplittingHusnain AliBelum ada peringkat
- Reversible Ammonia-Based Hydrogen Storage DavidDokumen22 halamanReversible Ammonia-Based Hydrogen Storage Davidkwayneolson6081Belum ada peringkat
- Methyl Ricinoleate MyDokumen10 halamanMethyl Ricinoleate Mygufran nagdawalaBelum ada peringkat
- Spring 2015 OMET Practice Problem Set KEYDokumen12 halamanSpring 2015 OMET Practice Problem Set KEYSay sayBelum ada peringkat
- Introduction To CatalysisDokumen44 halamanIntroduction To CatalysisGia Huy NguyenBelum ada peringkat
- Production of Maleic AnhydrideDokumen8 halamanProduction of Maleic AnhydrideZafran AliBelum ada peringkat
- How To Manage Your SCR Catalyst Effectively - Power Engineering InternationalDokumen10 halamanHow To Manage Your SCR Catalyst Effectively - Power Engineering InternationalJeeEianYannBelum ada peringkat
- Final Year Project Query: Compiled by Moosa Naseer & Assisted by TeamDokumen2 halamanFinal Year Project Query: Compiled by Moosa Naseer & Assisted by TeamMoosa NaseerBelum ada peringkat
- Notes Mechanism of Methanol Synthesis From Carbon Monoxide and Hydrogen On Copper CatalystsDokumen4 halamanNotes Mechanism of Methanol Synthesis From Carbon Monoxide and Hydrogen On Copper CatalystsArif HidayatBelum ada peringkat
- CATALYSISDokumen7 halamanCATALYSISJudy Ann Leyco BartolataBelum ada peringkat
- Sodium Carbonate, Milk and Indicator LipaseDokumen14 halamanSodium Carbonate, Milk and Indicator LipaseStephen AreriBelum ada peringkat

![Industrial _ Engineering Chemistry Process Design and Development Volume 19 issue 4 1980 [doi 10.1021_i260076a001] Garside, John; Shah, Mukund B. -- Crystallization Kinetics from MSMPR Crystallizers.pdf](https://imgv2-2-f.scribdassets.com/img/document/284126833/149x198/abec42366b/1444359067?v=1)