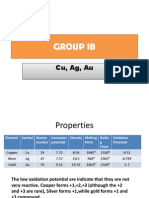
Electrolysis FRM Ag2so4
Diunggah oleh
Bhupesh MulikJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Electrolysis FRM Ag2so4
Diunggah oleh
Bhupesh MulikHak Cipta:
Format Tersedia
Carbon electrode
Silver sulphate
solution
Anode Cathode
+ -
Ag
+
, H
+
OH
-
, SO
4
2-
Electrolysis of SilverArgentu! Sulphate solution, Ag
2
SO
4
"ons present in the electrolyte# Cation# Ag
+
, H
+
$ Anion# OH
-
,
SO
4
2-
"ons !ove to anode# OH
-
, SO
4
2-
"on choose to be discharged at anode# OH
-
%position of ions&
Half e'uation# 4OH
-
O
2
+ 2H
2
O + 4e
Observation# (ubbles of colourless gas released$
"ons !ove to cathode# Ag
+
, H
+
$
"ons choose to be discharged at cathode# Ag
+
%position of ions&
Half e'uation# Ag
+
+ e Ag
Observation# Silver !etal deposited at the electrode$
Carbon electrode
)$* + potassiu!
bro!ide
Anode Cathode
+ -
OH
-
, (r
-
,
+
, H
+
Electrolysis of )$* + %concentrated& -otassiu! (ro!ide solution,
,(r
"ons present in the electrolyte# Cation# ,
+
, H
+
$ Anion# OH
-
, (r
-
"ons !ove to anode# OH
-
, (r
-
"on choose to be discharged at anode# (r
-
%concentrated ion&
Half e'uation# 2(r
-
(r
2
+ 2e
Observation# (ubbles of bro.nish gas released$
"ons !ove to cathode# ,
+
, H
+
$
"ons choose to be discharged at cathode# H
+
%position of ions&
Half e'uation# 2H
+
+ 2e H
2
Observation# (ubbles of colourless gas released
Silver electrode
Silver /itrate
solution
Anode Cathode
+ -
OH
-
, /O
)
-
Ag
+
, H
+
Electrolysis of Silver nitrate solution, Ag/O
)
.ith silver plate$
"ons present in the electrolyte# Cation# Ag
+
, H
+
Anion# OH
-
, /O
)
-
"ons !ove to anode# OH
-
, /O
)
-
"on choose to be discharged at anode# /o ion discharged$ Anode
is silver !etal, .hich is an active anode$ "t .ill dissolve in the
electrolyte$
Half e'uation# Ag Ag
+
+ e
Observation# 0he silver electrode beco!es thinner
"ons !ove to cathode# Ag
+
, H
+
$
"ons choose to be discharged at cathode# Ag
+
%position of ions&
Half e'uation# Ag
+
+ e Ag
Observation# Silver !etal deposited at the electrode$ Carbon
electrode beco!es thic1er$
0he colour of the solution re!ains unchanged$
-ure0ulenAsli
copper
Copper %""& sulphate
solution
Cu
2+
, H
+
, SO
4
2-
, OH
-
Anode Cathode
+ -
"!pure
copper
"!purities
0he i!pure copper plate dissolves to for! Cu
2+
ions$
Cu Cu
2+
2e
0he i!purities fall to the botto! of the bea1er$
0he Cu
2+
ions are discharged to for! copper at the pure copper plate$
Cu
2+
+ 2e Cu
2. Purification of metals
+ Anode
- Cathode
Carbon electrodes
+olten alu!iniu! o2ide, Al
2
O
)
Al
)+
, O
2-
+olten alu!iniu!
Alu!iniu! ions in the electrolyte .ill be
discharged at the carbon electrodes and
beco!e alu!iniu! ato!$ +olten
alu!iniu! .ill be collected at the botto!$
Al
)+
+ )e Al
O2ide ions, O
2-
in the electrolyte .ill be
discharged at the carbon lining and beco!e
o2ygen gas$
2O
2-
O
2
+ 2e
Carbon
lining
Extraction of metals
Application Of electrolysis in "ndustries
"ron spoon %sudu besi&
Copper %""& sulphate
solution
Cu
2+
, H
+
, SO
4
2-
, OH
-
Anode Cathode
+ -
Copper
electrode
0he copper anode dissolves to for! Cu
2+
ions$
Half e'uation# Cu Cu
2+
2e
At the cathode, Cu
2+
ions are discharged and deposited on the surface of
the iron spoon$
Half e'uation# Cu
2+
+ 2e Cu
Condition for good 'uality of plating#
Concentration of Cu
2+
ions !ust be lo.
0he electric current !ust be s!all
0he iron spoon !ust be turned steadily
0he surface of iron spoon !ust be clean$
3. Electroplating of metals
Penyaduran logam
CA0"O/ Half reaction
,
+
%-otassiu!& ,
+
+ e ,
/a
+
%Sodiu!& /a
+
+ e /a
Ca
2+
%Calciu!& Ca
2+
+ 2e Ca
+g
2+
%+agnesiu!& +g
2+
+2e +g
Al
)+
%Alu!iniu!& Al
)+
+ )e Al
3n
2+
%3inc& 3n
2+
+ 2e 3n
4e
2+
%"ron& 4e
2+
+ 2e 4e
Sn
2+
%Stanu!& Sn
2+
+ 2e Sn
-b
2+
%5ead& -b
2+
+ 2e -b
H
+
%Hydrogen& 2H
+
+ 2e H
2
Cu
2+
%Copper& Cu
2+
+ 2e Cu
Ag
+
%Silver& Ag
+
+ e Ag
A/"O/ Half 6eaction
4
-
%4luoride& 2 4
-
4
2
+ 2e
SO
4
2-
%Sulphate& SO
4
2-
SO
2
+ O
2
+ 2e
/O
)
-
%/itrate& 2/O
)
-
2/O
2
+ O
2
+ 2e
Cl
-
%Chloride& 2Cl
-
Cl
2
+ 2e
(r
-
%(ro!ide& 2(r
-
(r
2
+ 2e
"
-
%"odide& 2"
-
"
2
+ 2e
OH
-
%hydro2ide& 4OH
-
O
2
+ H
2
O + 4e
Anda mungkin juga menyukai
- The Silversmith's Handbook: Containing full instructions for the alloying and working of silverDari EverandThe Silversmith's Handbook: Containing full instructions for the alloying and working of silverBelum ada peringkat
- Charges of Cation and AnionDokumen2 halamanCharges of Cation and AnionJonathan Delos SantosBelum ada peringkat
- Brady Solution Chapter 20Dokumen31 halamanBrady Solution Chapter 20NurrahmisrBelum ada peringkat
- KimiaDokumen46 halamanKimiaErvina RetnaningtyasBelum ada peringkat
- (Template) Proposal PKM-K CHEESEADokumen18 halaman(Template) Proposal PKM-K CHEESEAniswatuhasanahBelum ada peringkat
- Chapter6-Electrochemistry (Part 2)Dokumen27 halamanChapter6-Electrochemistry (Part 2)Uswatun KhasanahBelum ada peringkat
- Electrolysis of Silver SulphateDokumen5 halamanElectrolysis of Silver SulphateJackson_de_Roz_6005100% (1)
- Electrolysis Aqueous SolutionDokumen40 halamanElectrolysis Aqueous SolutionVictor OkosunBelum ada peringkat
- Electrolysis 090618180154 Phpapp01Dokumen20 halamanElectrolysis 090618180154 Phpapp01jiivi87Belum ada peringkat
- Form 4A Notes: Cations Migrate To The Cathode and Are Reduced by Gaining of ElectronsDokumen9 halamanForm 4A Notes: Cations Migrate To The Cathode and Are Reduced by Gaining of ElectronsGono TakaduuBelum ada peringkat
- Electrolysis: Term MeaningDokumen22 halamanElectrolysis: Term MeaningYeen ChengBelum ada peringkat
- ELECTROLYSISDokumen12 halamanELECTROLYSISKatlo KgosiyangBelum ada peringkat
- Chapter 6 Electrochemistry SPMDokumen62 halamanChapter 6 Electrochemistry SPMhanifzainol100% (1)
- ELECTROCHEMISTRYDokumen73 halamanELECTROCHEMISTRY2020 CubingBelum ada peringkat
- Chemistry - Notes Icse 10Dokumen25 halamanChemistry - Notes Icse 10Suneet MohanBelum ada peringkat
- New Electrolysis 1Dokumen18 halamanNew Electrolysis 1Rethabile LekgethoBelum ada peringkat
- Esis 2Dokumen35 halamanEsis 2Amir Abd KadirBelum ada peringkat
- 6.3 (A) Electrolysis of An Aqueous SolutionDokumen18 halaman6.3 (A) Electrolysis of An Aqueous SolutionFid AwanBelum ada peringkat
- 10 Position of Carbon in Reactivity SeriesDokumen4 halaman10 Position of Carbon in Reactivity SeriesEverest Lim Yong KeanBelum ada peringkat
- Chemistry Homework Material (Electrolysis) by Adaugo Olaedo UbahDokumen3 halamanChemistry Homework Material (Electrolysis) by Adaugo Olaedo UbahAdaugo UbahBelum ada peringkat
- Jadualberkala BermaklumatDokumen9 halamanJadualberkala BermaklumatZjuhaida IdaBelum ada peringkat
- ElectrolysisDokumen24 halamanElectrolysisstudent purposesBelum ada peringkat
- Notes Updates SaltsDokumen29 halamanNotes Updates SaltsJaybeeAngelBelum ada peringkat
- Electrolysi S Electrolyte Electrode DischargeDokumen28 halamanElectrolysi S Electrolyte Electrode Dischargeanwar9602020100% (1)
- Electrolysis of Aqeous Solutions (Copper Sulfate) PosterDokumen1 halamanElectrolysis of Aqeous Solutions (Copper Sulfate) Posternnilam1308Belum ada peringkat
- SS2 Note ElectrolysisDokumen7 halamanSS2 Note ElectrolysisIbukun OlaitanBelum ada peringkat
- Electrolysis of An Aqueous SolutionDokumen18 halamanElectrolysis of An Aqueous SolutionLieza IejaBelum ada peringkat
- Two More Uses of Electrolysis: When Electrodes Are Not InertDokumen2 halamanTwo More Uses of Electrolysis: When Electrodes Are Not InertShahid Ur RehmanBelum ada peringkat
- Gcse : H O (L) H (Aq) + OH (Aq)Dokumen9 halamanGcse : H O (L) H (Aq) + OH (Aq)Takudzwa ChademanaBelum ada peringkat
- Chapter 6b Electrolysis of Aqueous SolutionDokumen16 halamanChapter 6b Electrolysis of Aqueous SolutionKavitha ThayagarajanBelum ada peringkat
- Aluminium: Extraction and UsesDokumen12 halamanAluminium: Extraction and UsesIan SembadaBelum ada peringkat
- Group IbDokumen21 halamanGroup IbFadilahdilaa RizqiBelum ada peringkat
- ElectrochemistryDokumen16 halamanElectrochemistryitsshaunboteBelum ada peringkat
- 4.2 ElectrolysisDokumen5 halaman4.2 Electrolysis211273wBelum ada peringkat
- Electrolysis in SolutionsDokumen13 halamanElectrolysis in SolutionsTeandraBelum ada peringkat
- ELECTROLYSIS Notes Condensed 2Dokumen3 halamanELECTROLYSIS Notes Condensed 2Diya ShahBelum ada peringkat
- Preferential Discharge TheoryDokumen4 halamanPreferential Discharge TheoryRitesh Mittra33% (3)
- Advantages of CorrosionDokumen3 halamanAdvantages of CorrosionYudhisthiraBelum ada peringkat
- 2.1.10 Electrolysis IDokumen15 halaman2.1.10 Electrolysis Idniel9430Belum ada peringkat
- ElectrolysisDokumen19 halamanElectrolysisNurhi ShahBelum ada peringkat
- Notes Updates SaltsDokumen33 halamanNotes Updates SaltsFebian HenryBelum ada peringkat
- 4th Form Qualitative Analysis Sheet Summary SheetDokumen3 halaman4th Form Qualitative Analysis Sheet Summary SheetFrank MassiahBelum ada peringkat
- Electrolysis Notes For SdaDokumen13 halamanElectrolysis Notes For Sdatmoatshe96Belum ada peringkat
- Chapter 4Dokumen11 halamanChapter 4J.K HomerBelum ada peringkat
- Electrolysis of AueqousDokumen8 halamanElectrolysis of AueqousSelinawong Win Jing SelinawongBelum ada peringkat
- Chem 7Dokumen4 halamanChem 7kel17Belum ada peringkat
- Electrolysis of Solutions: Earning UtcomesDokumen13 halamanElectrolysis of Solutions: Earning UtcomesNicaliaBelum ada peringkat
- Summary - ElectrolysisDokumen7 halamanSummary - ElectrolysisKeertana SNBelum ada peringkat
- Electrolysis. Olevel ChemistryDokumen53 halamanElectrolysis. Olevel ChemistrySaraYasinBelum ada peringkat
- Chemistry Form 4 Experiment Chapter 6 (6.3) - ElectrolysisDokumen15 halamanChemistry Form 4 Experiment Chapter 6 (6.3) - ElectrolysisPearl Hasleigh100% (1)
- 78 128Dokumen51 halaman78 128Anonymous qKeDFDBelum ada peringkat
- Electrochemsitry NotesDokumen9 halamanElectrochemsitry NotesAhmad Shafiq ZiaBelum ada peringkat
- Acidity of Metal Ions in Aqueous SolutionDokumen14 halamanAcidity of Metal Ions in Aqueous SolutionKSmklBelum ada peringkat
- Ettel 1981Dokumen54 halamanEttel 1981SaladinBelum ada peringkat
- ElectrolysisDokumen20 halamanElectrolysisGowriram RamBelum ada peringkat
- ELECTROLYSIS o Level 2Dokumen33 halamanELECTROLYSIS o Level 2Tom TommmaBelum ada peringkat
- ElectrochemistryDokumen38 halamanElectrochemistryShannon SmithBelum ada peringkat
- Chemistry Lesson23 (Electrochemistry3)Dokumen23 halamanChemistry Lesson23 (Electrochemistry3)Siang DanielBelum ada peringkat
- IGCSE Chemistry - UNIT 8 - ELECTROLYSISDokumen5 halamanIGCSE Chemistry - UNIT 8 - ELECTROLYSISRaffaella LaxaldeBelum ada peringkat
- Electrolysis of Aqueous SolutionsDokumen5 halamanElectrolysis of Aqueous SolutionsnoraBelum ada peringkat
- 3 Cell RCPT ApparatusDokumen13 halaman3 Cell RCPT ApparatusBhupesh MulikBelum ada peringkat
- Calculator Z ScoreDokumen3 halamanCalculator Z ScoreBhupesh MulikBelum ada peringkat
- 3 Cell RCPT ApparatusDokumen13 halaman3 Cell RCPT ApparatusBhupesh MulikBelum ada peringkat
- ACI Design & Aggregates Worksheet & Mix LEGAZPIDokumen6 halamanACI Design & Aggregates Worksheet & Mix LEGAZPIBhupesh MulikBelum ada peringkat
- DNSKNKDokumen1 halamanDNSKNKBhupesh MulikBelum ada peringkat
- Sieve Analysis of Fine and Coarse Aggregates: Test Procedure ForDokumen10 halamanSieve Analysis of Fine and Coarse Aggregates: Test Procedure ForPaul MaduagwuBelum ada peringkat
- NSNCBKBCBDokumen1 halamanNSNCBKBCBBhupesh MulikBelum ada peringkat
- NZCNK ZNCKNCKNKDokumen1 halamanNZCNK ZNCKNCKNKBhupesh MulikBelum ada peringkat
- EN1348Dokumen17 halamanEN1348GiriPrasath67% (3)
- MMDNNDDokumen1 halamanMMDNNDBhupesh MulikBelum ada peringkat
- MMDNNDDokumen1 halamanMMDNNDBhupesh MulikBelum ada peringkat
- NB NBBDokumen1 halamanNB NBBBhupesh MulikBelum ada peringkat
- Effect of Fine Materials On The Compressive StrengthDokumen20 halamanEffect of Fine Materials On The Compressive StrengthHashem EL-MaRimeyBelum ada peringkat
- Xaannnskc MNKM, SNCKS, C NSNC Sncs CLSNCLSN Cs C S, M Cks KC SLDokumen1 halamanXaannnskc MNKM, SNCKS, C NSNC Sncs CLSNCLSN Cs C S, M Cks KC SLBhupesh MulikBelum ada peringkat
- Molarity of HCLDokumen1 halamanMolarity of HCLBhupesh MulikBelum ada peringkat
- Blaine Vs ResiduesDokumen5 halamanBlaine Vs ResiduesBhupesh Mulik67% (3)
- Effect of Fine Materials On The Compressive StrengthDokumen20 halamanEffect of Fine Materials On The Compressive StrengthHashem EL-MaRimeyBelum ada peringkat
- Mix Design For Plaster PDFDokumen4 halamanMix Design For Plaster PDFBhupesh MulikBelum ada peringkat
- Excel Skills - Loan Amortization TemplateDokumen9 halamanExcel Skills - Loan Amortization Templatezzduble1Belum ada peringkat
- Molarity of HCLDokumen1 halamanMolarity of HCLBhupesh MulikBelum ada peringkat
- JKL GHJG Gemon Ginger Lemon CamphorDokumen2 halamanJKL GHJG Gemon Ginger Lemon CamphorBhupesh MulikBelum ada peringkat
- 114 QDokumen6 halaman114 QBhupesh MulikBelum ada peringkat
- Blaine Vs ResiduesDokumen5 halamanBlaine Vs ResiduesBhupesh Mulik67% (3)
- 96 Kuli MarathaDokumen37 halaman96 Kuli MarathaBhupesh MulikBelum ada peringkat
- Flexure Stainless ExampleDokumen34 halamanFlexure Stainless Examplejh50000Belum ada peringkat
- JFGKFGNJKJGFJDK NNKNKSNFSKSKFSNSKN Nnsnsnks NNSFNSKNSKFNDokumen1 halamanJFGKFGNJKJGFJDK NNKNKSNFSKSKFSNSKN Nnsnsnks NNSFNSKNSKFNBhupesh MulikBelum ada peringkat
- Software Inventory and AssessmentDokumen20 halamanSoftware Inventory and AssessmentBhupesh MulikBelum ada peringkat
- Nied 2016Dokumen10 halamanNied 2016Bhupesh MulikBelum ada peringkat
- College of Arts Science & CommerceDokumen2 halamanCollege of Arts Science & CommerceBhupesh MulikBelum ada peringkat
- Mix Design Using Rheology MeterDokumen5 halamanMix Design Using Rheology MeterBhupesh MulikBelum ada peringkat
- S ELITE Nina Authors Certain Ivey This Reproduce Western Material Management Gupta Names Do OntarioDokumen15 halamanS ELITE Nina Authors Certain Ivey This Reproduce Western Material Management Gupta Names Do Ontariocarlos menaBelum ada peringkat
- Heat Transfer ExampleDokumen4 halamanHeat Transfer Examplekero_the_heroBelum ada peringkat
- Management of Preterm LaborDokumen2 halamanManagement of Preterm LaborpolygoneBelum ada peringkat
- Method Statement (RC Slab)Dokumen3 halamanMethod Statement (RC Slab)group2sd131486% (7)
- AtelectasisDokumen37 halamanAtelectasisSandara ParkBelum ada peringkat
- Reading Practice 6Dokumen5 halamanReading Practice 6Âu DươngBelum ada peringkat
- Viscoline Annular UnitDokumen4 halamanViscoline Annular UnitjoquispeBelum ada peringkat
- D6228 - 10Dokumen8 halamanD6228 - 10POSSDBelum ada peringkat
- Mixed Topic Revision 4Dokumen18 halamanMixed Topic Revision 4YaakkwBelum ada peringkat
- 41403A - Guide - Rev - 12-20-17 - With Edits - 2-16-18Dokumen167 halaman41403A - Guide - Rev - 12-20-17 - With Edits - 2-16-18Ronald KahoraBelum ada peringkat
- REV Description Appr'D CHK'D Prep'D: Tolerances (Unless Otherwise Stated) - (In)Dokumen2 halamanREV Description Appr'D CHK'D Prep'D: Tolerances (Unless Otherwise Stated) - (In)Bacano CapoeiraBelum ada peringkat
- Pe 3 Syllabus - GymnasticsDokumen7 halamanPe 3 Syllabus - GymnasticsLOUISE DOROTHY PARAISO100% (1)
- Yogananda Scientific HealingDokumen47 halamanYogananda Scientific HealingSagar Pandya100% (4)
- Introduction and Vapour Compression CycleDokumen29 halamanIntroduction and Vapour Compression Cycleمحسن الراشدBelum ada peringkat
- Dungeon World ConversionDokumen5 halamanDungeon World ConversionJosephLouisNadeauBelum ada peringkat
- Electri RelifDokumen18 halamanElectri Relifsuleman247Belum ada peringkat
- Topic of Assignment: Health Wellness and Yoga AssignmentDokumen12 halamanTopic of Assignment: Health Wellness and Yoga AssignmentHarsh XBelum ada peringkat
- The Vapour Compression Cycle (Sample Problems)Dokumen3 halamanThe Vapour Compression Cycle (Sample Problems)allovid33% (3)
- Sop For Enlistment of Engineering ConsultantsDokumen1 halamanSop For Enlistment of Engineering Consultantssatheb319429Belum ada peringkat
- انظمة انذار الحريقDokumen78 halamanانظمة انذار الحريقAhmed AliBelum ada peringkat
- J130KDokumen6 halamanJ130KBelkisa ŠaćiriBelum ada peringkat
- SPA - MichaelDokumen1 halamanSPA - Michaelgilberthufana446877Belum ada peringkat
- Ventricular Septal DefectDokumen8 halamanVentricular Septal DefectWidelmark FarrelBelum ada peringkat
- Neopuff PDFDokumen4 halamanNeopuff PDFoechimBelum ada peringkat
- Chia (Salvia Hispanica L.) Oil Stability Study of The Effect of NaturDokumen7 halamanChia (Salvia Hispanica L.) Oil Stability Study of The Effect of NaturInta Nur IlmiBelum ada peringkat
- Dressmaking - Q1 TASK-SHEET v1 - Schalemar OmbionDokumen2 halamanDressmaking - Q1 TASK-SHEET v1 - Schalemar OmbionAlvaCatalinaBelum ada peringkat
- Chemistry Xi: Short Questions and 20% Long QuestionsDokumen3 halamanChemistry Xi: Short Questions and 20% Long QuestionsSyed Nabeel HassanBelum ada peringkat
- Uas MR1Dokumen2 halamanUas MR1IvanBelum ada peringkat
- Arsenal Strength Catalog 6.2-1Dokumen41 halamanArsenal Strength Catalog 6.2-1Mohammed NavedBelum ada peringkat
- Entitlement Cure SampleDokumen34 halamanEntitlement Cure SampleZondervan100% (1)