Sepsis: Menu of New Approaches Replaces One Therapy For All: Review
Diunggah oleh
Dita Putri WidyantoroJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Sepsis: Menu of New Approaches Replaces One Therapy For All: Review
Diunggah oleh
Dita Putri WidyantoroHak Cipta:
Format Tersedia
C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2 65
HE FIRST AGENT for treating sepsis,
recombinant human activated protein
C (rhAPC; drotrecogin alfa, Xigris) was
recently approved by the US Food and Drug
Administration. This paper reviews lessons
learned from recent trials, including work
with rhAPC, and the pathophysiology of
sepsis.
We are finally making progress in treating
sepsis, after a long string of disappointments.
Although improved understanding of the
pathophysiology of sepsis had given rise to
hopes that agents that block inflammation and
coagulation could reduce mortality, until
recently, clinical trials of these agents had dis-
appointing results. More recent trials are bet-
ter designed to determine an agents effects in
particular infections and populations.
Now, instead of a one therapy for all
approach, we are finding that therapy directed
against specific biochemical derangements must
be targeted to patients who actually have these
derangements. Management of sepsis may even-
tually involve a menu of treatments, depending
on the presence of inflammatory markers, com-
pensatory anti-inflammatory activity, the sever-
ity of disease, and other factors.
I SEPSI S DEFI NED
In 1992, a consensus panel of the American
College of Chest Physicians and the Society of
Critical Care Medicine convened to develop
definitions of critical illness for the purposes of
clinical trial design:
Systemic inflammatory response syn-
drome (SIRS) is the host response to critical
illness of either infectious or noninfectious ori-
gin. (Noninfectious causes include burns, trau-
ma, and pancreatitis.) SIRS can be readily
STEVEN P. LAROSA, M D
*
Associ at e St af f Physi ci an, Depart ment of Inf ect i ous
Di seases, The Cl evel and Cl i ni c
Sepsis: Menu of new approaches
replaces one therapy for all
REVI EW
I ABSTRACT
Ef f ect i ve t herapi es f or sepsi s are bei ng devi sed, af t er many
f ai l ures. However, i nst ead of a one t herapy f or al l
approach, management of sepsi s wi l l i nvol ve a menu of
t reat ment s, dependi ng on t he presence of i nf l ammat ory
markers, t he severi t y of di sease, and ot her f act ors. Thi s
art i cl e l ooks at promi si ng new t herapi es, i ncl udi ng
recombi nant human act i vat ed prot ei n C (rhAPC; drot recogi n
al f a, Xi gri s), recent l y approved by t he US Food and Drug
Admi ni st rat i on.
I KEY POI N TS
In a recent cl i ni cal t ri al , pat i ent s who recei ved rhAPC had a
28-day mort al i t y rat e of 24.7%, compared wi t h 30.8% i n
t he pl acebo group, a rel at i ve ri sk reduct i on of nearl y 20%.
Of i mport ance, at basel i ne, nearl y al l t he pat i ent s i n t he
st udy had bi ochemi cal evi dence of coagul opat hy and
i nf l ammat i on, 75% had t wo or more organ f ai l ures, and
70% were i n sept i c shock.
Fut ure t herapi es f or sepsi s coul d i ncl ude cort i cost eroi d
repl acement t herapy, ent eral f eeds cont ai ni ng argi ni ne and
omega-3 f at t y aci ds, i nt ravenous i nf usi ons of Ri ngers et hyl
pyruvat e sol ut i on, mechani cal vent i l at i on wi t h l ow t i dal
vol umes, hemoperf usi on col umns t hat bi nd bact eri al t oxi ns,
and novel ant i endot oxi n and ant i -i nf l ammat ory agent s.
Sepsi s i s caused by cascades of i nf l ammat i on and
coagul at i on i n whi ch t umor necrosi s f act or al pha and
i nt erl euki n-1 pl ay a cent ral rol e.
T
*
The aut hor has i ndi cat ed t hat he ow ns st ock i n El i Li l l y and Company.
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
66 C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2
diagnosed at the bedside by the presence of at
least two of the following four signs:
Hyperthermia or hypothermia
Tachycardia
Tachypnea
Leukocytosis or leukopenia.
Sepsis is SIRS due to a presumed or
known infection.
Severe sepsisis sepsis with an acute asso-
ciated organ failure.
Septic shock, a subset of severe sepsis, is
defined as persistently low mean arterial
blood pressure despite adequate fluid resusci-
tation.
Refractory septic shock is persistently
low mean arterial blood pressure despite vaso-
pressor therapy and adequate fluid resuscita-
tion.
1
I THE LEADI NG CAUSE OF DEATH
I N I NTENSI VE CARE UNI TS
Sepsis is the leading cause of death in non-
coronary intensive care units and the 13th
leading cause of death in the United States
overall.
2
More than 700,000 cases of severe
sepsis are estimated to occur each year in the
United States.
3
The incidence of sepsis is expected to
increase over the next decade owing to the
following factors: an aging population, an
increasing immunosuppressed population,
increased use of invasive catheters and pros-
thetic materials, and the growing problem of
antimicrobial resistance.
The mortality rate in severe sepsis remains
between 28% and 50%, depending on the
country and institution.
4
I PATHOPHYSI OLOGY OF SEVERE SEPSI S
The inf lammat ion cascade
Severe sepsis can start with an infection in any
part of the body, including the lungs,
abdomen, skin, soft tissue, urinary tract, or
blood (eg, in meningococcemia). The patho-
gens are usually bacteria, although fungi,
viruses, and parasites can also cause sepsis.
Trouble starts when components of the
outer membrane of bacteria bind to the CD14
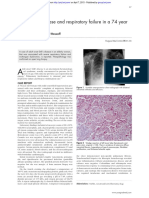
receptor on the surface of monocytes (FIGURE 1).
These outer-membrane components include
the lipid A moiety of lipopolysaccharide
(endotoxin) in gram-negative organisms, and
lipoteichoic acid and peptidoglycan in gram-
positive organisms.
Binding of the CD14 receptor sends a sig-
nal to the interior of the monocyte via the
recently described Toll-like receptors, telling
it to produce the inflammatory cytokines
tumor necrosis factor alpha (TNF-alpha) and
interleukin-1 (IL-1).
5,6
These cytokines have a direct toxic effect
on tissues. They also activate phospholipase
A
2
, leading to increased concentrations of
platelet-activating factor. In addition, they
promote nitric oxide synthase activity, tissue
infiltration by neutrophils, and neutrophil
activity.
7,8
Link bet ween inf lammat ion
and coagulat ion
Interleukin-1 and TNF-alpha also promote
coagulation in several ways. They directly
stimulate the endothelium and monocytes to
express tissue factor, the first step in the
extrinsic pathway of coagulation. Tissue factor
leads to production of thrombin, which itself
is inflammatory. Thrombin leads to fibrin clots
in the microvasculature, a sequelae most easi-
ly recognized in meningococcal septic shock
with purpura fulminans.
Further, these cytokines impair fibrinolysis
by promoting production of plasminogen acti-
vator inhibitor-1, a potent inhibitor of fibri-
nolysis.
9
Inflammatory cytokines also disrupt the
bodys natural modulators of coagulation and
inflammation, activated protein C and
antithrombin.
Protein C circulates as an inactive zymo-
gen, a form of proenzyme. In the presence of
thrombin and the endothelial surface-bound
protein thrombomodulin it is converted to acti-
vated protein C. Recent studies showed that
inflammatory cytokines can shear thrombo-
modulin from the endothelial surface and even
lead to down-regulation of this molecule, thus
preventing activation of protein C.
10
Activated protein C, with its cofactor pro-
tein S, turns off thrombin production by
cleaving factors Va and VIIIa.
11
Activated
protein C also restores fibrinolytic potential
by inhibiting plasminogen activator inhibitor-
SEPSI S LAROSA
There are
> 700,000 cases
of severe sepsis
each year in t he
US, and 1/3 of
pat ient s die
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2 67
I Pathophysiology of severe sepsis
CCF
2002
FIGURE 1
1.
12
In vitro studies reveal that activated pro-
tein C has direct anti-inflammatory proper-
ties, including inhibiting the production of
inflammatory cytokines by lipopolysaccha-
ride-stimulated monocytes, inhibiting leuko-
cyte adhesion and rolling, and inhibiting neu-
trophil accumulation.
1315
Antithrombin, the second natural
endothelial regulator affected during sepsis,
inhibits thrombin production at multiple steps
in the coagulation cascade; it also binds to and
inhibits thrombin directly.
16
When bound to
the endothelial cell surface, antithrombin
leads to production of the anti-inflammatory
molecule prostacyclin.
17
Evidence exists that
neutrophil elastase cleaves glycosaminogly-
cans off the surface of the endothelial lining,
thus limiting the anti-inflammatory properties
of antithrombin.
18
Severe sepsis: The f inal common pat hway
The vicious cycle of inflammation and coagu-
lation leads to cardiovascular insufficiency,
multiple organ failure, and, in 28% to 50% of
patients, death.
4
Cardiovascular insufficiency
can occur both at the level of the myocardium
as a result of the myocardial depressant effects
of TNF-alpha, or at the level of the vessel due
to vasodilatation and capillary leak.
19
I DI SAPPOI NTI NG RESULTS
FROM CLI NI CAL TRI ALS
Armed with this knowledge of the cascade of
events in sepsis, researchers conducted at
least 30 phase III clinical trials of various
agents expected to interrupt the process,
mostly anti-inflammatory molecules (TABLE
1).
2035
Approximately 20,000 patients were
APC
APC
APC
Gram-negat i ve
baci l l i
Li popol ysacchari de (endot oxi n)
Li pot ei choi c aci d
I L-1
Tol l -l i ke recept or
Plasminogen
act ivat or inhibit or-1
Fi bri nol ysi s
Plat elet -act ivat ing f act or TNF-alpha
Leukot rienes, arachidonic acid
Thrombi n
Gram-posi t i ve
cocci
CD14
recept or
Monocyt e
Ant i t hrombi n
M icrovascular
coagulopat hy
Direct t issue injury
Ti ssue f act or
Sepsi s l eads t o organ f ai l ure and deat h vi a a
cascade of i nf l ammat i on and coagul at i on;
act i vat ed prot ei n C (APC) bl ocks t he cascade at
several poi nt s. A f ormul at i on of recombi nant
human APC was recent l y approved f or t reat i ng
sepsi s.
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
68 C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2
entered, most of whom had a clinical diagno-
sis of severe sepsis.
The results were disappointing. At best,
these agents reduced the 28-day all-cause
mortality rate only modestly, and the Food
and Drug Administration has approved none
of them for treating sepsis.
I LESSONS LEARNED
Why were these agents not as effective as
hoped? Some possible reasons involve the
agents tested, while others involve the popu-
lations in which they were tested.
M olecule-specif ic issues
Lack of biologic activity. Some of the
agents tested were simply not biologically
active. E5 and HA-1A, two endotoxin
binders, were found to bind and inhibit endo-
toxin in vitro, but only weakly.
36
In a study of
a monoclonal antibody to TNF, circulating
bioactive TNF-alpha levels did not decrease
in patients receiving the monoclonal anti-
body.
27
Pathogen-specific efficacy. Another rea-
son is that many of the anti-inflammatory
agents vary in efficacy in different types of
infections. Most of them had modest benefit
in patients with gram-negative infections but
little or no benefit in gram-positive infec-
tions.
37
In one trial,
30
patients with gram-pos-
itive infections who received the p75
TNF:IgG fusion protein actually had a higher
mortality rate than did patients who received
placebo.
30
Drug-drugand drug-disease interactions
may also explain the failure of these agents in
sepsis trials. In a recent trial of antithrombin
in sepsis,
38
no treatment effect was demon-
strated in the overall group, but a trend
towards efficacy was seen in patients who did
not receive heparin concomitantly.
This finding is explainable: heparin
negates the anti-inflammatory properties of
antithrombin by diverting the antithrombin
away from endothelial surface glycosamino-
glycans, thus preventing production of prosta-
cyclin.
16
In addition, during sepsis, neu-
trophils release elastase, a molecule that
cleaves antithrombin, especially when
heparin is given exogenously.
18
Populat ion-specif ic issues
Severe sepsis is not homogeneous. The
enrollment criterion used in nearly all of the
sepsis trials to date was the clinical diagnosis of
severe sepsis. However, one therapy may not
benefit all patients with this diagnosis. It is
important that the intended target for therapy
be present in the population being studied.
27
For example, in trials of antiendotoxin
agents and anticytokine therapy, the inflam-
matory cytokine targeted was absent in a large
percentage of patients enrolled.
39
This is
explainable, as the net state of inflammation
during a septic episode is a dynamic process.
Early in sepsis, inflammatory mediators pre-
dominate. Later, anti-inflammatory mediators
may predominate, or monocytes may lose their
responsiveness and lymphoid tissue may
undergo apoptosis. These later events may
result in immunoparalysis. In fact, during
this so-called compensatory anti-inflammatory
response stage of the disease, an anti-inflam-
matory therapy might be harmful.
40
Non-sepsis-related deaths. The studies
used the end point of death from any cause at
28 days. If a trial using this end point is to
determine whether an agent is effective in sep-
sis, sepsis must be the primary cause of death in
the study population. In many trials, however,
a substantial percentage of deaths were due to
severe underlying diseases and not to sepsis.
41
The statistical power of these studies to detect
an agents efficacy was thus diminished.
Therapy may be less beneficial in less-
severe disease. Many of the agents tested in
humans were first tested and found effective
in animals. Of interest, the animals who
received placebo in these trials had mortality
rates of 80% to 100%considerably higher
than in humans. In tests of the same agents in
which the animals that received placebo had
mortality rates comparable with those in
humans with severe sepsis (30%35%), these
agents showed little benefit or actually
showed harm.
42
Human clinical trials of anti-
TNF molecules and IL-1 receptor antagonists
have demonstrated this phenomenon as
well.
29,31
It would make sense that patients with
less-severe disease and less inflammation
would be closer to homeostasis and could be
potentially harmed by an intervention.
SEPSI S LAROSA
Early in sepsis,
inf lammat ory
mediat ors
predominat e
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2 69
Ne g a t i v e t r i a l s o f t he r a p y i n se p si s
AGENTS NO. OF RESULTS AND COM M ENTS
TRI ALS
High-dose cort icost eroids
20
9 Trend t owards w orse out come i n t reat ed group
Ant iendot oxin ant ibody
J5 ant i serum
21
1 Benef i t di d not correspond w i t h presence of J5 ant i bodi es
HA-1A
22,23
2 Benef i t i n gram-negat i ve i nf ect i ons w i t h shock i n f i rst t ri al ,
but not conf i rmed i n second t ri al
E5
4,2426
3 Benef i t i n pat i ent s w i t hout shock i n earl y t ri al s,
but not conf i rmed
Ant i-TNF monoclonal ant ibodies 10
*
Consi st ent 3.5%4.0% decrease i n mort al i t y (NS)
(muri ne and human)
27
TNF recept or:I gG const ruct s
P55
28,29
2 Benef i t i n pat i ent s w i t h earl y shock i n f i rst t ri al ,
but not conf i rmed i n second t ri al
P75
30
1 No benef i t overal l ; harm i n gram-posi t i ve i nf ect i ons
I L-1ra ant agonist s
31,32
2
Consi st ent 4% decease i n mort al i t y (NS)
Plat elet -act ivat ing 2 Benef i t i n pat i ent s w i t h gram-negat i ve i nf ect i ons i n f i rst t ri al ,
f act or ant agonist s
33,34
but not conf i rmed i n second t ri al
I buprof en
35
1 3% reduct i on i n mort al i t y (NS)
Improvement i n met abol i c paramet ers
*
Phase II and III
Phase III
T A B L E 1
I ADVANCES I N SEPSI S TRI ALS
Three recent trials, the MONARCS,
43
PROWESS,
44
and Ger-inf-05 trials,
45
demon-
strate advances in sepsis trial design and,
potentially, in the treatment of severe sepsis.
The M ONARCS t rial
The Monoclonal Anti-TNF: a Randomized
Controlled Sepsis (MONARCS) trial,
43
con-
ducted in 157 sites in North America, examined
the safety and efficacy of afelimomab, an anti-
TNF monoclonal antibody, in severe sepsis.
What is novel about this trial is the use of
a marker to target the therapy and select for a
population with a greater disease severity. It
used a rapid test to identify patients with IL-6
levels greater than 1,000 pg/mL. IL-6 is a
cytokine that is a marker of the net state of
inflammation and of disease severity.
At 28 days, the overall mortality rate in
the afelimomab group was 35.9%, compared
with 32.9% in the placebo group (P = .049),
a difference consistent with the treatment
effect observed in other anti-TNF trials.
The PROWESS t rial
The Protein C Worldwide Evaluation in
Severe Sepsis (PROWESS) trial
44
was a phase
III trial of recombinant human activated pro-
tein C (rhAPC). The trial was stopped when
the second interim analysis found overwhelm-
ing evidence of efficacy: the 28-day mortality
rate in the rhAPC group was 24.7%, com-
pared with 30.8% in the placebo group, a rel-
ative risk reduction of 19.4% and an absolute
risk reduction of 6.1% (P =.005).
The success of this agent in this trial
appears to be due not only to the biologic
effect of rhAPC but also the population in
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
which it was studied. All the patients in this
study had a clinical diagnosis of severe sepsis,
and nearly 100% had biochemical evidence of
coagulopathy and inflammation, the target
that rhAPC was intended to treat. The popu-
lation studied was also of appropriate disease
severity, with nearly 75% having two or more
organ failures at baseline, and 70% in septic
shock. In fact, the majority of the benefit seen
in the trial occurred in patients with greater
disease severity (with Acute Physiology and
Chronic Health Evaluation [APACHE] II
scores 25).
rhAPC seemed to have potent biologic
activity. D-Dimer levels were measured to
assess rhAPCs effect on thrombin generation,
and IL-6 levels were measured to assess its
effect on inflammation. The agent reduced
both of these markers significantly.
Of interest, the pathogen type did not
affect the efficacy of rhAPC: treated patients
had a lower mortality rate regardless of
whether they had pure gram-positive infec-
tions or pure gram-negative infections.
44
70 C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2
I I NDI CATI ONS
To be consi dered f or rhAPC t herapy t he pat i ent shoul d meet al l t hree of t he f ol l ow i ng cri t eri a
1 A known or suspect ed sit e of inf ect ion indicat ed by one or more of t he f ollowing:
Purul ent sput um or respi rat ory sampl e
A chest radi ograph w i t h new i nf i l t rat es not expl ai ned by a noni nf ect i ous process
Spi l l age of bow el cont ent s not ed duri ng an operat i on
Radi ographi c or physi cal exami nat i on evi dence of an i nf ect ed col l ect i on
Whi t e bl ood cel l s i n a normal l y st eri l e body f l ui d
Posi t i ve bl ood cul t ure
Evi dence of i nf ect ed mechani cal hardware by physi cal or radi ol ogi c exami nat i on
2 Evidence of syst emic inf lammat ory syndrome as indicat ed by at least t hree of t he f ollowing:
Hypot hermi a or f ever: core body t emperat ure 36C (96.8F) or 38C (100.4F)
Tachypnea: 20 breat hs/mi nut e or on mechani cal vent i l at i on f or an acut e process
Tachycardi a: 90 beat s per mi nut e (unl ess t he pat i ent has a pacemaker or i s on pharmacol ogi c t herapy
f or preexi st i ng t achycardi a)
Whi t e bl ood cel l count 12,000 or 4,000 cel l s/mm
3
or > 10% bands on di f f erent i al count
3 Sepsis-induced organ f ailure crit eria
(Note: Organ failure may not be explained by another non-sepsis illness, and cannot be greater than 48 hours in duration.)
Severe sepsi s (cri t eri a 1 and 2) w i t h an APACHE II score 25
AND
Cardi ovascul ar syst em dysf unct i on: pat i ent must ei t her have:
Evidence of sept ic shock, def i ned as mean art eri al pressure < 60 mm Hg or syst ol i c art eri al pressure < 90 mm Hg or t he
need f or vasopressors t o mai nt ai n t hese bl ood pressures i n t he f ace of adequat e i nt ravascul ar vol ume (cent ral venous
pressure > 8 mm Hg or pul monary art ery occl usi on pressure > 12 mm Hg), or af t er an adequat e f l ui d chal l enge (12 mL/kg)
has been gi ven
OR
Two or more of t he f ollowing organ f ailures:
Respi rat ory syst em dysf unct i on: PaO
2
/Fi O
2
rat i o < 200
Renal dysf unct i on: uri ne out put < 0.5 mL/kg/hour f or 1 hour i n t he f ace of adequat e i nt ravascul ar vol ume (cent ral venous
pressure > 8 mm Hg or pul monary art ery occl usi on pressure > 12 mm Hg, or af t er an adequat e f l ui d chal l enge (12 mL/kg)
has been gi ven
Hemat ol ogi c dysf unct i on: t hrombocyt openi a (< 80,000 pl at el et s/mm
3
or a 50% drop i n t he l ast 3 days), or
an i nt ernat i onal normal i zed rat i o (INR) > 1.2 not expl ai ned by l i ver di sease or concomi t ant warf ari n usage
Unexpl ai ned met abol i c aci dosi s: pH < 7.30 w i t h an el evat ed pl asma l act at e l evel t hat i s > 1.5 t i mes t he upper l i mi t
of normal
Cl e v e l a nd Cl i ni c g ui d e l i ne s f o r usi ng r hAPC ( d r o t r e co g i n a l f a , Xi g r i s)
T A B L E 2
SEPSI S LAROSA
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2 71
I CONTRAI NDI CATI ONS
Act i ve i nt ernal bl eedi ng
Surgery i n t he previ ous 12 hours
Thrombocyt openi a ( 20,000 pl at el et s/mm
3
)
Evi dence of post operat i ve bl eedi ng
Evi dence of gast roi nt est i nal bl eedi ng
Hi st ory of cent ral nervous syst em mass l esi on or evi dence of cerebral herni at i on
Hi st ory of st roke, art eri ovenous mal f ormat i on, cerebral aneurysm, i nt racrani al or i nt raspi nal surgery, or severe head t rauma
requi ri ng hospi t al i zat i on, w i t hi n l ast 3 mont hs
Ci rrhosi s (hi st ory of esophageal vari ces, port al hypert ensi on)
Evi dence of a bl eedi ng compl i cat i on f ol l ow i ng a percut aneous procedure, eg, decreasi ng hemogl obi n, f l ank hemat oma af t er
f emoral l i ne pl acement
Nephrost omy t ubes i n pl ace
Unexpl ai ned ment al st at us changes or neurol ogi c exami nat i on changes
Therapeut i c doses of hepari n ( 15,000 U/day) wi t hi n t he previ ous 8 hours, l ow-mol ecul ar-wei ght hepari n at a dose hi gher t han
prophyl axi s w i t hi n t he previ ous 12 hours
Syst emi c t hrombol yt i c t herapy wi t hi n t he past 3 days, aspi ri n > 650 mg/day wi t hi n t he past 3 days, gl ycoprot ei n IIb/IIIa ant ago-
ni st s w i t hi n t he past 7 days, warf ari n w i t hi n t he past 4 days, or cl opi dogrel or t i cl opi di ne w i t hi n t he past 4 days
End-st age di sease processes i n w hi ch t here i s not a commi t ment t o aggressi ve management or i n w hi ch t he pat i ent has an
advanced di rect i ve t o w i t hhol d l i f e-sust ai ni ng t reat ment
Enrol l ment i n a cl i ni cal t ri al i nvol vi ng a mol ecul e t hat t arget s t he coagul at i on cascade
Pul monary, spl eni c, or l i ver cont usi ons f ol l ow i ng t rauma
Presence of an epi dural cat het er
Pedi at ri c pat i ent s (< 18 years) unl ess t reat i ng purpura f ul mi nans
I DOSAGE AND GUI DELI NES FOR M ONI TORI NG rhAPC USE
Gi ve rhAPC 24 g/kg/hour as a cont i nuous i nf usi on f or a t ot al of 96 hours
If a surgery or percut aneous procedure needs t o be perf ormed, st op rhAPC 2 hours bef ore t he procedure
rhAPC can be rest art ed 1 hour af t er a percut aneous procedure and 12 hours af t er a surgi cal procedure i f adequat e hemost asi s
has been achi eved
If evi dence of gast roi nt est i nal bl eedi ng occurs, st op rhAPC i mmedi at el y; an endoscopy shoul d be perf ormed
Pat i ent s shoul d recei ve st ress ul cer prophyl axi s, such as sucral f at e, a hi st ami ne-2 ant agoni st , or a prot on-pump i nhi bi t or
If a pat i ent requi res f ul l -dose t herapeut i c hepari n f or t he t reat ment of a t hrombot i c event , t he rhAPC i nf usi on shoul d be st opped
If a pat i ent requi res renal repl acement t herapy, at t empt s shoul d be made t o run t he di al ysi s wi t h rhAPC al one; i f cl ot t i ng occurs,
t he l ow est dose of hepari n necessary t o mai nt ai n t he pat ency of t he di al ysi s f i l t er shoul d be used
TABLE 2 contains guidelines for the use of
rhAPC.
The Ger-inf -05 st udy
The Ger-inf-05 study
45
evaluated the effect
of replacement doses of corticosteroids
(hydrocortisone 50 mg intravenously every 6
hours plus fludrocortisone 50 g by mouth
daily for 7 days) in patients with septic shock
that was refractory to fluids and vasopressors.
The specific population of interest in this
study was patients who did not respond to
the adrenocorticotropin (ACTH) stimula-
tion test, a group in which it would be scien-
tifically reasonable to consider adrenal
replacement.
In this small study (N =299), adrenal
replacement therapy was associated with a sta-
tistically significant reduction in mortality in
the 76% of patients who were adrenal hypore-
sponders (relative risk =0.670, P =.023).
This study is another example of a thera-
py being given in an appropriate target popu-
lation.
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
72 C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2
I FUTURE DI RECTI ONS
The success of rhAPC in severe sepsis and
steroid replacement therapy in refractory sep-
tic shock with adrenal hyporesponsiveness
represents not the end of severe sepsis research
and therapy but the beginning. On the basis of
ongoing research, one can envision multi-tar-
geted treatment for severe sepsis akin to what
is done in the treatment of cancer and HIV.
Such treatment might include, depending on
the clinical setting, many of the agents cur-
rently being studied.
Lessons from past sepsis trials should allow
the design of clinical trials that demonstrate
the true efficacy of new agents.
Pot ent ial f ut ure t reat ment s
f or severe sepsis
Immunonutrition. Enteral feeds contain-
ing arginine and omega-3 fatty acids have
been shown to decrease hospital days, ventila-
tor days, and infectious complications in criti-
cally ill patients.
46
Prescription intravenous fluids. Ringers
ethyl pyruvate solution has been shown to be
a scavenger of reactive oxygen species that
can lead to oxidant-mediated cellular and
organ injury in a rat model of mucosal
injury.
47
Ventilator strategies. In a landmark
study,
48
patients with acute respiratory distress
syndrome who received mechanical ventila-
tion with lower tidal volumes (6 mL/kg) had a
lower mortality rate and more ventilator-free
days than patients who received traditional
tidal volumes (12 mL/kg).
Anti-endotoxin agents. Novel agents
include more potent inhibitors of endotoxin,
including highly conserved antibodies to
gram-negative outer membrane proteins, as
well as lipid A partial structure antagonists
that compete with endotoxin for the CD14
receptor and Toll-like receptor on the surface
of monocytes.
Endothelial modulators. rhAPC and tis-
sue factor pathway inhibitor could potentially
be used to decrease thrombin generation in
sepsis and its resulting inflammation.
Anti-inflammatory agents. Novel anti-
inflammatory agents include inhibitors of
toxic neutrophilic enzymes such as elastase,
and enzymes and proteins involved in
cytokine signaling.
Bindingcolumns. Several hemoperfusion
columns are being developed to bind bacterial
products involved in sepsis. One of these
columns binds the toxin responsible for
hemolytic uremic syndrome, another binds
the superantigens responsible for streptococcal
and staphylococcal toxic shock, and a
polymyxin column binds the endotoxin moi-
ety of gram-negative organisms.
Corticosteroid replacement therapy.
Patients with septic shock and adrenal
hyporesponsiveness could receive physiologic
replacement of a glucocorticoid and mineralo-
corticoid.
SEPSI S LAROSA
I REFERENCES
1. The ACCP/SCCM Consensus Conference Committee.
Definitions for sepsis and organ failure and guidelines for
the use of innovative therapies in sepsis. Chest 1992;
101:16441655.
2. Sands KE, Bates DW, Lanken PN, et al. Epidemiology of
sepsis syndrome in 8 academic medical centers. JAMA
1997; 278:234240.
3. Angus DC, Linde-Zwirble WT, Lidicker J, et al.
Epidemiology of severe sepsis in the United States: analy-
sis of incidence, outcome, and associated cost of care. Crit
Care Med 2001; 29:13031310.
4. Angus DC, Birmingham MC, Balk RA, et al. E5 murine
monoclonal antiendotoxin antibody in gram-negative
sepsis: a randomized controlled trial. JAMA 2000;
283:17231730.
5. Heumann D, Glauser MP. Pathogenesis of sepsis. Sci Am
Sci Med 1994; 1:2837.
6. Schwandner R, Dzirski R, Wesche H, et al. Peptidoglycan-
and lipoteichoic acid-induced cell activation is mediated
by Toll-like receptor 2. J Biol Chem 1999;
374:1740617409.
7. Endo S, Inada K, Nakae H, et al. Plasma levels of type II
phospholipase A2 and cytokines in patients with sep-
sis. Res Commun Mol Pathol Pharmacol 1995;
90:413421.
8. Dinarello CA. Biological basis for interleukin-1 in disease.
Blood 1996; 87:20952147.
9. Vervolet MG, Thijs LG, Hack CE. Derangements of
coagulation and fibrinolysis in critically ill patients
with sepsis and septic shock. Semin Thromb Hemost
1998; 24:3344.
10. Boehme MWJ, Deng Y, Raeth U, et al. Release of throm-
bomodulin from endothelial cells by concerted action of
TNF-alpha and neutrophils: in vivo and in vitro studies.
Immunology 1996; 87:134140.
11. Esmon CT, Xu J, GU JM, et al. Endothelial protein C
receptor. Thromb Haemost 1999; 82:251258.
12. Sakata Y, Loskutoff DJ, Gladson CL, Hekman CM, Griffin
JH. Mechanism of protein C dependent clot lysis: role of
plaminogen activator inhibitor. Blood 1986;
68:12181223.
13. Mizutani A, Okajima K, Noguchi T. Activated protein C
The success
of rhAPC
is not t he
end of sepsis
research, but
t he beginning
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
C LEV ELA N D C LIN IC JO U R N A L O F M ED IC IN E V O LU M E 6 9 N U M B ER 1 JA N U A RY 2 0 0 2 73
The f ut ure
t reat ment
f or sepsis
may resemble
mult i-drug
t reat ment f or
HIV or cancer
reduces ischemia/reperfusion induced acute renal injury
by inhibiting leukocyte activation in rats [abstract].
Anesthesiology 1999; 91(suppl 3A):A266.
14. Murakami K, Okajima K, Uchiba M, et al. Activated pro-
tein C prevents LPS-induced pulmonary vascular injury by
inhibiting cytokine production. Am J Physiol 1997; 272:
L197L202.
15. Grinnell BW, Hermann RB, Yan SB. Human protein C
inhibits selectin-mediated cell adhesion: role of unique
fucosylated oligosaccharide. Glycobiology 1994;
4:221225.
16. Rosenberg RD. Biochemistry of heparin antithrombin
interactions, and the physiologic role of this natural anti-
coagulant mechanism. Am J Med 1989; 87(suppl
3B):2S9S.
17. Uchiba M, Okajima K. Antithrombin III (AT III) prevents
LPS-induced pulmonary vascular injury: novel biological
activity of ATIII. Semin Thromb Hemost 1997;
23:583590.
18. Jordan RE, Nelson RM, Kilpatrick J, Newgren JO, Esmon
PC, Fournel MA. Inactivation of human antithrombin by
neutrophil elastase: kinetics of the heparin-dependent
reaction. J Biol Chem 1989; 264:1049310500.
19. Parillo J, Parker M, Natanson C, et al. Septic shock in
humans: advances in the understanding of pathogenesis,
cardiovascular dysfunction, and therapy. Ann Intern Med
1990; 113:227242.
20. Cronin L, Cook DJ, Carlet J, et al. Corticosteroid trestment
for sepsis: a critical appraisal and meta-analysis of the lit-
erature. Crit Care Med 1995; 23:14301439.
21. Ziegler EJ, McCutchan A, Fierer J. Treatment of gram-neg-
ative bacteremia and shock with human antiserum to a
mutant Escherichia coli. N Engl J Med 1982;
307:12251230.
22. Ziegler EJ, Fisher CJ Jr, Sprung CL, et al. Treatment of
gram-negative bacteremia and septic shock with HA-1A
human monoclonal antibody against endotoxin. N Engl J
Med 1991; 324:429436.
23. McCloskey RV, Straube RC, Sanders C, Smith SM, Smith
CR, and the CHESS Trial Study Group. Treatment of septic
shock with human monoclonal antibody HA-1A. Ann
Intern Med 1994; 121:15.
24. Greenman RL, Schein RMH, Martin MA, et al. A con-
trolled clinical trial of E5 murine monoclonal IgM anti -
body to endotoxin in the treatment of gram-negative
sepsis. JAMA 1991; 266:10971102.
25. Greenberg RN, Wilson KA, Kunz AY, Wedel KI, Gorelick
KJ. Observations using antiendotoxin antibody (E5) as
adjuvant therapy in humans with suspected, serious,
gram-negative sepsis. Crit Care Med 1992; 20:730735.
26. Bone RC, Balk RA, Fein AM, et al. A second large con-
trolled clinical study of E5, a monoclonal antibody to
endotoxin: results of a prospective, multicenter, ran-
domized, controlled trial. Crit Care Med 1995;
23:9941006.
27. Marshall JC. Clinical trials of mediator-directed therapy in
sepsis: what have we learned? Intensive Care Med 2000;
26(suppl 1):S75S83.
28. Abraham E, Glauser MP, Butler T, et al. P55 tumor necrosis
factor receptor fusion protein in the treatment of
patients with severe sepsis and septic shock. JAMA 1997;
277:15311538.
29. Abraham E, Laterre PF, Garbino J, et al. Lenercept (p55
tumor necrosis factor fusion protein) in severe sepsis and
early septic shock: a randomized, double-blind, placebo-
controlled, multicenter phase III trial with 1342 patients.
Crit Care Med 2001; 29:503510.
30. Fisher CJ Jr, Agosti JM, Opal SM, et al. Treatment of sep-
tic shock with the tumor necrosis factor receptor: Fc
fusion protein. N Engl J Med 1996; 334:16971702.
31. Fisher CJ Jr, Dhainaut JF, Opal SM, et al. Recombinant
human interleukin-1 receptor antagonist in the treatment
of patients with sepsis syndrome. JAMA 1994;
271:18361843.
32. Opal SM, Fisher CJ Jr, Dhainaut JF, et al. Confirmatory
interleukin-1 receptor antagonist trial in severe sepsis: a
phase III, randomized, double-blind, placebo-controlled,
multicenter trial. Crit Care Med 1997; 25:11151124.
33. Dhainaut JF, Tenaillon A, Le Tulzo Y, et al. Platelet-activat-
ing factor receptor antagonist BN 52021 in the treatment
of severe sepsis: a randomized double-blind, placebo-con-
trolled, multicenter clinical trial. Crit Care Med 1994;
22:17201728.
34. Dhainaut JF, Tenaillon A, Hemmer M, et al. Confirmatory
platelet-activating factor receptor antagonist trial in
patients with severe gram-negative bacterial sepsis: a
phase III, randomized, double-blind, placebo-controlled,
multicenter trial. Crit Care Med 1998; 26:19631971.
35. Bernard GR, Wheeler AP, Russell JA, et al. The effects of
ibuprofen on the physiology and survival of patients with
sepsis. N Engl J Med 1997; 336:912918.
36. Warren HS, Amato SF, Fitting C, et al. Assessment of abili-
ty of murine and human anti-lipid A monoclonal antibod-
ies to bind and neutralize lipopolysaccharide. J Exp Med
1993; 177:8997.
37. Opal SM, Cohen J. Clinical gram-positive sepsis: Does it
fundamentally differ from gram-negative sepsis? Crit Care
Med 1999; 27:16081616.
38. Warren BL, Eid A, Singer P, et al. Caring for the critically ill
patient. High-dose antithrombin III in severe sepsis: a ran-
domized controlled trial. JAMA 2001; 286:18691878.
39. Wortel CH, von der Mohlen MA, van Deventer SJ, et al.
Effectiveness of a human monoclonal antibody (HA-1A) in
gram-negative sepsis: relationship to endotoxin and
cytokine levels. J Infect Dis 1992; 166:13671374.
40. Van der Poll T, van Deventer SJH. Cytokines and anticy-
tokines in the pathogenesis of sepsis. Infect Dis Clin North
Am 1999; 13:413426.
41. Sprung CL, Eidelman LA, Pizov R, et al. Influences of
alterations in forgoing life-sustaining treatment practices
on a clinical sepsis trial. Crit Care Med 1997; 25:383387.
42. Cui X, Parent C, Banks S, et al. Compared to its use alone,
TNF soluble receptor (TNFR: FC) with fluid therapy
reduces survival in septic rats [abstract]. Crit Care Med
2000; 28(suppl 12):A132.
43. Panacek E, Marshall J, Fischkoff S, et al. Neutralization of
TNF by monoclonal antibody improves survival and
reduces organ dysfunction in human sepsis [abstract].
Chest 2000; 118(suppl 4):88S.
44. Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safe-
ty of recombinant human activated protein C for severe
sepsis. N Engl J Med 2001; 344:699709.
45. Annane D. Effects of the combination of hydrocortisone
(HC)-fludro-cortisone (FC) on mortality in septic shock
[abstract]. Crit Care Med 2000; 28(suppl 12):A46.
46. Beale RJ, Bryg DJ, Bihari D. Immunonutrition in the criti-
cally ill: a systematic review of clinical outcome. Crit Care
Med 1999; 27:27992805.
47. Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM,
Fink MP. Ringers ethyl pyruvate ameliorates ischemia/per-
fusion-induced intestinal mucosal injury in rats. Crit Care
Med 2001; 29:15131518.
48. The Acute Respiratory Distress Network. Ventilation with
lower tidal volumes as compared with traditional tidal
volumes for acute lung injury and the acute respiratory
distress syndrome. N Engl J Med 2000; 342:13011308.
ADDRESS: Steven P. LaRosa, MD, Department of Infectious
Disease, S32, The Cleveland Clinic Foundation, 9500 Euclid
Avenue, Cleveland, OH 44195; e-mail larosas@ccf.org.
on June 16, 2014. For personal use only. All other uses require permission. www.ccjm.org Downloaded from
Anda mungkin juga menyukai
- Sepsis: Definition, Epidemiology, and Diagnosis: Clinical ReviewDokumen5 halamanSepsis: Definition, Epidemiology, and Diagnosis: Clinical ReviewhfathiardiBelum ada peringkat
- Sepsis and The Systemic Inflammatory Response SyndromeDokumen9 halamanSepsis and The Systemic Inflammatory Response SyndromeHandi Tri EffendiBelum ada peringkat
- Risk and Setting For Multiple Organ Failure in Medical PatientsDokumen2 halamanRisk and Setting For Multiple Organ Failure in Medical PatientsabdullahBelum ada peringkat
- Septic ShockDokumen12 halamanSeptic ShockAndi Tri Sutrisno100% (1)
- Evidence-Based Management of Severe Sepsis and Septic ShockDokumen8 halamanEvidence-Based Management of Severe Sepsis and Septic ShockFra1312Belum ada peringkat
- Sepsis - The American Journal of Medicine (2007)Dokumen11 halamanSepsis - The American Journal of Medicine (2007)Jmv VegaBelum ada peringkat
- Antiphospholipid Syndrome:A Challenging Hypercoagulable Statewithsystemic ManifestationsDokumen8 halamanAntiphospholipid Syndrome:A Challenging Hypercoagulable Statewithsystemic ManifestationsFernando Re TaBelum ada peringkat
- Manejo Choque Septico y Sepsis GraveDokumen17 halamanManejo Choque Septico y Sepsis GravewilliamsbarriosBelum ada peringkat
- jacobi2002Dokumen6 halamanjacobi2002Oswaldo MascareñasBelum ada peringkat
- SepsisDokumen36 halamanSepsisWan FaizuddinBelum ada peringkat
- 2016 Sepsis Guideline: Key Updates from 2001Dokumen68 halaman2016 Sepsis Guideline: Key Updates from 2001Falayna Ithu DheisyaBelum ada peringkat
- 1 24 13 MosierDokumen59 halaman1 24 13 MosierRiski DohartuaBelum ada peringkat
- SepsisDokumen2 halamanSepsisNannai02Belum ada peringkat
- Management of Severe Sepsis: Learning ObjectivesDokumen5 halamanManagement of Severe Sepsis: Learning ObjectivesVictoriano Valiente100% (1)
- Literature AbdoDokumen20 halamanLiterature AbdothestaffforpediatricptBelum ada peringkat
- Rheumatology 2010 Hepburn 2243 54Dokumen12 halamanRheumatology 2010 Hepburn 2243 54Desti Siliawati IIBelum ada peringkat
- Antiphospholipid SyndromeDokumen8 halamanAntiphospholipid SyndromeVijeyachandhar DorairajBelum ada peringkat
- Piis0002934310002937 PDFDokumen2 halamanPiis0002934310002937 PDFMuhammad TamlikhaBelum ada peringkat
- Editorial: Editorial Current Drug Targets, 2007, Vol. 8, No. 4 491Dokumen76 halamanEditorial: Editorial Current Drug Targets, 2007, Vol. 8, No. 4 491laraalvarenga5832Belum ada peringkat
- Pathophysiology of Sepsis American Journal of PathologyDokumen10 halamanPathophysiology of Sepsis American Journal of PathologyStella Gracia OctaricaBelum ada peringkat
- Lupus Research PaperDokumen5 halamanLupus Research Paperttdqgsbnd100% (3)
- Il-6, Mmp-3 Y Pronóstico en Pacientes Con Sepsis Previamente SanosDokumen8 halamanIl-6, Mmp-3 Y Pronóstico en Pacientes Con Sepsis Previamente SanosJohn Edison Coronado BalagueraBelum ada peringkat
- Sepsis Diagnosis and Monitoring: Fast Early ReliableDokumen24 halamanSepsis Diagnosis and Monitoring: Fast Early ReliableI Nyoman Gede Semarajana100% (1)
- Dis IcDokumen11 halamanDis IcyeandunBelum ada peringkat
- Autoimmune PancreatitisDokumen7 halamanAutoimmune PancreatitisWinstonBelum ada peringkat
- Assessment of Splenic Function: ReviewDokumen9 halamanAssessment of Splenic Function: Reviewjosias_aragao3527Belum ada peringkat
- Manejo de La Sepsis 2Dokumen5 halamanManejo de La Sepsis 2Rachmi Pratiwi Febrita PartiBelum ada peringkat
- Ruiz-Iraztorza Et Al (2010) - Antiphospholipid SyndromeDokumen13 halamanRuiz-Iraztorza Et Al (2010) - Antiphospholipid SyndromextraqrkyBelum ada peringkat
- Idiopathic Thrombocytopenic Purpura (ITP)Dokumen7 halamanIdiopathic Thrombocytopenic Purpura (ITP)Rizqka PertiwiBelum ada peringkat
- Essentials of Sepsis Management: Early Recognition and TreatmentDokumen11 halamanEssentials of Sepsis Management: Early Recognition and TreatmentcastillojessBelum ada peringkat
- Biomarcadores en InmunosupresionDokumen10 halamanBiomarcadores en InmunosupresionRicardo GarciaBelum ada peringkat
- Gnaps EmedicineDokumen13 halamanGnaps Emedicineharyanti lupitaBelum ada peringkat
- AAFP SepsisDokumen13 halamanAAFP SepsisANISA RACHMITA ARIANTI 2020Belum ada peringkat
- Coagulopatia y Sepsis EngDokumen10 halamanCoagulopatia y Sepsis Engadrian mendoza croesBelum ada peringkat
- El Glucocalix en La SepsisDokumen8 halamanEl Glucocalix en La SepsisAndrea GaytánBelum ada peringkat
- The Effectiveness of The Treatment of Idiopathic Thrombocytopenic Purpura With Modern DrugsDokumen6 halamanThe Effectiveness of The Treatment of Idiopathic Thrombocytopenic Purpura With Modern DrugsIJRASETPublicationsBelum ada peringkat
- Neutropenia Febril PediatricsDokumen15 halamanNeutropenia Febril Pediatricschaos05Belum ada peringkat
- AdultOI CryptococcosisDokumen18 halamanAdultOI CryptococcosisricardoatejassBelum ada peringkat
- Evaluation and Management of Suspected Sepsis and Septic Shock in Adults - UpToDateDokumen37 halamanEvaluation and Management of Suspected Sepsis and Septic Shock in Adults - UpToDatebarcanbiancaBelum ada peringkat
- Sepsis and Septic Shock StrategiesDokumen41 halamanSepsis and Septic Shock StrategiesBolivar IseaBelum ada peringkat
- The Role of Cytokine in SepsisDokumen16 halamanThe Role of Cytokine in Sepsisammar aboghalionBelum ada peringkat
- Spleen MCQDokumen22 halamanSpleen MCQShriyansh Chahar0% (1)
- Pathogenesis of The Disease Process Sepsis: A New Hypothesis ForDokumen11 halamanPathogenesis of The Disease Process Sepsis: A New Hypothesis ForLuis Alejandro Chanona EspinosaBelum ada peringkat
- Hematological Findings and Complications of COVID-19: AffiliationsDokumen32 halamanHematological Findings and Complications of COVID-19: Affiliationsnicky_kelly_3Belum ada peringkat
- Martin 2016Dokumen12 halamanMartin 2016rositha prabandariBelum ada peringkat
- Anti-Inflammatory Therapies For CardiovascularDokumen12 halamanAnti-Inflammatory Therapies For Cardiovasculariancaruso27Belum ada peringkat
- DIC Pregnancy 2009 PDFDokumen2 halamanDIC Pregnancy 2009 PDFGebbyBelum ada peringkat
- Lupus Nephritis ThesisDokumen6 halamanLupus Nephritis ThesisWriteMyPaperReviewsSouthBend100% (2)
- Staphy 2Dokumen5 halamanStaphy 2Alexandra MariaBelum ada peringkat
- MGX of SepticemiaDokumen30 halamanMGX of SepticemiaAChRiS--FiTnEsSBelum ada peringkat
- Moriarty2020 Article LipoproteinAAndItsPotentialAssDokumen8 halamanMoriarty2020 Article LipoproteinAAndItsPotentialAssdariusBelum ada peringkat
- Acute Lymphoblastic Leukemia ThesisDokumen5 halamanAcute Lymphoblastic Leukemia Thesisljctxlgld100% (2)
- Sepsis y Shock SepticoDokumen41 halamanSepsis y Shock SepticoSilvia GarciaBelum ada peringkat
- Adult Still's Disease and Respiratory Failure in A 74 Year Old WomanDokumen3 halamanAdult Still's Disease and Respiratory Failure in A 74 Year Old WomanEduardo Romero StéfaniBelum ada peringkat
- Sepsis y ShockDokumen10 halamanSepsis y ShockJose Alonso Alcoser ArcilaBelum ada peringkat
- Sepsis, Sirs and ModsDokumen4 halamanSepsis, Sirs and ModsMayra Alejandra Prada SerranoBelum ada peringkat
- Handbook of SepsisDari EverandHandbook of SepsisW. Joost WiersingaBelum ada peringkat
- Fast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseDari EverandFast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseBelum ada peringkat
- Stains and Staining Lab3 and 4Dokumen43 halamanStains and Staining Lab3 and 4Abdurraouf SaidBelum ada peringkat
- Rabies - MicrobiologyDokumen30 halamanRabies - MicrobiologyMohammed ShakeelBelum ada peringkat
- Childrens FBC Reference Ranges PDFDokumen1 halamanChildrens FBC Reference Ranges PDFdimitratendraBelum ada peringkat
- 1993 - Mad A Heterodimeric Partner For Max That Antagonizes Myc Transcriptional ActivityDokumen12 halaman1993 - Mad A Heterodimeric Partner For Max That Antagonizes Myc Transcriptional ActivityRaymond LaBoyBelum ada peringkat
- Chem InvestigatoryDokumen22 halamanChem InvestigatoryDevendra Yadav78% (40)
- Neurons and Glial Cells: Structure and FunctionsDokumen59 halamanNeurons and Glial Cells: Structure and FunctionsMelissa Aina Mohd YusofBelum ada peringkat
- Cell WallDokumen6 halamanCell WallBeatrice SposatoBelum ada peringkat
- Monoclonal ANTI-FLAG™ M2 From Mouse (F1804) - BulletinDokumen5 halamanMonoclonal ANTI-FLAG™ M2 From Mouse (F1804) - BulletinSigma-Aldrich100% (1)
- Blood Smear ExaminationDokumen65 halamanBlood Smear Examinationqlephon100% (2)
- Micro Danica FriasDokumen48 halamanMicro Danica Friasailene agustinBelum ada peringkat
- GB1 Q2 TosDokumen2 halamanGB1 Q2 TosRuby UriarteBelum ada peringkat
- Chemistry of Prostaglandins, Leukotrienes and ThromboxanesDokumen20 halamanChemistry of Prostaglandins, Leukotrienes and ThromboxanesAbhimanyu AwasthiBelum ada peringkat
- IB Biology Cell Respiration GuideDokumen4 halamanIB Biology Cell Respiration GuideMalak ABelum ada peringkat
- Modern Pharmacology and Toxicology Series Covers Latest Developments in Interferon ResearchDokumen343 halamanModern Pharmacology and Toxicology Series Covers Latest Developments in Interferon ResearchSergey LyakhovBelum ada peringkat
- Cell Part DefinitionsDokumen2 halamanCell Part Definitionsyemaya hannaBelum ada peringkat
- Mrs. Anamika Sahu GulbakeDokumen40 halamanMrs. Anamika Sahu GulbakeAnamika SahuBelum ada peringkat
- Affinity ChromatographyDokumen19 halamanAffinity ChromatographyReemBelum ada peringkat
- Cell Adhesion MoleculesDokumen14 halamanCell Adhesion MoleculesSecret Agent100% (1)
- Happ Week 2 TransesDokumen7 halamanHapp Week 2 TransesJohn TacordaBelum ada peringkat
- B Ca CL Cu Fe MG MN Mo Ni: Carbon, Hydrogen, Oxygen BoronDokumen4 halamanB Ca CL Cu Fe MG MN Mo Ni: Carbon, Hydrogen, Oxygen BoronWayne SalvadorBelum ada peringkat
- Chemistry of Lipid Nanoparticles For RNA DeliveryDokumen11 halamanChemistry of Lipid Nanoparticles For RNA DeliveryJohnBelum ada peringkat
- V3 - PLANETS of ORTHODONTICS - Volume III - Biomechanics and Tooth MovementDokumen89 halamanV3 - PLANETS of ORTHODONTICS - Volume III - Biomechanics and Tooth MovementPhanQuangHuyBelum ada peringkat
- Session No. 1.2. Cell Types and ModificationsDokumen48 halamanSession No. 1.2. Cell Types and ModificationsShekaina Faith Cuizon LozadaBelum ada peringkat
- Biology Form 4 KSSM: Chapter 6 CellDokumen19 halamanBiology Form 4 KSSM: Chapter 6 CellPrithika Shankar100% (1)
- Stem Cell FactorDokumen22 halamanStem Cell FactoractivnetBelum ada peringkat
- Histology Final ExamDokumen33 halamanHistology Final ExamAlok Kumar100% (3)
- JEM TCells Special IssueDokumen118 halamanJEM TCells Special IssueAbdurRahmanBelum ada peringkat
- Enzymes - Structure, Classification, and FunctionDokumen1 halamanEnzymes - Structure, Classification, and Functionamanuel melakuBelum ada peringkat
- 1-s2.0-S2666246922000027-Main Expanding The Landscape of E3 Ligases For Targeted Protein DegradationDokumen5 halaman1-s2.0-S2666246922000027-Main Expanding The Landscape of E3 Ligases For Targeted Protein DegradationTsung-Shing WangBelum ada peringkat
- Alex - S Protein Folding For SCIENCE EPQ StuffDokumen3 halamanAlex - S Protein Folding For SCIENCE EPQ StuffFunkymaleBelum ada peringkat