Discussion
Diunggah oleh
Bro SmileDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Discussion
Diunggah oleh
Bro SmileHak Cipta:
Format Tersedia
DISCUSSION
The photosynthetic pigments are responsible for absorbing and trapping light energy uses its radiant
energy to power up the process of photosynthesis. The main photosynthetic pigments are chlorophyll a
and b. There are accessory pigments like carotene and xanthophyll which transmit absorbed light energy
to chlorophyll a. Chlorophyll absorbs certain wavelengths of light within the visible light spectrum.Plant
appear green because chlorophyll reflect yellow and green wavelength of light,while red and blue
wavelength are absorbed and provide energy used for photosynthesis.
In order to identify the pigments that are present in leaves, they need to be separated and
characterized. Separation of the pigments can be done by paper chromatography. This separates
pigments of a spinach leaf .In order to separate the pigments in spinach leaves and carrot extract, grind
them separately in acetone medium. In a chromatography paper, draw a mark line at around 2 cm from
the tip of the paper as well as a dot in the middle of that line. With a capillary tube, place a spot of the
pigment on the pencil mark of spinach in middle paper and of carrot in the other paper. Let the
pigments dry. Now place each paper in separate arrangements of chromatography solvents and keep
each closed. Let the solvent front rise to around 3 - 6 cm and then remove the papers. Allow them to
dry. Once dried, measure the distances of the pigments moved and then calculate the Rf values.. The
separation is due to the solubility of the pigment in the chromatography solvent (acetone) and the
affinity of the pigment to the paper surface In paper chromatography, a paper strip has its tip dipped in
a non-polar solvent that contains the pigments to be separated. The water that is bound to the cellulose
fiber of the paper acts as the polar medium that attracts polar molecules. When the solvent moves
through the paper, more polar pigments are bound easily to the water in the cellulose of the paper and
stops moving.The pigments dissolve and move along with it. Less polar or non-polar pigments have
more affinity to the solvent rather than the paper. Thus they move farther from the pigment origin
compared to the distance travelled by the different pigments in the extract. The distance travel by
solvent and pigments are measured to calculate the Rf value (Retention Factor).
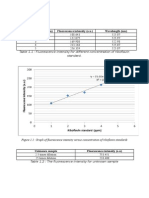
Rf = Distance moved by pigment/ Distance moved by solvent
To determine distance traveled by the pigment you must measure from the middle of the pigment band
to the markline and to determine the distance traveled by the solvent you must measure from the
solvent line to the mark line.A small RF value indicate a strong affinity of the compound for the
chromatography paper, move very slowly and crossed a small distance from the mark line.Those
pigments that moved the farthest are non-polar while those that stayed near are polar in
nature.Carotene is a non polar as well as the highest Rf value because it travel the furthest from the
mark line and move fastly.
In case of the spinach leaf extract, the chromatography paper showed a first band of chlorophyll b, then
xanthophyll and finally carotene at the top. This shows the polarity of the pigments. We can see that
chlorophylls are more polar than xanthophyll or carotene.For the carrot extract,only xanthophyll and
carotene appear on the chromatograhphy paper. Carotene is responsible for the orange colour
of carrots . Carotene is the most soluble and travels furthest, followed by Xanthophyll and finally
chlorophyll b.
In conclusion,we can separate different kind of pigment by using the chromatography paper and
calculate the Rf value by measuring the distance travel by solvent and pigment from the mark
line.Carotene travelled furthest than any other pigment because it is a non polar,most soluble and has
the largest value of Rf.
Anda mungkin juga menyukai
- Bíblia Sagrada Novo Testamento Inglês-Português Bilingue PDFDokumen272 halamanBíblia Sagrada Novo Testamento Inglês-Português Bilingue PDFAdson Richardson86% (22)
- Chem Lab Project Paper ChromatographyDokumen14 halamanChem Lab Project Paper ChromatographyFarah Kharuddin100% (1)
- Bio320 Lab 2Dokumen4 halamanBio320 Lab 2Mirza KarmilaBelum ada peringkat
- FULL REPORT Food Chemistry Food ColorantDokumen10 halamanFULL REPORT Food Chemistry Food ColorantWong Su ZuanBelum ada peringkat
- Thin Layer Chromatography of InkDokumen10 halamanThin Layer Chromatography of InkWanny HermioneBelum ada peringkat
- Proposal Furnihome SDN BHD FSGDokumen10 halamanProposal Furnihome SDN BHD FSGAmirul AdhamBelum ada peringkat
- Gas Chromatography Lab Report Experiment 05Dokumen5 halamanGas Chromatography Lab Report Experiment 05PDPPPMAT0621 Ruhilin Binti Nasser100% (1)
- Recrystallization Lab Report FinalDokumen6 halamanRecrystallization Lab Report Finalapi-255889385Belum ada peringkat
- Gas Chromatography (GC)Dokumen4 halamanGas Chromatography (GC)Mohd Izwan67% (3)
- Idaho State UniversityDokumen12 halamanIdaho State Universitysydneymarie710Belum ada peringkat
- Richard Meier - Beyond AutonomyDokumen33 halamanRichard Meier - Beyond AutonomyOtis Sloan BrittainBelum ada peringkat
- Separation and Identification of Plant Pigments by TLC MainDokumen5 halamanSeparation and Identification of Plant Pigments by TLC MainnaomiBelum ada peringkat
- MIC481 Lab ReportDokumen6 halamanMIC481 Lab ReportAbg Khairul Hannan Bin Abg AbdillahBelum ada peringkat
- Extraction of Caffeine From Tea LeavesDokumen8 halamanExtraction of Caffeine From Tea LeavesCesarah CabungcalBelum ada peringkat
- Intro CASE STUDY CHM361Dokumen1 halamanIntro CASE STUDY CHM361Syaiful Ashraf Mohd AshriBelum ada peringkat
- 4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFDokumen27 halaman4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFWanqing HeBelum ada peringkat
- CHM260 Basic Instrumental AnalysisDokumen6 halamanCHM260 Basic Instrumental Analysisfatin harris100% (1)
- Determinacion de La Vainilina Por HPLCDokumen4 halamanDeterminacion de La Vainilina Por HPLCAlfredo CruzBelum ada peringkat
- Faculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Dokumen9 halamanFaculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Husna Insyirah Bt SamadBelum ada peringkat
- Thin Layer ChromatographyDokumen2 halamanThin Layer ChromatographyOdessa Vidallon100% (3)
- Bio310 Lab 1Dokumen19 halamanBio310 Lab 1nursyahirahBelum ada peringkat
- Basic Analytical Chemistry CHM 256: Laboratory Case Study Report (Chromatography)Dokumen3 halamanBasic Analytical Chemistry CHM 256: Laboratory Case Study Report (Chromatography)ShafikaBelum ada peringkat
- S Determination of Caffeine in BeveragesDokumen5 halamanS Determination of Caffeine in BeveragesVioleta Grigoras100% (1)
- CHM 260 Lab Report Exp 4Dokumen7 halamanCHM 260 Lab Report Exp 4Warina 01Belum ada peringkat
- Spectro ReportDokumen30 halamanSpectro ReportIbrahim Muhamad100% (2)
- Chm580 Experiment 3Dokumen9 halamanChm580 Experiment 3ohhiBelum ada peringkat
- Phase Transfer 0Dokumen3 halamanPhase Transfer 0Jeannine CoxBelum ada peringkat
- Suggested Answer For Tutorial 3Dokumen3 halamanSuggested Answer For Tutorial 3Echizen100% (1)
- Isolation of Caffeine From Tea Leaves (Experiment)Dokumen6 halamanIsolation of Caffeine From Tea Leaves (Experiment)Laichi ArrietaBelum ada peringkat
- Experiment 3 - Carboxylic Acid and DerivativesDokumen3 halamanExperiment 3 - Carboxylic Acid and DerivativesFaris SyahmiBelum ada peringkat
- Chm260 Exp 1Dokumen6 halamanChm260 Exp 1Ilya ZafirahBelum ada peringkat
- VBVBFNDokumen8 halamanVBVBFNSuhailyShukriBelum ada peringkat
- BIO 462 Experiment 2Dokumen6 halamanBIO 462 Experiment 2Nurul Farhah RadzuwanBelum ada peringkat
- Results For Experiment 3Dokumen5 halamanResults For Experiment 3Syahriezan HaminBelum ada peringkat
- Qualitative Analysis of Organic CompoundsDokumen13 halamanQualitative Analysis of Organic CompoundsChristopher YepmoBelum ada peringkat
- Titration LabDokumen3 halamanTitration LabResonationBelum ada peringkat
- Basic Instrumental Analysis Experiment 2: Uv-Visible Determination of An Unknown Concentration of Kmno4 SolutionDokumen7 halamanBasic Instrumental Analysis Experiment 2: Uv-Visible Determination of An Unknown Concentration of Kmno4 SolutionSiti Maizatul AkmaBelum ada peringkat
- Past Year Chm260 Oct2016 PDFDokumen2 halamanPast Year Chm260 Oct2016 PDFaisyahBelum ada peringkat
- ConclusionDokumen1 halamanConclusionenieynaz0% (1)
- Lab Report Exp. 6 CHM457 Fund. Organic ChemistryDokumen8 halamanLab Report Exp. 6 CHM457 Fund. Organic ChemistryHusnul HakimBelum ada peringkat
- CHM510 - SpeDokumen7 halamanCHM510 - SpeafifiBelum ada peringkat
- Exp6 chm361 PDFDokumen11 halamanExp6 chm361 PDFShafiqahFazyaziqahBelum ada peringkat
- A Survey About Online and Distance Learning (Odl) On Uitm Tapah StudentsDokumen23 halamanA Survey About Online and Distance Learning (Odl) On Uitm Tapah StudentsAzdy HaiqalBelum ada peringkat
- Lab Report Chm256 Exp 4Dokumen6 halamanLab Report Chm256 Exp 4Miss KillerBelum ada peringkat
- Lab CHM 420 Exp 2Dokumen4 halamanLab CHM 420 Exp 2nana izzBelum ada peringkat
- Experiment 5 AASDokumen15 halamanExperiment 5 AASnn bbBelum ada peringkat
- Print Tuto 6Dokumen3 halamanPrint Tuto 6fatin harrisBelum ada peringkat
- Basic Analytical Chemistry Laboratory ReportDokumen5 halamanBasic Analytical Chemistry Laboratory Reportfarah0% (1)
- Chm421-Experiment 9 - Separation of Amino Acid Mixture by Paper ChromatographyDokumen8 halamanChm421-Experiment 9 - Separation of Amino Acid Mixture by Paper Chromatographynipale hiBelum ada peringkat
- Bachelor of Science (Hons) Applied ChemistryDokumen20 halamanBachelor of Science (Hons) Applied Chemistryfaiqah hasbullah100% (1)
- Gas Chromatography (GC) Optimization of Flow Rate and Column TemperatureDokumen6 halamanGas Chromatography (GC) Optimization of Flow Rate and Column TemperatureDang HumairahBelum ada peringkat
- Objective: The Objective of This Laboratory Is: - To Standardise of A Hydrochloric Acid (HCL) SolutionDokumen12 halamanObjective: The Objective of This Laboratory Is: - To Standardise of A Hydrochloric Acid (HCL) SolutionShaker HusienBelum ada peringkat
- PH Measurement and Buffer PreparationDokumen3 halamanPH Measurement and Buffer Preparationpnduban18Belum ada peringkat
- Thin Layer ChromatographyDokumen28 halamanThin Layer ChromatographyKeith Coral100% (1)
- Thin Layer ChromatographyDokumen16 halamanThin Layer ChromatographyDr. M. Prasad Naidu100% (1)
- Experiment 2 Sodium Borohydride Reduction of CyclohexanoneDokumen6 halamanExperiment 2 Sodium Borohydride Reduction of CyclohexanoneSarah HannisBelum ada peringkat
- chm510 Exp2Dokumen10 halamanchm510 Exp2May LeeBelum ada peringkat
- Atomic Adsorption SpectrosDokumen14 halamanAtomic Adsorption SpectrosRzi Danil Ishutin0% (1)
- Title Uv-Vis Determination of An Unknown Concentration Kmno SolutionDokumen4 halamanTitle Uv-Vis Determination of An Unknown Concentration Kmno SolutionMuhammad Amirul AfifiBelum ada peringkat
- Introductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionDari EverandIntroductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionBelum ada peringkat
- Chromotography Lab 2018Dokumen2 halamanChromotography Lab 2018AndrewBelum ada peringkat
- Photo 2 Co CHROMDokumen5 halamanPhoto 2 Co CHROMsmithsashay74Belum ada peringkat
- Lab Report 6 Plant PhysiologyDokumen7 halamanLab Report 6 Plant Physiologyapi-384857069Belum ada peringkat
- 8th Grade Summer Review Quiz (Grade 8) - Free Printable Tests and Worksheets PDFDokumen4 halaman8th Grade Summer Review Quiz (Grade 8) - Free Printable Tests and Worksheets PDFLerato TlakaneBelum ada peringkat
- Vipassana Meditation CenterDokumen37 halamanVipassana Meditation CenterOvais AnsariBelum ada peringkat
- Layer GuideDokumen5 halamanLayer GuidedemdiinBelum ada peringkat
- SextantDokumen26 halamanSextantChollo Anacio100% (1)
- James Study IntroDokumen23 halamanJames Study IntroMariaBelum ada peringkat
- Abbreviations: Abbreviation Used in Architectural Floor Plan and Building MaterialDokumen4 halamanAbbreviations: Abbreviation Used in Architectural Floor Plan and Building MaterialTooba KhalilBelum ada peringkat
- Manuscript Releases Vol 3Dokumen254 halamanManuscript Releases Vol 3chechi10Belum ada peringkat
- Terence McKenna New York Times - ObituaryDokumen2 halamanTerence McKenna New York Times - Obituarygalaxy5111100% (1)
- Photographic Papers in The 20th Century: Methodologies For Authentication, Understanding, and DatingDokumen11 halamanPhotographic Papers in The 20th Century: Methodologies For Authentication, Understanding, and DatingSlobodanStojkovicBelum ada peringkat
- Đề Tuyển Sinh Lớp 10 Môn Tiếng AnhDokumen6 halamanĐề Tuyển Sinh Lớp 10 Môn Tiếng Anhvo nguyen thanh tranhBelum ada peringkat
- Bible Lesson: Jehoshaphat's Dangerous FriendshipDokumen3 halamanBible Lesson: Jehoshaphat's Dangerous FriendshipJanice Alvarez Gacusan AbbasBelum ada peringkat
- MM Computer Journal 150 2006 AugustDokumen128 halamanMM Computer Journal 150 2006 AugustTechne PhobosBelum ada peringkat
- Spirit: Stallion of The CimmaronDokumen5 halamanSpirit: Stallion of The CimmaronShashwata DattaBelum ada peringkat
- Microsoft Picture It PublishingDokumen22 halamanMicrosoft Picture It PublishingDanielle Elish GocoBelum ada peringkat
- The Story of PingDokumen16 halamanThe Story of Pingwoolfman100% (14)
- The Master and The SlaveDokumen330 halamanThe Master and The SlaveRalph Ellectual100% (5)
- Old Yeller by Fred Gipson - Teacher Study GuideDokumen3 halamanOld Yeller by Fred Gipson - Teacher Study GuideHarperAcademic67% (3)
- WWII Airborne H2H RAREDokumen116 halamanWWII Airborne H2H RAREJay Mike100% (11)
- Linea de Tiempo ShakiraDokumen2 halamanLinea de Tiempo ShakiraAndres F Patiño SanchezBelum ada peringkat
- Cactus Explorer 18 - CompleteDokumen88 halamanCactus Explorer 18 - Completekhun sakBelum ada peringkat
- The Reality of Drama MinistryDokumen15 halamanThe Reality of Drama MinistryApostle UcheBelum ada peringkat
- Mo JaresDokumen3 halamanMo JaresGr MacopiaBelum ada peringkat
- Authentic Portraits GuideDokumen20 halamanAuthentic Portraits GuideLis100% (1)
- Khalil GibranDokumen2 halamanKhalil GibranLutfiArdiningTyasBelum ada peringkat
- Rizal 4 5 6Dokumen7 halamanRizal 4 5 6aljonBelum ada peringkat
- DN D5 eDokumen7 halamanDN D5 espinggeitBelum ada peringkat
- Group of CompaniesDokumen6 halamanGroup of Companiesraisul100% (1)