Corneal Wound Healing: A Review: Epithelial Wound Healing (See Table 1) - There Are Many
Diunggah oleh
Maizan Khairun NissaJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Corneal Wound Healing: A Review: Epithelial Wound Healing (See Table 1) - There Are Many
Diunggah oleh
Maizan Khairun NissaHak Cipta:
Format Tersedia
C H R I S S T E E L E B S c ( H o n s ) , M C O p t o m , D C L P
H E A D O F O P T O M E T RY, S U N D E R L A N D E Y E I N F I R M A RY
Corneal wound healing: a review
In comparison to cutaneous wound healing, corneal wound healing is more complex as a result of the greater differentiation
and organisation of the corneal sub-structures. Our understanding of corneal wound healing continues to improve as the
importance of the various physiological processes involving cellular and sub-cellular events, occurring under the influence of
extra-cellular matrix proteins and growth factors, is developed.
Much of the knowledge concerning corneal
wound healing is derived from experimental
work on animals such as rabbits and
monkeys, although there have been
occasional reported studies involving human
subjects. There are, however, certain
anatomical differences between these
species, in particular the absence of
Bowmans layer in rabbits and therefore
caution should be exercised when
extrapolating animal models to human
clinical findings.
Corneal wound healing, as in other parts
of the body, is the end result of a sequence of
events which are controlled by many factors.
Elsewhere in the body, wound healing
culminates in scar formation and
vascularisation whereas one of the most
crucial aspects of corneal wound healing is
how the healing processes aim to minimise
these end results, which would otherwise
have serious visual consequences.
EPITHELIAL WOUND HEALING
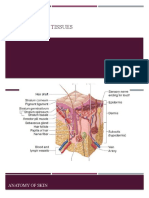
The human epithelium is arranged in five to
seven layers of cells and is approximately
50m thick (Figures 1a and b). It consists
of a single layer of mitotically active
Figure 1a. A 3D representation of corneal
anatomy (by permission from Martin Dunitz in
Excimer Lasers in Ophthalmology)
28
Part 1
columnar basal cells covered by
a layer of wing cells that are
usually one to three cell layers
thick. These are overlaid by two
to four outer flattened cell
layers of squamous cells. The
basal epithelial cells adhere to
their basement membrane Figure 1b. Diagrammatic representation of basil and wing
cells in the cornea
through a series of adhesion
complexes (Figure 2).
The processes involved in the
healing of corneal epithelial
wounds can be described in
three separate phases that, in
reality, are part of a continuous
process. Animal studies have
shown that these stages in
epithelial healing can be
described as the latent phase,
cell migration and adhesion
and, finally, cell proliferation
(see Table 1). There are many Figure 2. Attachment of human corneal epithelium to stroma
factors that will affect the
healing process including the size and depth
of the wound and the causative agent. Tear
quality and integrity is particularly
important, as any tear deficiency may
compromise the wound healing process1,2.
observed before the basal cells start
flattening and separating7,8. In studies
THE LATENT PHASE
conducted on rabbits, it has been
The initial, or latent phase describes the
demonstrated that hemidesmosomal
movement of existing basal epithelial cells
attachments between the basement
at the corneal wound margin that occurs
membrane and the basal cells completely
within the four to six hours following corneal
disappear to approximately 70m outward
debridement in rabbits and monkeys3.
(i.e. towards the corneal periphery) from the
Throughout this phase, polymorphonuclear
epithelial defect margin, and are
leukocytes, derived from tear fluid, are often
considerably reduced for a further
associated with the wound margins and deal
200m9,10 in epithelial defects greater than
with the removal of necrotic cells4,5,6. These
4-5mm in diameter. The final stage of the
cells may actually cause a microscopic
latent phase commences with the
enlargement of the epithelial defect with
production of cellular processes on the basal
removal of necrotic cells and debris.
edges of cells bordering the wound. These
However, polymorphonuclear cells usually
can be finger-like filopodia or wider shaped
disappear once a monolayer of epithelial
lamellipodia (Figures 3a and 3b) and their
cells covers the wound area. Rounding and
appearance marks the beginning of the
retraction of epithelial cells at the wound
second phase of cell movement 8,11,12.
edge, with loss of surface microvilli, can be
SEPTEMBER 24 1999 OPTOMETRY TODAY
C O R N E A L
W O U N D
H E A L I N G :
R E V I E W
Table 1
A summary of the major events in corneal epithelia wound healing
Stage 1:
The latent phase
Occurs within
four to six hours
Stage 2:
Cell migration
and adhesion
Lasts 24-36 hours
Stage 3:
Cell proliferation
Lasts 36 hours to
several months
Epithelial debridement
Intracellular formation of
actin filaments
(composed of fodrin,
vinculin and ankyrin)
Activation of limbal
stem cells
Polymorphonuclear
leukocyte invasion
Removal of necrotic
cells/debris
Retraction/ rounding of
epithelial cells at wound
edge
Reduction in
hemidesmosome
attachments 200m
outward around wound
margins
Commencement of
lamellipodial
and filopodial
extensions
formation of filopodial
and lamellipodial
extensions completed
Actin filaments
accumulate at leading
edges of lamellipodia
and filopodia giving
cytoskeletal support
Appearance of
fibronectin,
usually within one hour
temporary anchor
formation cyclical
process commences
as cells start to
advance
centripetal migration of
leading epithelial cells
across stromal surface.
energy derived from
glycogen metabolism
stem cells produce
transient amplifying
cells (TACs)
TACs give rise to post
mitotic cells (PMCs)
PMCs give rise to
terminally differentiated
cells (TDCs)
further establishment of
hemidesmosomes
possible epithelial
hyperplasia
replacement of corneal
nerve axon terminal
endings
Hypersensitivity of
corneal epithelial
nerves for several
months
formation of Y-X contact
lines
completion of epithelial
monolayer covering
wound area
fibronectin disappears
synthesis of new
hemidesmosomes and
other anchoring
complexes
appearance of type III
collagen
epithelial/ stromal
adhesion
restored from six to
eight weeks although
abnormalities can
persist for up to 15
months
Figure 3a. Diagrammatic representation of formation of
lamellipodia and filopodiastroma
CELL MIGRATION
The second phase involves the
migration of the epithelial cells
across the wound area before
mitosis
commences,
thus
producing linear cell healing of
the corneal wound. The
movement of these cells is
mediated by the intracellular
formation and contraction of actin
filaments13 that are composed of
proteins including fodrin, vinculin
and ankyrin14,15. The increased
synthesis of these and other
proteins, such as glycoproteins,
requires energy that is derived
from glycogen metabolism16. It is
these actin filaments which give
the cells the cytoskeletal support
required during this kinetic second
phase and these are concentrated
in the leading edges of the
migrating cells, in particular lining
the filopodia and lamellipodia13.
The importance of actin filament
synthesis to cell motion has been
demonstrated by the negative
effect of cytochalasin B which
inhibits actin filament formation17.
The migrating epithelial cells are
thought to move across the wound
surface by a cyclical process
involving the filopodia and
lamellipodia. These form temporary
anchors, or focal adhesions, whilst
intracellular
contractile
mechanisms draw the trailing cell
forward. These anchor points are
then cleaved, enabling the
filopodial and lamellipodial cellular
processes to extend once more to
repeat the cycle3 (Figure 2).
Soon after corneal wounding,
extracellular matrix proteins such
as fibronectin, fibrinogen/fibrin,
laminin and tenascin appear on
the wound site surface 18-25. There
is evidence to suggest that the
extracellular
glycoprotein
fibronectin plays an essential role
by providing a transient subepithelial matrix onto which the
migrating epithelial cells can
adhere during these frequent
cyclical processes of cleaving and
attaching of the migrating
epithelial cells. Fibronectin
appears on the wound surface
within an hour after injury18 and it
has been found to have cell
receptor sites as well as binding
sites for certain basement
membrane components which
include heparin sulphate and type
IV
collagen.
Fibronectin
stimulates epithelial cells to
produce plasminogen activator
which, in turn, converts
plasminogen to plasmin which
breaks down adhesions between
cells and underlying
sub-epithelial matrix26,27. In this
way
the
filopodial
and
lamellipodial processes bind with
and cleave from the underlying
matrix during the cyclical process
described above. Once corneal
healing is complete, fibronectin
disappears18.
Only once migration of the
epithelial monolayer is complete
does it become more firmly
anchored to the basement
membrane and to Bowmans layer
by
newly
synthesised
hemidesmosomes and anchoring
filaments containing type VII
collagen28. Until this point, the
adhesions have been relatively
weak, allowing the new epithelium
to be easily rubbed off (Figure 2).
The precise process by which these
anchoring complexes are formed is
not yet fully understood. Research
has indicated the presence of
various other protein molecules in
these anchoring complexes. These
include talin, fimbrin, integrins
(i.e. heterodimeric transmembrane
glycoproteins), laminin and
kalinin23,29,30.
Figure 3b. Electron micrograph of actual lamellipodia and filopodia minutes after
wound healing (courtesy Acta Ophthalmologica (Suppl) in Gipson et al, 1992:70:75)
SEPTEMBER 24 1999 OPTOMETRY TODAY
29
C O R N E A L
W O U N D
H E A L I N G :
R E V I E W
Figures 5a and b. Diagram representing corneal epithelial
wound healing - with formation of X and Y suture lines
(a and b)
Figure 4. Scanning EM of a wound margin
four hours after wounding (x1500)
(by permission from Portfolio Publishing Co, Texas)
An important pre-requisite for stability of
regenerated corneal epithelium is tight
adhesion to the underlying stroma. The
main structures involved in epithelial
adhesion include hemidesmosomes, which
are electron dense adhesion structures
located along the basal cell membranes,
basal laminae and anchoring fibrils31 (Figure
2). Anchoring fibrils are extracellular matrix
structures composed of type VII collagen
which spread out into the stroma forming
inter-linking meshworks with collagen fibrils
giving rise to anchoring plaques32-36.
On the basis of rabbit models, it has been
shown that adhesion structures between
corneal epithelium and stroma are fully
restored approximately six to eight weeks
after injury37-39. Although certain adhesion
structures do regenerate, the overall
architecture of the basement membrane may
be altered and perhaps this may be
permanent37.
Restoration of epithelial cell adhesion
structures is a complex process which can
proceed for many months following injury.
The reappearance of anchoring fibrils and
basal lamina has been shown to be directly
proportional to wound healing duration,
whereas hemidesmosome reformation occurs
simultaneously with basal lamina formation.
Other factors affecting adhesion complex
formation include the age, depth of the
corneal wound and the nature of any
underlying condition such as anterior
basement membrane dystrophies.
How quickly permanent hemidesmosomal
attachments form depends on whether the
basement membrane remained intact at the
time of corneal wounding. Studies in
monkey eyes, for example, have shown that
30
despite their formation, fragmented regions
of epithelial basement membrane can be
devoid of type VII collagen anchoring fibrils
even as long as 18 months after wounding in
post photokeratectomy32. This may help
explain the symptoms of foreign body
sensation, lacrimation or tenderness in some
photorefractive keratectomy patients who
later suffer epithelial breakdown35.
Several studies have indicated that
migration of cells occurs in a centripetal
fashion from the limbus towards the centre
of the cornea40,41 (Figure 4). In the case of
large central corneal abrasions, centripetal
epithelial sheet movement may occur from
more than one direction with the leading
edges forming convex shapes. Eventually,
these various leading edges meet to close
the wound forming Y or X shaped contact
lines observable with the slit lamp42
(Figures 5a and b). Animal studies
indicate that the rate of epithelial cell
migration varies with species, e.g. 17mm/day
in the mouse and 64mm in the rabbit7.
Epithelial cells usually migrate en masse as a
continuous sheet with individual cells
remaining in constant unchanging positions
relative to each other9,52. Occasionally, small
groups or columns of cells migrate
independently giving rise to whorls of
variable size which can easily be observed
with the use of fluorescein stain normally
very close to the Y or X contact lines43,44.
This second, migratory and adhesive phase
lasts for between 24 and 36 hours3.
SEPTEMBER 24 1999 OPTOMETRY TODAY
EPITHELIAL CELL PROLIFERATION
The final phase in the healing of an
epithelial defect involves proliferation of the
epithelial cells until normal epithelial
thickness is restored. The basal epithelial
cells are the main participants in this
proliferative process45. All self-regenerating
tissues of the body are thought to have stem
cells which are responsible for cell
replacement and tissue regeneration. In the
cornea, these are thought to be located near
the limbus. Evidence to support this
hypothesis includes observations that large
corneal epithelial wounds appear to repair
more quickly the closer the wound is to the
limbus when compared to smaller more
central corneal wounds46. The two main
categories of epithelial cell present in the
cornea can be classified as the basal cells and
the overlying suprabasal cells. Stem cells
first produce rapidly dividing cells termed
transient amplifying cells (TACs) which
refer to corneal basal cells. These further
divide into more differentiated cells termed
post mitotic cells (PMCs). Similarly, these
cells produce the final, fully differentiated
corneal epithelial cells known as terminally
differentiated cells (TDCs)47. Both PMCs
and TDCs have been described as the
suprabasal cells of the corneal epithelium48.
Any protuberances from the underlying
stroma are smoothed out to give a regular
corneal surface with proliferation of
epithelial cells. Finally, wound healing is said
to be complete with the further
C O R N E A L
establishment
of
hemidesmosomes
permanently anchoring the corneal
epithelium to the underlying stroma. In
extensive corneal wounds, including the
limbus and conjunctival epithelium, human
studies have shown that conjunctival cells
are capable of migrating centripetally into
the cornea. However, these cells appear to
be not well differentiated, irregular and
relatively thin42. Because the normal
phenotype of conjunctival epithelium is that
of columnar epithelium with numerous
goblet cells, the transformation to normal
stratified squamous corneal epithelium
without goblet cells implies that the
conjunctival epithelium retains the
capability of phenotypic change to that of
corneal epithelium. Such epithelial
differentiation
involves
not
only
morphologic change but the acquisition of
the biochemical and physiological properties
of corneal epithelium as well47.
STROMAL WOUND HEALING
The human corneal stroma is approximately
500m thick and forms approximately 90%
of the total corneal thickness. The stroma
can be sub-divided into the 8-10m thick
Bowmans layer and the 8-12m thick
Descemets layer. Sandwiched in between is
the lamellar stroma.
Healing of corneal stromal wounds is
slower than in other connective tissues
presumably because of the avascularity.
After an incisional injury, the stromal matrix
imbibes fluid and becomes oedematous in
the area adjacent to the wound. The
severed acellular Bowmans layer and
Descemets membrane (if sufficiently deep)
do not heal but instead the cut ends of the
membranes remain retracted.
Stromal regeneration depends upon a
co-ordinated interaction between epithelial
cells and keratocytes where polypeptide
growth factors play an important role (see
later). Following stromal wounding,
keratocytes undergo proliferation and
migration stimulated by the release of
certain cytokines. Keratocyte activity is not
seen until the corneal wound has been fully
covered by new epithelium. These
remaining keratocytes start to undergo
fibroblastic transformation with resulting
expansion of the fibroblast population by
mitosis after approximately 48 to 72 hours,
which reaches its peak between three to six
days, whilst new connective tissue is
synthesised35,49. Keratocyte mitosis decreases
with increasing keratocyte re-population
and rather than migrating from the basal
layers of the corneal stroma, the new
W O U N D
H E A L I N G :
R E V I E W
keratocytes are created by mitotic division of
cells peripheral to the epithelial defect4,50,51.
The mechanism by which keratocytes
initially disappear is not yet fully understood,
although the fact that keratocytes disappear
very quickly, with a lack of detritus, may
indicate a mechanism of self-destruction,
i.e apoptosis rather than necrosis.
The fibroblasts produce collagens,
glycoproteins and proteoglycans which form
the new stromal extracellular matrix. The
human corneal stroma contains collagen
types I, III, V, and VI, all of which are now
thought to co-polymerise within the same
fibrils throughout the stroma. Collagen type
VI constitutes approximately 30% of the
corneal stromal collagen content and is
predominant in the connecting filaments
interlinking stromal collagen fibrils52. This
hybrid nature has led to the suggestion that
fibril diameters in the cornea are controlled
by the inclusion of one or more of these
minor collagen types with type I collagen,
which predominates (71%) in human
corneal stroma. In this early phase, the newly
synthesised collagen fibrils are usually larger
in diameter than normal, due to the higher
concentration of chondroitin/dermatan
sulphate which lasts for up to three to four
months. This is in contrast to the normally
low levels of sulphated dermatan sulphate in
normal corneal stroma53,54. The resulting
variation in collagen fibril diameter is one
factor which may cause a disruption of
corneal transparency and scarring.
Initially, the extra cellular matrix, which
normally contains high levels of type IV
collagen
filaments,
laminin
and
proteoglycans, including keratan sulphate,
dermatan sulphate and heparan sulphate, is
also quite disorganised. Proteoglycans are
composed of acidic macromolecules which
incorporate sulphated glycosaminoglycans
(GAGs), which possess strongly hydrophilic
properties. These play an important role in
the regulation of early, post wounding
corneal deturgesence which can therefore
add to the irregularity of the normal spacing
between collagen fibrils giving rise to a
vacuolar appearance.
Stromal remodelling is thought to be
controlled, in part at least, by various matrix
metalloproteinases, e.g. collagenase,
stromelysin and gelatinase55. Removal of
damaged collagen fibres is controlled by the
presence of polymorphonuclear leukocytes
and the proteolytic enzymes they contain, as
in epithelial wound healing.
The formation of a stromal scar in humans
is therefore a dynamic process and clinical
changes can be noted by slit-lamp
biomicroscopy for several years post
wounding. After two years, the lamellar
collagen pattern is almost back to normal
dimensions but with shorter and narrower
lamellae. The strength of corneal scars never
reaches that of uninjured corneal tissue.
ENDOTHELIAL WOUND HEALING
Damage to the corneal endothelium
following various types of trauma has been
well documented84. Endothelial integrity is
crucial in maintaining stromal transparency.
The corneal stroma which can absorb
considerable amounts of fluid, is kept in its
normally deturgescent state by the
endothelial active transport and barrier
functions. It has been estimated that as
much as 80% of endothelial cells can be lost
before
decompensation
ensues56.
Endothelial cells in humans and primates
have minimal or no capacity to replicate by
mitosis and therefore endothelial wound
healing is largely dependant on enlargement
and movement of surrounding cells to cover
a wound site. This is in contrast to rabbits
whose endothelial cells are capable of
extensive mitosis, and cats which are
capable of limited endothelial mitosis57.
Human endothelial wound repair is thus
achieved by endothelial cells sliding over
the stromal or Descemets surface, or more
usually over a fibronectin sub-matrix. The
endothelium is responsible for the
deposition of a new Descemets layer
throughout the wound area. Under certain
circumstances, a tissue layer forms posterior
to the newly formed Descemets membrane
which also contains fibroblast-like cells,
collagen fibrils, basement membrane
proteins and junctional complexes. These
have been termed retrocorneal fibrous
membranes (RCFM)34.
AN INTRODUCTION TO GROWTH FACTORS
AND OCULAR WOUND HEALING
Growth factors are a heterogenous group of
proteins capable of stimulating growth and
multiplication of cells. There is mounting
evidence that peptide growth factors have
an important role by regulating many of the
processes involved in normal corneal
wound healing including migration, mitosis
and differentiation of cells. The eye is a
target tissue for all of the major families of
growth factors58-62. These include
epidermal growth factor (EGF), which was
the first to be isolated and described in
196263, followed by fibroblast growth
factors (FGF), platelet derived growth
factors (PDGF), insulin like growth factors
(IGF) and transforming growth factors beta
SEPTEMBER 24 1999 OPTOMETRY TODAY
31
C O R N E A L
W O U N D
H E A L I N G :
R E V I E W
Figure 6. Model for growth factor action in corneal wound healing
Part 2 of this article
will appear in our October 8 issue.
For a full set of references, please fax 01252-816176
(TGF). TGF is a potent inducer of lysl
oxidase mRNA in cultured scleral
fibroblasts, which plays a major role in the
synthesis of corneal extracellular matrix
components after injury90.
EGFs are one of the most biologically
potent families of growth factors and the
best characterised, although its precise
physiologic activity and clinical efficacy is
not even now fully understood89. Many
animal studies have shown that EGFs
stimulate wound healing but the efficacy is
dependant on the type of wound sustained,
i.e. chemical, thermal or traumatic90.
Epithelial wound healing rates in animal
models have been reported to accelerate by
as much as 25% to 45% in the presence of
EGFs88,89,64-68. EGFs also seem to have a
positive effect in certain non-dystrophic
corneal conditions, although the effect of
EGFs in humans has not been well
documented69. EGFs are normally present in
tear fluid whereas attempts to identify EGF
in the anterior chamber have been relatively
unsuccessful70. The lacrimal gland is
thought to be the main source of tear fluid
EGF. It is possible that it is stored possibly in
granules and released in response to neural
stimuli; or alternatively that it is
continuously synthesised and secreted71.
Transforming growth factor alpha (TGF) is
also found in human tear fluid and also
originates from the lacrimal gland72. This is
another polypeptide hormone very similar
structurally to EGF. These two main growth
factors probably control corneal wounding
by several pathways. These include an
exocrine response produced by growth
factors acting on epithelial cells following
secretion by the lacrimal gland. There is the
autocrine pathway, where epithelial cells
produce growth factors which act upon
themselves for which the evidence is the
identification of mRNA in epithelial cells,
giving them the potential to produce their
own EGFs. An example of a growth factor
thought to act in this way is TGF. The
paracrine pathway would enable epithelial
32
cells synthesising growth factors to influence
neighbouring cells of which TGF is again
an example58 (Figure 6).
Epithelial cells, fibroblasts and endothelial
cells have EGF receptors which consist of
transmembrane glycoproteins. Binding of
EGF to the extracellular domain of the EGF
receptors gives rise to EGF-receptor
complexes in a molecular ratio of 1:1. This
results in activation of protein kinase activity
involving a process of dimerisation within
the cytoplasmic section of the receptor. The
ensuing chain of events leading to cell
migration, mitosis and proliferation is not
well understood58.
Healing of stromal injuries is similar to
epithelial injuries. However, a major
difference is the extensive production by
stromal fibroblasts of extracellular matrix
components such as collagen90. Studies of
dermal incision healing in skin have shown
that transforming growth factor (TGF-)
may be the factor most responsible for
increased synthesis of collagen and therefore
increased strength of stromal wounds73,74.
Fibroblast growth factor (FGF) and insulinlike growth factor (IGF) are present
particularly in the corneal stroma. Both FGF
and EGF stimulate the proliferation of
corneal epithelium, keratocytes and
endothelium. However, because EGF is the
more potent growth factor, it has superior
properties in increasing tensile strength of
corneal stromal wounds and even has a
greater effect on endothelium than FGF. IGF
stimulates in particular the growth of
keratocytes and enhances the effect of EGF
on endothelial cells. Mesodermal growth
factor (MGF) stimulates the proliferation of
keratocytes and increases the healing rates of
endothelial cells89,93.
Growth factors are rapidly emerging as an
exciting new generation of ophthalmic
pharmaceuticals. Although early results
were rather disappointing, it is likely that
they will increasingly play a major role in
wound healing management throughout the
practice of ophthalmology in the future.
SEPTEMBER 24 1999 OPTOMETRY TODAY
SUMMARY
Corneal wound healing is a complex process
that is controlled by many factors. The
processes involved in corneal epithelial
wound healing can be described by three
separate phases that, in reality, are part of a
continuous process. Wound healing is
affected by many factors including the size
of the wound, its depth, causative agent and
tear quality. The healing of corneal stromal
wounds is slower than in other connective
tissues and stromal re-modelling is thought
to be partly controlled by various matrix
metalloproteinases. A variety of peptide
growth factors appear to play an important
role in the regulation of normal corneal
wound healing.
ACKNOWLEDGEMENTS
The author wishes to thank Martin Dunitz
for agreeing to the reproduction of extracts
of Chapter Four written by C. Steele in
Excimer Lasers: Principles and Practice,
Editors: McGhee, Taylor, Gartry and Trokel.
First published in 1997.
He also thanks Stephen Morgan,
Consultant Ophthalmologist, Sunderland
Eye Infirmary, for his comments during the
preparation of this article.
IN PART II
Part II of this article will address commonly
encountered causes of corneal wounding and
the role of TCLs where appropriate in the
management of these conditions. In particular,
the pathogenesis of recurrent corneal erosion
will be discussed, including the principles of
managing such patients in practice and the
various management options available.
These include medical therapy, superficial
keratectomy, anterior stromal puncture,
excimer laser phototherapeutic keratectomy
and the use of therapeutic contact lenses
(TCLs). The use of TCLs in persistent
epithelial defects, chemical injuries, corneal
lacerations and post corneal surgery will also
be briefly discussed.
Anda mungkin juga menyukai
- Wound Healing: Ziv Peled, M.DDokumen8 halamanWound Healing: Ziv Peled, M.Dapi-26007957Belum ada peringkat
- Chapter 7: Wound HealingDokumen12 halamanChapter 7: Wound HealingpoddataBelum ada peringkat
- Tissue Repair and Wound Healing ProcessDokumen70 halamanTissue Repair and Wound Healing ProcessRegi SeptianBelum ada peringkat
- Wound Repair and RegenrationDokumen9 halamanWound Repair and RegenrationShivanshu SiyanwalBelum ada peringkat
- Corneal Epithelial Wound Healing: PerspectiveDokumen8 halamanCorneal Epithelial Wound Healing: PerspectiveHanaBelum ada peringkat
- Biology of Periodontal Wound HealingDokumen78 halamanBiology of Periodontal Wound HealingSudip SenBelum ada peringkat
- Healing: RegenerationDokumen14 halamanHealing: RegenerationElvisBelum ada peringkat
- Wound Healing, or Wound Repair, Is An Intricate Process in Which The Skin (OrDokumen20 halamanWound Healing, or Wound Repair, Is An Intricate Process in Which The Skin (OrDeepak AhujaBelum ada peringkat
- Wound Healing and Tissue RepairDokumen27 halamanWound Healing and Tissue RepairKELVIN MAYOMBOBelum ada peringkat
- Review ArticleDokumen12 halamanReview ArticleIkHa RakhmayaniBelum ada peringkat
- Healing of extraction sockets in 4 stagesDokumen34 halamanHealing of extraction sockets in 4 stagesIndrani Das93% (14)
- Wound HealingDokumen23 halamanWound HealingMarry M. MarquesBelum ada peringkat
- Wound Healing: Healing by First Intention (Primary Union)Dokumen31 halamanWound Healing: Healing by First Intention (Primary Union)Muhammad Masoom AkhtarBelum ada peringkat
- Dr. Suhad Faisal HatemDokumen13 halamanDr. Suhad Faisal HatemIlyes RaniBelum ada peringkat
- A Closer Look: The Corneal EpitheliumDokumen4 halamanA Closer Look: The Corneal Epitheliumshay sBelum ada peringkat
- Ecm&migrationDokumen6 halamanEcm&migrationNanda SapitriBelum ada peringkat
- Giles 2007 Wound Healing in Spontaneous Perforation or Myringotomy Middle Ear ReconstructionDokumen3 halamanGiles 2007 Wound Healing in Spontaneous Perforation or Myringotomy Middle Ear Reconstructionprashantpatel.jaipurBelum ada peringkat
- Skin and Soft TissuesDokumen122 halamanSkin and Soft TissuesNagulan ChanemougameBelum ada peringkat
- Meibomian Gland Disease. Classification and Grading of Lid ChangesDokumen17 halamanMeibomian Gland Disease. Classification and Grading of Lid Changesnadya sahnazBelum ada peringkat
- Hope 2007Dokumen15 halamanHope 2007raden chandrajaya listiandokoBelum ada peringkat
- JCM 08 02083 v2Dokumen27 halamanJCM 08 02083 v2Veronica WongBelum ada peringkat
- Martin Paul PDFDokumen8 halamanMartin Paul PDFSteven NoriegaBelum ada peringkat
- Cytokines and The Skin Barrier: Molecular SciencesDokumen26 halamanCytokines and The Skin Barrier: Molecular SciencesFelp ScholzBelum ada peringkat
- Mechanisms of Wound Healing in The PeriodontiumDokumen2 halamanMechanisms of Wound Healing in The Periodontiumwiwied sambodoBelum ada peringkat
- CH 3 PDFDokumen2 halamanCH 3 PDFKgerbBelum ada peringkat
- Wound HealingDokumen16 halamanWound HealingHAWO MOHAMEDBelum ada peringkat
- Skin Cell Proliferation Stimulated by MicroneedlesDokumen6 halamanSkin Cell Proliferation Stimulated by MicroneedlesAbouOrtizBelum ada peringkat
- Wound Healing Reading ChaptersDokumen56 halamanWound Healing Reading ChaptersChris MegapanosBelum ada peringkat
- Relation Between Hypothermia and SSIDokumen7 halamanRelation Between Hypothermia and SSIRekkiBelum ada peringkat
- Glaucoma, Mesenchymal Stem Cell, Retinal Cell, Stem Cell TherapyDokumen13 halamanGlaucoma, Mesenchymal Stem Cell, Retinal Cell, Stem Cell TherapykaharBelum ada peringkat
- Cellular Human Tissue-Engineered Skin Substitutes Investigated For Deep and Dif Ficult To Heal InjuriesDokumen23 halamanCellular Human Tissue-Engineered Skin Substitutes Investigated For Deep and Dif Ficult To Heal InjuriesNovelas, Series y PelículasBelum ada peringkat
- Healing and Repair Flash PointsDokumen3 halamanHealing and Repair Flash PointsHassan AhmadBelum ada peringkat
- Membrane and Cell Wall Deposition at The Division SiteDokumen2 halamanMembrane and Cell Wall Deposition at The Division SiteNatalia LadinoBelum ada peringkat
- Skin Wound HealingDokumen9 halamanSkin Wound HealingOEDIEN-kmbBelum ada peringkat
- Patho 3Dokumen13 halamanPatho 3ambiliBelum ada peringkat
- Tissue Injury and Healing: Brent Kincaid, DDS, John P. Schmitz, DDS, PHDDokumen10 halamanTissue Injury and Healing: Brent Kincaid, DDS, John P. Schmitz, DDS, PHDAmith HadhimaneBelum ada peringkat
- Balsa 2015Dokumen17 halamanBalsa 2015Pipe VodBelum ada peringkat
- Healing of Oral WoundsDokumen73 halamanHealing of Oral WoundsMadhvendra Singh80% (5)
- A Review of Skin and The Effects of Aging On Skin Structure and Function - Skin Turnover TimeDokumen10 halamanA Review of Skin and The Effects of Aging On Skin Structure and Function - Skin Turnover TimeEUNPYO LEEBelum ada peringkat
- Abd 91 05 0614Dokumen7 halamanAbd 91 05 0614Selvy Anriani GasperszBelum ada peringkat
- Innate Immune Cell-Epithelial Crosstalk During Wound RepairDokumen11 halamanInnate Immune Cell-Epithelial Crosstalk During Wound Repairvanessa_werbickyBelum ada peringkat
- Establishment of A Novel in Vitro Model of Stratified Epithelial Wound Healing With Barrier FunctionDokumen9 halamanEstablishment of A Novel in Vitro Model of Stratified Epithelial Wound Healing With Barrier FunctionBaladika muda28Belum ada peringkat
- Understanding Trabecular Meshwork Physiology: A Key To The Control of Intraocular Pressure?Dokumen5 halamanUnderstanding Trabecular Meshwork Physiology: A Key To The Control of Intraocular Pressure?Nail AlheloBelum ada peringkat
- Anatomy, Physiology and Biochemistry of Cornea: Presenter-Yordanos (R1) Moderator-Dr YaredDokumen106 halamanAnatomy, Physiology and Biochemistry of Cornea: Presenter-Yordanos (R1) Moderator-Dr YaredAmare GetnetBelum ada peringkat
- 0003IADDokumen12 halaman0003IADRika AzyenelaBelum ada peringkat
- Mathematical Biosciences: Stephen J. Gilmore, Benjamin L. Vaughan JR., Anotida Madzvamuse, Philip K. MainiDokumen7 halamanMathematical Biosciences: Stephen J. Gilmore, Benjamin L. Vaughan JR., Anotida Madzvamuse, Philip K. MainiDewinsBelum ada peringkat
- BR Med Bull 2011 Sakabe BMB Ldr025Dokumen15 halamanBR Med Bull 2011 Sakabe BMB Ldr025VersoBelum ada peringkat
- Integumentary System: Stratum BasaleDokumen21 halamanIntegumentary System: Stratum BasalerinaBelum ada peringkat
- Anatomi Fisiologi KorneaDokumen11 halamanAnatomi Fisiologi KorneaMauVeeBelum ada peringkat
- Ern Mrcs Bk1Dokumen21 halamanErn Mrcs Bk1aeages100% (1)
- Referat Cystoid Macular Edema Periode 17 Juni - 20 Juli 2019Dokumen25 halamanReferat Cystoid Macular Edema Periode 17 Juni - 20 Juli 2019CharlotteGraceNusiferaBelum ada peringkat
- Fascial Closure-Dumanian2020Dokumen15 halamanFascial Closure-Dumanian2020Kevin QuinterosBelum ada peringkat
- Wound Healing in The Oral Mucosa: Patricio C. Smith and Constanza MartínezDokumen14 halamanWound Healing in The Oral Mucosa: Patricio C. Smith and Constanza MartínezNadira NurinBelum ada peringkat
- Wound Healing and Its Impairment in The Diabetic Foot: ReviewDokumen9 halamanWound Healing and Its Impairment in The Diabetic Foot: ReviewJoey TsaiBelum ada peringkat
- Atherosclerosis: Pre-Lesional PhaseDokumen2 halamanAtherosclerosis: Pre-Lesional PhaseprogfabBelum ada peringkat
- The Multifunctional Role of Fibroblasts During Wound Healing in Hirudo Medicinalis (Annelida, Hirudinea)Dokumen13 halamanThe Multifunctional Role of Fibroblasts During Wound Healing in Hirudo Medicinalis (Annelida, Hirudinea)Agung Tri LaksonoBelum ada peringkat
- Natomy and Physiology of Cornea: Dr.K.Mahalakshmi 1 Yr PGDokumen61 halamanNatomy and Physiology of Cornea: Dr.K.Mahalakshmi 1 Yr PGMaha lakshmiBelum ada peringkat
- Wound HealingDokumen45 halamanWound HealingReda IsmaeelBelum ada peringkat
- Lalalalalala LL All A LalDokumen1 halamanLalalalalala LL All A LalMaizan Khairun NissaBelum ada peringkat
- Daftar PustakaDokumen1 halamanDaftar PustakaMaizan Khairun NissaBelum ada peringkat
- Presentasi Kasus Dipersiapkan: Stroke IskemikDokumen1 halamanPresentasi Kasus Dipersiapkan: Stroke IskemikMaizan Khairun NissaBelum ada peringkat
- Presentasi Kasus Dipersiapkan: Stroke IskemikDokumen1 halamanPresentasi Kasus Dipersiapkan: Stroke IskemikMaizan Khairun NissaBelum ada peringkat
- Lalalalala LL A A OkooDokumen1 halamanLalalalala LL A A OkooMaizan Khairun NissaBelum ada peringkat
- Diseases, Vol. 45, No. 2, Pp. 241-249, 2007Dokumen1 halamanDiseases, Vol. 45, No. 2, Pp. 241-249, 2007Maizan Khairun NissaBelum ada peringkat
- Diskusi Topik Toxoplasma EnsefalisDokumen1 halamanDiskusi Topik Toxoplasma EnsefalisMaizan Khairun NissaBelum ada peringkat
- Pepe PepDokumen1 halamanPepe PepMaizan Khairun NissaBelum ada peringkat
- Presentasi Kasus Langsung: Space Occupying LesionDokumen1 halamanPresentasi Kasus Langsung: Space Occupying LesionMaizan Khairun NissaBelum ada peringkat
- Presentasi Kasus Dipersiapkan: Stroke IskemikDokumen1 halamanPresentasi Kasus Dipersiapkan: Stroke IskemikMaizan Khairun NissaBelum ada peringkat
- RRR RRRRRRRDokumen1 halamanRRR RRRRRRRMaizan Khairun NissaBelum ada peringkat
- Diskusi Topik Toxoplasma EnsefalisDokumen1 halamanDiskusi Topik Toxoplasma EnsefalisMaizan Khairun NissaBelum ada peringkat
- Error! No Text of Specified Style in DocumentDokumen1 halamanError! No Text of Specified Style in DocumentMaizan Khairun NissaBelum ada peringkat
- Error! No Text of Specified Style in DocumentDokumen1 halamanError! No Text of Specified Style in DocumentMaizan Khairun NissaBelum ada peringkat
- BagusDokumen33 halamanBagusMaizan Khairun NissaBelum ada peringkat
- 12-Huang Et AlpdfDokumen12 halaman12-Huang Et AlpdfMaizan Khairun NissaBelum ada peringkat
- HYDROPS FETALIS ASSOCIATED WITH HOMOZYGOSITY FOR HB ADANA (A59 (E8) Gly®Asp (A2) )Dokumen8 halamanHYDROPS FETALIS ASSOCIATED WITH HOMOZYGOSITY FOR HB ADANA (A59 (E8) Gly®Asp (A2) )Maizan Khairun NissaBelum ada peringkat
- HTTPDokumen1 halamanHTTPMaizan Khairun NissaBelum ada peringkat
- One-Step Multiplex RT-PCR For Detection of BCR - ABL Gene in Malay Patients With Chronic Myeloid LeukaemiaDokumen4 halamanOne-Step Multiplex RT-PCR For Detection of BCR - ABL Gene in Malay Patients With Chronic Myeloid LeukaemiaMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- SEO-Optimized Title for Document on Sexually Transmitted InfectionsDokumen59 halamanSEO-Optimized Title for Document on Sexually Transmitted InfectionsMaizan Khairun NissaBelum ada peringkat
- Panitia Uin Medical Olympiad and Essay CompetitionDokumen1 halamanPanitia Uin Medical Olympiad and Essay CompetitionMaizan Khairun NissaBelum ada peringkat
- Post Partum ExamDokumen4 halamanPost Partum ExamAhby Vitug de Luna100% (1)
- Veterinarian Doctor Resume - Bassem BazzezDokumen2 halamanVeterinarian Doctor Resume - Bassem Bazzezkouloud dibiBelum ada peringkat
- Elderly Care IndiaDokumen3 halamanElderly Care IndiakasurvarBelum ada peringkat
- Juvenile Sex Offenders A Complex PopulationDokumen6 halamanJuvenile Sex Offenders A Complex PopulationRoberto GalleguillosBelum ada peringkat
- An Intergenerational Approach to Family TherapyDokumen31 halamanAn Intergenerational Approach to Family TherapyBeatriz Eugenia Guerra100% (5)
- FCPS Ii Major Tentative Schedule 2a Feb 2024Dokumen1 halamanFCPS Ii Major Tentative Schedule 2a Feb 2024haseeb ur rehmanBelum ada peringkat
- CefepimeDokumen2 halamanCefepimeMae Ann Bueno CastillonBelum ada peringkat
- (ANES) Mon 01 Epidural For Labor Analgesia (A2021)Dokumen3 halaman(ANES) Mon 01 Epidural For Labor Analgesia (A2021)Miguel SantosBelum ada peringkat
- Tugas Bahasa Inggris Wound CareDokumen2 halamanTugas Bahasa Inggris Wound CareBela Asa100% (1)
- Chapter33Walker2015 PDFDokumen12 halamanChapter33Walker2015 PDFMai AngelBelum ada peringkat
- Desai - Palliative Medicine in Myelodysplastic Syndromes - Patients and Caregivers - A Qualitative StudyDokumen5 halamanDesai - Palliative Medicine in Myelodysplastic Syndromes - Patients and Caregivers - A Qualitative StudyRafael TerceiroBelum ada peringkat
- HEALTHY JUICES AND THEIR BENEFITSDokumen7 halamanHEALTHY JUICES AND THEIR BENEFITSdeepaliBelum ada peringkat
- Demand and ElasticityDokumen5 halamanDemand and ElasticityCorey Crismon100% (1)
- 16-Week Marathon Training Program with Weekly SessionsDokumen4 halaman16-Week Marathon Training Program with Weekly SessionsMaria Del Carmen García FermínBelum ada peringkat
- Triage in Medicine PDFDokumen13 halamanTriage in Medicine PDFElsya ApriliaBelum ada peringkat
- CPV/CCV Ag (3 Lines) : VcheckDokumen2 halamanCPV/CCV Ag (3 Lines) : VcheckFoamfab WattikaBelum ada peringkat
- Create Consent Form (39 charactersDokumen3 halamanCreate Consent Form (39 charactersJan Diel100% (2)
- Adverse Reactions To DiureticsDokumen8 halamanAdverse Reactions To DiureticsDimas RizkyBelum ada peringkat
- Beximco Pharma FinalDokumen14 halamanBeximco Pharma FinalAli Asgor Raton67% (3)
- NCP Infection NewDokumen3 halamanNCP Infection NewXerxes DejitoBelum ada peringkat
- MRCP UK PACES EXAM GUIDEDokumen124 halamanMRCP UK PACES EXAM GUIDEJayachandran P KBelum ada peringkat
- DHI Score PDFDokumen2 halamanDHI Score PDFAnonymous HNGH1oBelum ada peringkat
- Pi Is 0002937814002713Dokumen5 halamanPi Is 0002937814002713Ibnu YazidBelum ada peringkat
- Espiritismo Leonora Piper Coisas Que Acertou Questiona Sobrinha Que Não Existia-Resposta Está em Outro Arquivo PDFDokumen155 halamanEspiritismo Leonora Piper Coisas Que Acertou Questiona Sobrinha Que Não Existia-Resposta Está em Outro Arquivo PDFMariana MuradBelum ada peringkat
- Ross University 2010-2011 Pre-Residency Planning GuideDokumen61 halamanRoss University 2010-2011 Pre-Residency Planning GuidescatteredbrainBelum ada peringkat
- Subfalcine Herniation Damages Cingulate GyrusDokumen34 halamanSubfalcine Herniation Damages Cingulate GyrusLorenzo FrancisBelum ada peringkat
- Beggs Stage 1 - Ortho / Orthodontic Courses by Indian Dental AcademyDokumen33 halamanBeggs Stage 1 - Ortho / Orthodontic Courses by Indian Dental Academyindian dental academyBelum ada peringkat
- Food and Exercise LogDokumen24 halamanFood and Exercise LogmhetfieldBelum ada peringkat
- 1 - General Indications and Contraindications - 2019 - Lumbar Interbody FusionsDokumen12 halaman1 - General Indications and Contraindications - 2019 - Lumbar Interbody FusionsSergiu MalinBelum ada peringkat
- Ecg InterpretationDokumen3 halamanEcg Interpretationman0billi0% (1)