Production of MTBE (Methyl Tert-Butyl Ether)
Diunggah oleh
Aaron SinghDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Production of MTBE (Methyl Tert-Butyl Ether)
Diunggah oleh
Aaron SinghHak Cipta:
Format Tersedia
Production of MTBE (Methyl tert-butyl ether)
Introduction
When oil is distilled, the fraction that can be used as petrol (the naphtha fraction) is made up mainly of
C4 to C10 straight chain alkanes, which have suitable volatility
These burn in a way that leads to explosion in the engine cylinders
One way to improve the performance of the petrol is to add compounds that contain oxygen & MTBE is
used for this purpose
Overall Production & Reaction Conditions
MTBE is manufactured from 2-methylpropene (isobutene) and methanol using an acid catalyst.
The catalyst is an anionic ion-exchange resin
The reaction is carried out at 340-360 K and 8 atm pressure, with methanol in excess. The unused
methanol is recovered and recycled.
Obtaining 2-methylpropene required for the reaction
2-methylpropene is obtained by cracking (breaking up of larger distillation oil fractions into smaller
ones) or by reforming (joining of smaller distillation oil fractions to make bigger ones).
2-methylpropene is obtained by dehydrogenation of 2-methylpropane. The vapour is passed over a
catalyst (platinum and palladium on an inert support)
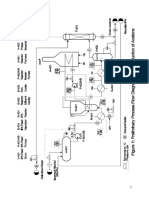
Equipment Focus
Fresh C4 feed from the dehydrogenation unit, methanol (MeOH) and a recycle stream are combined and
sent through the two main reactors where over 90% of the conversion is achieved
The reactor effluent enters the column below the resin beds
Additional MeOH can be injected at the top of the column
In addition to separating MTBE from the C4 and MeOH overhead stream, the column allows any
remaining conversion reactions to continue in multiple catalyst beds located above the feed injection
point
The reaction beds consists of an anionic ion-exchange resin (acidified) that accelerates the rates of the
exothermic reactions
Reaction rates are also increased by higher temperatures but reaction favours lower temperatures hence
catalyst is used
Product Specification
98% MTBE
Anda mungkin juga menyukai
- Simulation of Different Types of Distillation Columns Usig Aspen Plus SoftwareDokumen61 halamanSimulation of Different Types of Distillation Columns Usig Aspen Plus SoftwareShashank TiwariBelum ada peringkat
- Acetylene, the Principles of Its Generation and Use A Practical Handbook on the Production, Purification, and Subsequent Treatment of Acetylene for the Development of Light, Heat, and PowerDari EverandAcetylene, the Principles of Its Generation and Use A Practical Handbook on the Production, Purification, and Subsequent Treatment of Acetylene for the Development of Light, Heat, and PowerBelum ada peringkat
- Conversion of Methanol To Light Olefins On Sapo-34 Kinetic Modeling and Reactor DesignDokumen167 halamanConversion of Methanol To Light Olefins On Sapo-34 Kinetic Modeling and Reactor DesignHassan BahaaBelum ada peringkat
- Mek From N Butene PDFDokumen111 halamanMek From N Butene PDFAlexis PulhinBelum ada peringkat
- MTBEDokumen34 halamanMTBEphantanthanh67% (3)
- Hydrogenation of Fatty Acid Methyl Esters To FattyDokumen9 halamanHydrogenation of Fatty Acid Methyl Esters To FattyYulius Harmawan Setya PratamaBelum ada peringkat
- MTBE Production Material Balance Project: Process DescriptionDokumen39 halamanMTBE Production Material Balance Project: Process Descriptionmoheed100% (1)
- Turton AppBDokumen114 halamanTurton AppBAdesuwa O'sae0% (1)
- DME ProcessDokumen5 halamanDME ProcessAndres FragosoBelum ada peringkat
- EthylbenzeneDokumen4 halamanEthylbenzeneMouaath Al-Kalbani75% (4)
- Chapter 2Dokumen5 halamanChapter 2nfarBelum ada peringkat
- Mthanol ProductionDokumen61 halamanMthanol Productionvv vvBelum ada peringkat
- G 1 PDFDokumen199 halamanG 1 PDFKing HenryBelum ada peringkat
- Cumene A PDFDokumen4 halamanCumene A PDFdanena88Belum ada peringkat
- Methyl Methacrylate Plant CostDokumen3 halamanMethyl Methacrylate Plant CostIntratec Solutions50% (2)
- FYP CompleteDokumen104 halamanFYP CompleteAnonymous b9fcR5Belum ada peringkat
- LECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene OxideDokumen7 halamanLECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene Oxideمحمود محمدBelum ada peringkat
- Acido AceticoDokumen13 halamanAcido Aceticoting_tatBelum ada peringkat
- For Hysys UsersDokumen5 halamanFor Hysys UsersZohaib RanaBelum ada peringkat
- TOPIC: Acetic Acid Production Through Methanol Carbonylation Route Group MembersDokumen3 halamanTOPIC: Acetic Acid Production Through Methanol Carbonylation Route Group MembersThrese AreolaBelum ada peringkat
- Cumene ProductionDokumen26 halamanCumene ProductionAMOGH JHANWARBelum ada peringkat
- Mtbe UnlockedDokumen47 halamanMtbe UnlockedCaminito MallorcaBelum ada peringkat
- Design of EthylbenzeneDokumen5 halamanDesign of Ethylbenzenesahar vahdatifarBelum ada peringkat
- Direct Dimethyl Ether Synthesis: Takashi Ogawa, Norio Inoue, Tutomu Shikada, Yotaro OhnoDokumen9 halamanDirect Dimethyl Ether Synthesis: Takashi Ogawa, Norio Inoue, Tutomu Shikada, Yotaro OhnoM Alim Ur RahmanBelum ada peringkat
- Kinetics of Catalytic Dehydrogenation of Ethylbenzene To StyreneDokumen5 halamanKinetics of Catalytic Dehydrogenation of Ethylbenzene To Styreneibrahim3318Belum ada peringkat
- Vinyl AcetateDokumen5 halamanVinyl AcetateroxetteBelum ada peringkat
- Benzene Production Using Hydrodealkylation RouteDokumen3 halamanBenzene Production Using Hydrodealkylation RouteCluisantony Jayco DizeBelum ada peringkat
- Hydrodealkylation SimulationDokumen10 halamanHydrodealkylation SimulationSaiVenkatBelum ada peringkat
- MTBE Unit Expansion-ConversionDokumen13 halamanMTBE Unit Expansion-Conversiontunganh1110100% (1)
- Mtbe PDFDokumen47 halamanMtbe PDFYayee LalainheavenBelum ada peringkat
- University of Lagos: Process Description For The Production of Mtbe A Presentation by Group 10 ConsistingDokumen7 halamanUniversity of Lagos: Process Description For The Production of Mtbe A Presentation by Group 10 ConsistingJide Williams100% (1)
- ETHYLBENZENEDokumen19 halamanETHYLBENZENEolaBelum ada peringkat
- Nhóm (Đ.Anh+ Hiếu + Ý) Syngas to MethanolDokumen40 halamanNhóm (Đ.Anh+ Hiếu + Ý) Syngas to MethanolStrong NguyenBelum ada peringkat
- Ethyl Benzene Plant DesignDokumen45 halamanEthyl Benzene Plant DesignfaridzawiBelum ada peringkat
- Adnan Aljarallah 1988 Kinetic of MTBE Over AmberlystDokumen6 halamanAdnan Aljarallah 1988 Kinetic of MTBE Over AmberlystJason NunezBelum ada peringkat
- Production of Methyl MethacrylateDokumen63 halamanProduction of Methyl Methacrylateيزيد العزانيBelum ada peringkat
- AcetoneDokumen7 halamanAcetoneGeorgiana AndreeaBelum ada peringkat
- Mtbe 3 - DP 2Dokumen303 halamanMtbe 3 - DP 2Faiz ZainiBelum ada peringkat
- Acrolein Project Final PDFDokumen104 halamanAcrolein Project Final PDFPankaj RanaBelum ada peringkat
- Ethanolamines ProductionDokumen125 halamanEthanolamines Productionvraj ranaBelum ada peringkat
- Group Acetic Acid PresentationDokumen24 halamanGroup Acetic Acid PresentationNatko47Belum ada peringkat
- It1.Introduction & History:-: 1.1 Introduction To Cumene:-StructureDokumen12 halamanIt1.Introduction & History:-: 1.1 Introduction To Cumene:-StructureJaymin GoswamiBelum ada peringkat
- EnnnDokumen9 halamanEnnnSajid AliBelum ada peringkat
- Viewcontent11 PDFDokumen54 halamanViewcontent11 PDFEr Mayur PatilBelum ada peringkat
- Process Description DmeDokumen3 halamanProcess Description DmeFirdaus YahyaBelum ada peringkat
- Styrene From Ethane and BenzeneDokumen6 halamanStyrene From Ethane and BenzeneAmy Puah100% (2)
- Art:10 1134/S0965544111010038Dokumen10 halamanArt:10 1134/S0965544111010038CátiaLuzBelum ada peringkat
- Manfacture OF: Cyclo HexaneDokumen91 halamanManfacture OF: Cyclo HexaneNikhil Kumar Chennuri100% (4)
- Snamprogetti New MTBE Production Design PDFDokumen13 halamanSnamprogetti New MTBE Production Design PDFViệt HàBelum ada peringkat
- Project 6 - Ethylene Oxide PDFDokumen13 halamanProject 6 - Ethylene Oxide PDFStephanie Hawkins100% (1)
- Ether ProjectDokumen22 halamanEther ProjectekojamichaelBelum ada peringkat
- 1.project FullDokumen75 halaman1.project FullKolliparaDeepakBelum ada peringkat
- Side ReactionsDokumen22 halamanSide ReactionsAna Mariel VenturaBelum ada peringkat
- Heat Exchanger DesignDokumen74 halamanHeat Exchanger DesignChisom ChubaBelum ada peringkat
- Reactor ModelDokumen12 halamanReactor ModelTanuja ThanuBelum ada peringkat
- Process DescriptionDokumen4 halamanProcess DescriptionKen VenzonBelum ada peringkat
- Pro IiDokumen52 halamanPro IiMedranoIvanBelum ada peringkat
- MTBE Is Also Suitable As A Starting Material For The Preparation of Pure I-Butene Via The Ether CleavageDokumen3 halamanMTBE Is Also Suitable As A Starting Material For The Preparation of Pure I-Butene Via The Ether CleavageiffatBelum ada peringkat
- Synthesis of Biomass-Derived Gasoline Fuel Oxygenates by Microwave IrradiationDokumen18 halamanSynthesis of Biomass-Derived Gasoline Fuel Oxygenates by Microwave IrradiationDevesh Pratap ChandBelum ada peringkat