Anatoly I. Rusanov - Fluid State Equation
Diunggah oleh
FernandoEnriqueCalveteGonzálezHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Anatoly I. Rusanov - Fluid State Equation
Diunggah oleh
FernandoEnriqueCalveteGonzálezHak Cipta:
Format Tersedia
, 2003, 13(1), 1112
Fluid state equation
Anatoly I. Rusanov
Mendeleev Center, St. Petersburg State University, 199034 St. Petersburg, Russian Federation.
Fax: +7 812 428 6939; e-mail: rusanov@rus.usr.pu.ru
10.1070/MC2003v013n01ABEH001692
A thermodynamic approach based on the analysis of an excluded volume results in a general state equation, including the Planck
and van der Waals equations as particular cases, and a new precise state equation.
Let us consider an isotropic uniform molecular system. Since
a state equation may be always given by a set of isotherms, we
shall deal with an isotherm equation. We deduce it by integrating the trivial relationship
(1)
vdp = dm,
6
2
where v is the volume, p is the pressure, and m is the chemical
potential given by the standard expression
(2)
where m0 is the chemical potential of a molecule with resting
center of mass in the system under consideration, k is the
Boltzmann constant, T is the temperature, c and L are the concentration and the de Broglie wavelength, respectively (L is a
function of temperature). The traditional term with an activity
coefficient has been included in m0 whose only difference from
m is that m0 refers to molecules at rest. By analogy with (1), we
may write for such molecules (as if they were really present in
the system as a second component)
(3)
dm0 = vdp
with v as the partial molecular volume of a resting molecule,
i.e., the volume increment needed for restoring the initial pressure after introducing a single resting molecule into the system.
Putting now (2) in (1) and accounting for (3), we arrive at the
universal differential form of a state equation
dp
kT
=
.
1 vc
dc
(4)
To integrate equation (4), we have to know the concentration
dependence of v, which is strongly influenced by attraction
forces. To avoid this complication, we may integrate (4) as it
were no attraction forces (but with a given molecular structure
caused by attractive forces) and then add an attractive term
(which is well known) to a final expression. If there are no
attraction forces, v acquires the physical meaning of an excluded
volume vex. Indeed, among moving molecules, a single resting
and not interacting molecule (with a fixed centre of mass) plays
the role of a specific wall, which is evident to create an excluded volume. Thus, we can write the resulting state equation as
c
p = kT
dc
1 v
exc
ac2,
(5)
where a is the molecular attraction constant. Introducing the
intrinsic molecular volume v0 and the exclusion factor f ex vex/v0,
we can rewrite (5) in the dimensionless form
j
p~ =
dj
1 f
exj
a'j2,
(6)
where p~ pv0/kT is the ratio of the real pressure to the pressure
of an ideal gas of the same density, j v0c is the volume fraction of matter, and a' a/kTv0 is the dimensionless image of
the constant a.
Although we excluded an attractive contribution from the
integral in (6), the problem of the dependence of f ex on j is
maintained. Planck showed that f ex = 8 for a monatomic gas.1
However, the excluded volume can vary with concentration due
11
5
f ex
m = m0 + kT ln(cL3),
4
3
2
1
4
0.0
0.2
0.4
0.6
0.8
1.0
j
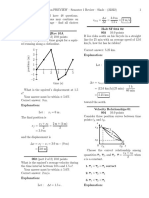
Figure 1 The dependence of the exclusion factor on the volume fraction
(solid lines) and the limits of applicability for the corresponding state
equations (dashed lines): (1) the Planck equation, (2) the van der Waals
equation, (3) equation (17), and (4) the line obtained from equation (6) and
the virial expansion for hard spheres with known ten coefficients.
to clustering, which starts already in the gaseous state of matter.
Let a system consist of spherical clusters containing n molecules on average and having a dense structure with a minimum
volume v0 per molecule. The cluster volume is nv0 and the
cluster radius is R = (3nv0/4)1/3 = ln1/3, where l is the radius
of an equivalent sphere corresponding to the volume v0. Depending on packing conditions, the volume of a free molecule can
differ from v0, but we ignore this for the sake of simplicity.
Defining then the excluded volume per molecule in a cluster as
v ex =
4(R + l)3 v0(n1/3 + 1)3
=
,
3n
n
(7)
we obtain an expression for the exclusion factor
(8)
f ex = 1 + 3n1/3 + 3n2/3 + n1.
ex
ex
According to (8), f monotonically decreases from f = 8 at
n = 1 to the limiting value f ex = 1 at n . The dependence of
f ex on j should be qualitatively the same since clustering
develops with increasing concentration.
Knowing the behaviour and the limits of f ex, we can compute
the integral in (6). The roughest approximation (applicable, if
any, only to some parts of the isotherm) is that f ex is not a
function but a constant. In this zero-order approximation, equation (6) becomes
ln (1 f exj)
p~ =
a'j2.
f ex
(9)
Equation (9) represents the Planck state equation at f ex = 8
(ref. 1) and a state equation typical of the lattice models of fluids
at f ex = 1.24 According to (8), the value f ex = 8 is attained at
a finite slope of the f ex(n) curve (df ex/dn = 4 at n = 1),
whereas the exclusion factor approaches unity asymptotically
(at df ex/dn 0). Therefore, equation (9) with f ex = 1 can prove
to be practical within a sufficiently wide range of state parameters
of a condensed phase. As for the Planck gas equation with f ex = 8,
it exhibits its particular usefulness by producing a correct value
, 2003, 13(1), 1112
Then, state equation (6) takes the form
for not only the first (b1 = 1) but also the second virial coefficient (b2 = 4) in the dimensionless virial expansion
p~ =
i b j .
i
(10)
f ex = 8 kj,
where k is a constant, which restricts the range of possible
variation of j. The whole range from zero to unity is embraced
at k = 7. This is a minimally possible value for k. As for a
maximum value, it can be easily calculated from the virial expansion and is k = 34. Putting (10) in (6) yields the state equation
in the first approximation
j
dj
1 8j +kj
a'j2,
(11)
The result of integration in (11) depends on the value of k.
Equation (11) is written for k > 16 as
1
p~ =
arctan
kj 4
arctan
k 16
k 16
a'j2
(12)
k 16
for k < 16 as
1
p~ =
ln
2 16 k
kj 4 16 k
kj 4 + 16 k
ln
4 + 16 k
a'j2 (13)
4 16 k
and for k = 16 as
p~ =
1
1
a'j2.
4 16j
4
(14)
The smaller the k value, the wider the state interval included,
and the lower the accuracy of the state equation. In the gas
region at k = 34, equation (12) correctly yields not only the first
and second but also the third virial coefficient (b3 = 10). At
k < 64/3, a van der Waals loop appears in the isotherm, indicating the possibility of a first-order phase transition and a critical
state. However, at the minimum value k = 7, when equation (10)
comprises the whole region 0 j 1, resultant state equation
(13) is practical only until j = 1/7.
As for equation (14), this is nothing else but the van der
Waals equation. This unexpected result supplies the famous
equation with a quite new interpretation. Traditionally, the van
der Waals equation is regarded as an equation with a constant
excluded volume (referring to the zero approximation in our
classification), which van der Waals equated, by mistake, not to
the eightfold but to fourfold molecule volume (the mistake was
corrected by Planck1). However, the van der Waals equation
yields a correct value for the second virial coefficient, and, when
belonging to the zero approximation, this could be explained
only by an accidental compensation of two mistakes: an incorrect functional dependence and an incorrect value of the
excluded volume. In reality, as we now see, the van der Waals
equation refers not to the zero approximation but to the first
approximation of the theory of excluded volume. The excluded
volume is not constant in the van der Waals equation, but it
changes according to a certain linear law. As for the functional
form of the van der Waals equation, it corresponds to an exact
particular solution of the first approximation.
Proceeding to the second approximation, we replace the
linear behaviour of f ex expressed in (10) by the ratio of two
linear functions chosen so as to satisfy the initial value f ex = 8
and the initial slope f ex = 34:
f ex =
(1 + kj)dj
1 (8 k)j + (34 8k)j
a'j2.
(16)
Proceeding to the first approximation, we assume a linear
decrease of f ex as
p~ =
p~ =
8 (34 8k)j
.
1 + kj
As in the first approximation, the form of the solution
depends on the value of k. The simplest solution corresponds
to k = (136)1/2 8 3.6619. For this particular value of k,
equation (16) becomes
p~ = 1.239
1
1 + 0.778ln (1 2.169j) a'j2.
1 2.169j
We present equation (17) as a new precise fluid state equation.
It exhibits a high accuracy both in the gaseous region (yielding
correctly first three virial coefficients) and in the critical region
(yielding the ratio of the Boyle point temperature to the critical
temperature 2.74 against the experimental value 2.75). In
contrast to the Planck and van der Waals equations, equation
(17) is valid in a much wider range of states up to j = 0.461 (cf.
the maximally possible value j = 0.74 for the packing of hard
spheres).
To compare the above three approximations, Figure 1 shows
the f ex(j) functions (solid lines) within the applicability limits
of the corresponding state equations (dashed lines). Figure 1
also demonstrates the dependence of f ex on j calculated from
equation (6) for a system of hard spheres (a' = 0) using the
values of first ten virial coefficients known in the literature5
(line 4). It can be seen that line 3 corresponding to equation
(17) is very close to line 4.
As a concluding remark, the above relationships formulated
for a one-component system may be easily expanded to a system with many chemical species. Equation (6) is easily generalised to a multicomponent system by averaging the excluded
and molecular volumes with respect to various chemical species
present in the system (v ex xiviex and v0 xivi0 , where xi is
the mole fraction of the ith species). The resultant equation is
j
p~ =
dj
1 f
ex j
a' j j ,
i,k
ik
(18)
where j is the aggregate volume fraction of all species and
a'ik aikv0/kTvi0vk0 is the dimensionless image of the pair attraction constant aik for molecules of the ith and kth species, f ex
visually maintaining its original definition as vex/v0. Since the
integrals in (6) and (18) look similarly, only the repulsive term
should be replaced, as shown in (18), when passing to a multicomponent system.
This work was supported by the Russian Foundation for
Basic Research (grant nos. 00-15-97357 and 01-03-32334).
References
1
2
3
4
M. Planck, Sitzungsber. Preuss. Akad. Wiss., 1908, no. 32, 633.
H. N. V. Temperley, Proc. Phys. Soc. A, 1954, 67, 233.
R. Brout, Phase Transitions, Univ. Brussels, New York, 1965.
N. A. Smirnova and A. I. Viktorov, in Equation of State for Fluids and
Fluid Mixtures, eds. J. V. Sengers, R. F. Kayser, C. J. Peters and H. J.
White, Jr., Elsevier, Amsterdam, 2000, part 1, p. 255.
5 J. P. M. Trusler, in Equation of State for Fluids and Fluid Mixtures, eds.
J. V. Sengers, R. F. Kayser, C. J. Peters and H. J. White, Jr., Elsevier,
Amsterdam, 2000, part 1, p. 35.
(15)
Received: 29th November 2002; Com. 02/2018
12
(17)
Anda mungkin juga menyukai
- Wilson-Presentation-2008.Tools For Evaluation PDFDokumen20 halamanWilson-Presentation-2008.Tools For Evaluation PDFGeral TorrezBelum ada peringkat
- Python For Petroleum Data Analytics: SPE Two-Days Short CourseDokumen2 halamanPython For Petroleum Data Analytics: SPE Two-Days Short CourseFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Rod PMDokumen65 halamanRod PMMohamed HeibaBelum ada peringkat
- 1053 Tubing Performance1Dokumen22 halaman1053 Tubing Performance1Dani GarnidaBelum ada peringkat
- Dalhousie University - Importance of Perforation Process & Its TechniquesDokumen100 halamanDalhousie University - Importance of Perforation Process & Its TechniquesFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Abu Rudeis - Drilling EngineeringDokumen87 halamanAbu Rudeis - Drilling EngineeringJhon Fredy SanabriaBelum ada peringkat
- Ballast & Rig Stability Handbook (T, 163)Dokumen163 halamanBallast & Rig Stability Handbook (T, 163)bolerjeff86% (14)
- Complete Final Report - Web PDFDokumen506 halamanComplete Final Report - Web PDFFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Production Optimization: January 21, 2016Dokumen45 halamanProduction Optimization: January 21, 2016Farhad Ali SafiBelum ada peringkat
- Fattah 4Dokumen24 halamanFattah 4camelion3Belum ada peringkat
- Casio FX-9700GH Calculator ManualDokumen188 halamanCasio FX-9700GH Calculator ManualLuna StoneBelum ada peringkat
- Fattah 4Dokumen24 halamanFattah 4camelion3Belum ada peringkat
- Norsok Well IntegrityDokumen162 halamanNorsok Well IntegrityAshish SethiBelum ada peringkat
- Cash Rules Learn and Manage The 7 Cash Flow Drivers For Your Company SuccessDokumen218 halamanCash Rules Learn and Manage The 7 Cash Flow Drivers For Your Company SuccessmonicaescobarroaBelum ada peringkat
- General JPSE PDFDokumen8 halamanGeneral JPSE PDFFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Prediction of Pressure Gradients For Multiphase Flow in TubiDokumen11 halamanPrediction of Pressure Gradients For Multiphase Flow in TubiFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Teknica - Problem Well Analysis 2001Dokumen148 halamanTeknica - Problem Well Analysis 2001FernandoEnriqueCalveteGonzálezBelum ada peringkat
- Petroleum Experts - Brochure Brochure IPM Suite-Integrated Production Modelling PDFDokumen28 halamanPetroleum Experts - Brochure Brochure IPM Suite-Integrated Production Modelling PDFFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Production Optimization BUETDokumen43 halamanProduction Optimization BUETAngelo GaonaBelum ada peringkat
- ESP Handbook Guide to Electrical Submersible Pump Operation and MaintenanceDokumen67 halamanESP Handbook Guide to Electrical Submersible Pump Operation and MaintenanceFernandoEnriqueCalveteGonzález100% (1)
- Halliburton - Real Time Production Optimization & Reservoir Management PDFDokumen21 halamanHalliburton - Real Time Production Optimization & Reservoir Management PDFFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Fekete - Fekete F.a.S.T. Software BrochureDokumen40 halamanFekete - Fekete F.a.S.T. Software BrochureFernandoEnriqueCalveteGonzález100% (2)
- Electric Submersible Pumps (ESP) PDFDokumen31 halamanElectric Submersible Pumps (ESP) PDFFernandoEnriqueCalveteGonzález100% (2)
- Subsea Exercise No.5Dokumen16 halamanSubsea Exercise No.5rverretBelum ada peringkat
- P613 05A Lec 06 Rate Relations (050302)Dokumen46 halamanP613 05A Lec 06 Rate Relations (050302)FernandoEnriqueCalveteGonzálezBelum ada peringkat
- How A Well FlowsDokumen34 halamanHow A Well FlowsfddddddBelum ada peringkat
- James L. Hunt - Production-Systems Analysis For Fractured WellsDokumen7 halamanJames L. Hunt - Production-Systems Analysis For Fractured WellsFernandoEnriqueCalveteGonzálezBelum ada peringkat
- Production Optimization BUETDokumen43 halamanProduction Optimization BUETAngelo GaonaBelum ada peringkat
- Second Annoucement WS25Dokumen10 halamanSecond Annoucement WS25FernandoEnriqueCalveteGonzálezBelum ada peringkat
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- ANSYS Autodyn Composite ModelingDokumen70 halamanANSYS Autodyn Composite Modelinggustavo5150Belum ada peringkat
- Optical Fiber and Antenna Fundamentals QuizDokumen7 halamanOptical Fiber and Antenna Fundamentals Quizsadke213Belum ada peringkat
- Phasor Circuit Analysis: Phasor Diagrams, Voltage and Current DivisionDokumen8 halamanPhasor Circuit Analysis: Phasor Diagrams, Voltage and Current DivisionDavis LiangBelum ada peringkat
- Full Download Fundamentals of Physics Extended 10th Edition Halliday Solutions ManualDokumen35 halamanFull Download Fundamentals of Physics Extended 10th Edition Halliday Solutions Manualpsyche.brazedjp7100% (40)
- Introduction to Seismic Signal Processing and InterpretationDokumen70 halamanIntroduction to Seismic Signal Processing and InterpretationNamwangala Rashid NatinduBelum ada peringkat
- Correction Factors For ASME ANSI-OM3 Stress-Velocity RelationshipDokumen8 halamanCorrection Factors For ASME ANSI-OM3 Stress-Velocity RelationshipMPhamBelum ada peringkat
- Fishing Vessel Hull Design and Towing Resistance Calculation by The CFD MethodsDokumen4 halamanFishing Vessel Hull Design and Towing Resistance Calculation by The CFD MethodsshahjadaBelum ada peringkat
- M5 3-Unified09Dokumen14 halamanM5 3-Unified09Chandana KarumanchiBelum ada peringkat
- MODULE 1.1 What Is SoundDokumen3 halamanMODULE 1.1 What Is SoundgaryfinkBelum ada peringkat
- 4 Cellular AutomataDokumen91 halaman4 Cellular AutomataSayed ElSheikhBelum ada peringkat
- Control Tutorials For MATLAB and Simulink - Motor Speed - System ModelingDokumen6 halamanControl Tutorials For MATLAB and Simulink - Motor Speed - System Modelingdisposable505Belum ada peringkat
- Fundamentals of Thermodynamics Fundamentals of ThermodynamicsDokumen32 halamanFundamentals of Thermodynamics Fundamentals of ThermodynamicsYep IdidthisBelum ada peringkat
- Hydrogen Reduction Cobalt-Chromium Spinel Oxides. Stoichiometric Cobalt ChromiteDokumen6 halamanHydrogen Reduction Cobalt-Chromium Spinel Oxides. Stoichiometric Cobalt ChromiteDuongBelum ada peringkat
- Gradient of A Scalar Field and Its Geometrical InterpretationDokumen3 halamanGradient of A Scalar Field and Its Geometrical InterpretationMichael100% (1)
- Thermodynamic ProcessesDokumen13 halamanThermodynamic ProcessesS Jayasuriya100% (1)
- A Review of Philosophy of Arkān (Basic Constituents) in The Formation of Universe and Life in Contemporary EraDokumen11 halamanA Review of Philosophy of Arkān (Basic Constituents) in The Formation of Universe and Life in Contemporary Erawasim ahmedBelum ada peringkat
- O Level Physics Syllabus Only From 2023Dokumen21 halamanO Level Physics Syllabus Only From 2023Md SafwatBelum ada peringkat
- Evaporation Explained: Process, Examples & Importance in 40 CharactersDokumen5 halamanEvaporation Explained: Process, Examples & Importance in 40 CharactersCristy Mie PagliawanBelum ada peringkat
- Juice HeatersDokumen3 halamanJuice HeatersqqyukiBelum ada peringkat
- ECSS E HB 31 01 - Part14A Cryogenic CoolingDokumen545 halamanECSS E HB 31 01 - Part14A Cryogenic CoolingGuillermo MartínezBelum ada peringkat
- CHEM1901/3 Worksheet 6: Molecular Geometry Model 1: Oxidation NumbersDokumen4 halamanCHEM1901/3 Worksheet 6: Molecular Geometry Model 1: Oxidation Numbersdeckbyte865Belum ada peringkat
- Assignment 2 - ENCI 427 Timber Engineering Design of Pres-Lam FrameDokumen2 halamanAssignment 2 - ENCI 427 Timber Engineering Design of Pres-Lam FrameBa Thanh DinhBelum ada peringkat
- The Standard Model of Particle PhysicsDokumen7 halamanThe Standard Model of Particle PhysicsTrisha BowenBelum ada peringkat
- Online Review AnswersDokumen15 halamanOnline Review AnswersdebshistoryfairBelum ada peringkat
- ★★Electron RF Linacs for Industrial Applications - ICABU11 - 17 - 포스텍Dokumen34 halaman★★Electron RF Linacs for Industrial Applications - ICABU11 - 17 - 포스텍KoseokhoBelum ada peringkat
- Byk Pencil TestDokumen2 halamanByk Pencil TestRichard RivasBelum ada peringkat
- Abstract-This Experiment Is To Examine The Time DomainDokumen1 halamanAbstract-This Experiment Is To Examine The Time DomainSajjad AhmedBelum ada peringkat
- Fault AnalysisDokumen27 halamanFault AnalysisFaiz The EndBelum ada peringkat
- High Performance Swing Velocity Tracking Control of Hydraulic ExcavatorsDokumen6 halamanHigh Performance Swing Velocity Tracking Control of Hydraulic ExcavatorsJack SonBelum ada peringkat