The Neurology of Eclampsia: Some Observations: Views and Reviews
Diunggah oleh
Fajar Rudy QimindraJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
The Neurology of Eclampsia: Some Observations: Views and Reviews
Diunggah oleh
Fajar Rudy QimindraHak Cipta:
Format Tersedia
VIEWS AND REVIEWS
The Neurology of Eclampsia : Some Observations
A. Chakravarty, S.D. Chakrabarti*
Department of Neurology
Vivekananda Institute of Medical Sciences and Medical College*
Calcutta -700 006, India
Summary
Nineteen patients admitted with diagnosis of eclampsia in a large general hospital between
1996 - 1999, were analyzed. Eight patients were referred to neurologists for assessment and
management. All these patients had recurrent generalized seizures. Five patients developed
visual disturbance. Neuroimaging (CT and/or MRI) revealed symmetrical occipital lesions
in all. One patient had a large pontine lesion. Seizure control was achieved in all with
intravenous phenytoin. All patients recovered fully without any residual neurological
deficit and their radiological brain lesions resolved completely, in all except one case. The
neurological manifestations and neuroimaging features in cases of eclampsia have been
reviewed. A brief note on the pathogenesis of the cerebral lesions is included and the
controversial aspect of seizure control in eclampsia highlighted.
Key words : Eclampsia, Neurologic manifestations, Visual loss, Seizure control.
Neurol India, 2002; 50 : 128-135
Introduction
Pre-eclampsia is a complex disorder characterized by
pregnancy induced hypertension, proteinuria and
edema occurring after twenty weeks of pregnancy.
The clinical presentation varies in severity and
multiorgan involvement is common. The
manifestations may include pulmonary edema,
oliguria, disseminated intravascular coagulopathy and
hepatic hemorrhages. Neurologic manifestations of
pre-eclampsia include headache, confusion,
hyperreflexia, visual hallucinations and blindness.
Eclampsia has been defined as the occurrence of
convulsion, not caused by any coincidental neurologic
disease (e.g. epilepsy) in a woman whose condition
Correspondence to : Dr. A. Chakravarty, 59, Beadon Street,
Calcutta - 700 006, India.
E-mail : sdchakra@yahoo.com
Neurology India, 50, June 2002
also meets the criteria for pre-eclampsia.1 Preeclampsia, in effect, becomes eclampsia with the
advent of a seizure or coma and would be briefly
highlighted later. The cerebral lesions causing
neurologic features, including seizures, presumably
occur as a result of cerebral circulatory dysregulation
and neuropathologic studies support this conjecture.
To the best of the authors knowledge, no
comprehensive study on the neurological and
neuroradiological aspect of eclampsia has been
published in the current neurologic literature in India.
Only a solitary case report highlighting reversible MR
imaging features was reported in 1997.2 The present
report describes the principal authors (AC)
experience with eight patients of eclampsia,
highlighting clinical neurologic aspects, neuroradiological features and seizure control measures
adopted, along with a brief review of the subject.
128
Neurology of Eclampsia
Material and Methods
During the period of study, a total of 19 patients were
admitted with diagnosis of eclampsia fulfilling the
criteria mentioned earlier. Total obstetric admissions
in the hospital during the same period had been
20,273 and total confinements were 15,411. Of these,
eight cases were referred to the neurological services,
principally because of recurrent seizures, prolonged
alteration of sensorium, severe headache and visual
loss in varying combinations. The patients were
evaluated clinically several times till full recovery and
were later followed up in OPD. All but one had initial
CT scan of brain and three patients (including the one
who did not have CT) had MR brain imaging at the
initial stage. During follow-up, imaging studies were
repeated between three to six months of discharge.
Results
General : The age range of the subjects varied from 24
to 30 years. Six were primigravida and two second
gravida. None had prior history of hypertension, renal
disease, diabetes or seizure disorder. All patients had
been attending the antenatal clinic of the hospital and
none except one showed any clinical evidence of preeclampsia upto the time of last clinic visit (3 to 4
weeks prior to admission). All were subsequently
admitted with history of having had either a seizure or
altered sensorium. Blood pressure on admission was
over 140 mm Hg systolic and 100 mm Hg diastolic in
all, with pedal edema and proteinuria. One patient had
spontaneous expulsion of a dead fetus and seven
underwent emergency cesarean section with only
three live births.
Neurologic evaluation : All patients had generalized
tonic clonic recurrent seizures (3 or more over 24
hours). The seizures started prepartum in 5 and
postpartum in 3 cases. At initial neurologic evaluation,
all were at varying stages of altered sensorium (GCS
8-10) without any localizing sign but with
hyperreflexia and bilateral extensor plantars. Six
patients complained of severe holocranial headache at
some stage of their illness and five complained of
blurred vision either prior to losing consciousness or
after improvement in level of consciousness. The
visual problem in all appeared to be cortical in nature.
Fundus examination revealed retinal arterial spasm in
all eight patients. Four patients had diffuse retinal
edema but no hemorrhages. None had any meningeal
sign. Improvement in sensorium started 36-48 hours
after seizure control and all were fully conscious after
72 hours. Blurred vision persisted for 5-7 days after
admission in 5 patients who had this symptom.
129
Neurologic management : All patients were prescribed
methyldopa by their obstetricians for control of
hypertension and one needed intravenous infusion of
glyceryl trinitrate. Obstetricians also put all patients
on diazepam infusion (20-30 mg over 12 hrs.) but
seizures persisted inspite of this. No patient was given
magnesium sulphate. Seizure control in 8 patients
referred to the neurologists was achieved with
intravenous phenytoin, using a similar protocol as
practiced in status epilepticus but at a slower rate.
Phenytoin 15 mg/kg was given by infusion pump over
60 minutes and was followed by bolus injections of
100 mg intravenous every 6 hours for 48 hours and
thereafter oral therapy was instituted. No patient had
breakthrough seizures. In addition, intravenous
infusion of mannitol was given intermittently in the
first 48 hours. At the time of discharge (10-14 days
after admission), no patient had any residual
neurologic sign or symptom. Oral phenytoin was
continued for 3 months after discharge.
Neuroradiological evaluation : CT scan of brain was
done within 48 hours after admission in seven
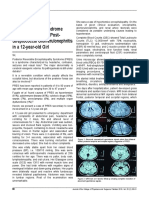
patients. All showed bilateral symmetrical occipital
hypodense lesions involving more of white matter
than the grey. In five patients, lesions extended to the
parieto-temporal areas of both sides (Fig. 1) but never
extended anteriorly beyond the sylvian fissure. No
lesions could be detected in the brain stem or in the
basal ganglionic regions. Areas of hyperdensity within
the hypodense areas (suggesting hemorrhages) were
seen in 3 patients. MR study could be done early in 3
cases only, including one case where CT was not
done. The lesions corroborated well with the CT
findings. Hypointense lesions on T1WI and
hyperintense lesions on T2WI and proton/flare
sequences were seen symmetrically in both occipital
regions with some extension in the parietotemporal
regions (Fig. 2). The lesion involved more of white
than grey matter. In one case, small hyperintense
lesions were seen on T2WI and proton density images
in posterior limb of internal capsule, both thalami and
subcortical white matter of right frontal lobe. The
pons also revealed hyperintense lesion on T2WI
(Fig. 3) and proton density images, with the larger
lesion in right paramedian aspect. The latter lesion
most likely represented an area of hemorrhage. MR
angiographic studies were not done in any patient.
Follow up neuroradiological evaluation : Seven
patients had follow up contrast CT scan of brain, 3-4
months after discharge. Neurologically, all were
normal with normal blood pressure, without any
medication. The repeat scan did not show any
evidence of the lesions detected earlier (Fig. 4). This
Neurology India, 50, June 2002
Chakravarty and Chakrabarti
Fig. 3 : MRI (T2WI) showing pontine lesion in patient SS.
Fig.1 : CT scan showing symmetrical hypodense lesions in
the parieto-occipital regions in an eclamptic patient.
completely resolved, a tiny area of hypointensity on
T1WI (Fig. 5) and hyperintensity in T2WI persisted in
the pons, suggesting presence of either a small infarct
or a posthemorrhagic gliotic area. She was clinically
asymptomatic and had no residual neurologic signs.
Discussion
Fig 2 : MR Imaging (T2WI) showing symmetrical occipital
hyperintense lesions (Patient SS).
suggested that the earlier hypodense lesions were
most likely areas of edema rather than infarction. The
hemorrhagic areas also resolved completely. The
single case with pontine lesion had a repeat MR scan
6 months later. While all the cerebral lesions
Neurology India, 50, June 2002
Pathogenesis and cerebral pathology : Preeclampsia/eclampsia is now considered to be
primarily a placental disorder. Both poor placentation
as well as hyper-placentosis (e.g. twins/molar
pregnancies) are associated with this condition.3 It is
believed that either an unknown factor (factor X, as
some call it) or villous debris from the malformed
placenta manage to reach the maternal circulation,
eliciting an immunological response from the
mothers system.4 Other variables like dietary factors,
namely protein and caloric deficiency, lack of
essential fatty acids, deficiency of magnesium,
calcium, zinc and an excess of sodium as well as
genetic factors like abnormal alleles for the genes of
TNF-, angiotensinogen, factor V and nitric oxide
synthase (NOS) predispose to this event.5-8 The
maternal response is primarily immunological with
increased activation and functional abnormalities of
maternal neutrophils, high circulating levels of the
cell-adhesion molecule VCAM-1 (which helps to
mediate neutrophil activity) and occurrence of
immune-complexes in the placenta, maternal serum
130
Neurology of Eclampsia
Fig. 4 : Resolution of CT lesions in follow up scan (patient
AD).
and various organs.9,10 The effect of this maternal
response is widespread cellular dysfunction with
multi-system involvement.11 There is endothelial
damage with increased capillary permeability, raised
fibronectin levels and electron microscopic evidence
of
damaged
endothelium.12,13
Coagulation
abnormalities occur, caused by increased activation
and consumption of platelets, low antithrombin III
levels, abnormal prostaglandin metabolism and in a
few cases, florid DIC.13,14 Endocrine anomalies
include activation of the renin-angiotensinaldosterone axis, abnormal catecholamines and
abnormalities in progesterone metabolism.13 These
pathophysiological changes lead to hypertension,
edema, proteinuria, bleeding tendencies, renal
dysfunction, liver damage and the neurological
abnormalities.
Neurological effects of pre-eclampsia/eclampsia can
be antepartum or post-partum, the latter being more
common.15 These are due to irregularities in the
autoregulation of cerebral circulation.16 Disruption of
the blood-brain barrier occurs due to both the
hypertension-induced capillary damage and the
immune-mediated endothelial dysfunction. This leads
to extravasation of red cells and plasma proteins into
perivascular space causing cerebral edema.17 Cerebral
vasospasm, produced by a combination of reaction to
hypertension, prostaglandin deficiency, defects in the
e-NOS gene (coding for nitric oxide systhase) and
endothelial damage, play an important role, producing
ischemia and infarction in the brain tissue.18-19 The
impaired blood coagulation system and the
131
Fig 5 : Follow up MR (T1WI) showing tiny residual pontine
lesion in patient SS.
abnormalities and deficiency of platelets predispose to
intra-cranial bleeds.20 Thus, a varied picture of
cerebral pathology, showing evidences of cerebral
edema, micro-infarcts, cortical petechiae and
pericapillary hemorrhages is observed in the brains of
patients with pre-eclampsia or eclampsia, which
clinically manifest as headache, visual disturbances,
confusion and seizures.
Neurologic features : The hallmark of eclampsia is
onset of seizures, which may be focal motor or
generalised tonic clonic. As indicated earlier, they
may appear before, during or after childbirth but
usually within first 24 hours postpartum. Delayed
onset of seizures has also been reported.21 In the
present series, however, seizure onset was more
prepartum (5 cases) than postpartum (3 cases). This
need not necessarily represent the general pattern as
only selected cases referred to neurologists were
included. All had generalised tonic clonic seizures.
None of the patients had any focal neurologic deficit
though all had bilateral symmetrical pyramidal signs.
Vision related symptoms are well recognised in
eclampsia.22 Retinal arterial dilatation, papilledema,
angiospasm, occlusion of central artery, hemorrhages,
exudates and edema - all have been described. While
all 8 cases in the present series had angiospam and 4
had retinal edema, none had papilledema,
hemorrhages or exudates on fundoscopy. The blurred
vision complained by 5 subjects was thought to be of
central origin and was corroborated with imaging
features of bilateral occipital involvement. Affection
of the posterior part of visual pathway has been well
recognized.22-26 The visual cortex was affected by
Neurology India, 50, June 2002
Chakravarty and Chakrabarti
edema, micro-infarctions and microhemorrhages in all
patients in the present series. Visual symptoms, of
course, normalised in all in a few days time.
Neuroimaging in severe pre-eclampsia/eclampsia :
The pathological features described earlier were
reflected in the neuroimaging features seen in the
present series. CT scan in eclampsia patients revealed
a wide spectrum of findings ranging from normal to
focal occipital or more widespread lesions which were
hypodense and non-enhancing and were ascribed to
localised edema.27 Repeat CT has shown partial or
complete resolution within 2 weeks. Cerebral
hemorrhage including subarachnoid hemorrhage has
also been described.28 A number of MR studies
confirm the presence of hyperintense lesions on T2WI
in eclampsia.29-31 Commonly, these have been found
in the posterior circulation territory with involvement
of cortical and subcortical white matter in occipital
and posterior parietal lobes. These lesions are
bilaterally symmetrical and follow the gyri in a
serpentine manner. Lesions have been described in the
deep white matter and basal ganglionic areas, which
are hyperintense in long TR images but occasionally
also in T1WI, suggesting microhemorrhages. Almost
all such lesions were transient, suggesting the
development of edema due to failure of autoregulatory
mechanism or transient vasospasm. Reversible
cerebral vasospasm by MR angiography has recently
been described in pre-eclampsia.32 On the other hand,
cerebral blood flow measured by SPECT in one
patient during an attack of visual disturbance showed
increased blood flow in the occipital cortex.33 The
combination of vasodilatation and vasospams in the
pathogenesis of ecalmpsia has already been
commented upon in the section on pathogenesis.
CT and MRI findings in the present series
corroborated with those described earlier. On CT
scanning, the lesions never extended anteriorly, but
frontal white matter lesions could be detected on MRI.
Similarly, central grey matter lesions were more
apparent on MRI than on CT scan. The observation of
pontine lesion on MRI in one subject, though she
never developed any classical pontine clinical
syndrome, was interesting. Such brain stem lesions
have not been highlighted in earlier studies. This was
also the only case in the present series where a tiny
residual abnormality presisted on repeat scanning
after 6 monhts. MRI, indeed, gives more accurate
assessment of the degree of CNS involvement in
eclampsia.
Seizure control in eclampsia : All eight patients in the
Neurology India, 50, June 2002
present series had been treated with intravenous
phenytoin (PHT) with initial loading dosage, followed
by intermittent intravenous bolus doses over next 48
hours. No doubt, some eyebrows would be raised in
this regard especially among obstetric colleagues.
Although five of the eight patients in this series had
antepartum onset of seizure, by the time they were
seen by the neurologists, one already had spontaneous
expulsion of the fetus and the rest 7 had emergency
cesarean section done. Attempt at initial seizure
control was made by the obstetricians with
intravenous diazepam and no patients received
magnesium sulfate (MgSO4). PHT was resorted to
later after neurologic referral, either for control of
ongoing seizure or prevention of further seizure. PHT
proved to be successful in this regard and no maternal
complications in the postpartum period could be
attributed to PHT therapy. There is a lot of controversy
in the literature and in medical practice about the
choice of anticonvulsants in eclampsia. An ideal
anticonvulsant should have good seizure control,
should not affect uterine function, should lower
overall maternal morbidity and mortality and should
not affect fetal morbidity or mortality, if used
antepartum. It is also important to consider whether
the drug has been used during the stage of preeclamspia to prevent onset of seizures or during
eclamptic phase to control seizures. These issues need
to be carefully judged and remembered while
examining the results of several trials done in recent
times. Rapid control of a single seizure episode can
certainly be achieved with a bolus dose of diazepam.
The efficacy of intravenous infusion in preventing
seizure recurrence is doubtful and repeated bolus
injections not usually practiced in pregnancy for its
possible adverse effects on the fetus e.g. hypotonia,
hypothermia, apnea etc.34 The need, therefore, is for a
longer acting drug and MgSO4 and PHT seem to be
the main contenders.
Most obstetricians all over the world probably still use
MgSO4 both in pre-eclampsia to prevent seizures and
in eclampsia to control and prevent recurrence of
seizures. Mechanism of action is not clearly known.
This may involve blockade of NMDA receptors
involved in seizure genesis35 or calcium channel
blocking, preventing cerebral vasospasm.36 MgSO4,
in experimental seizure models, does not appear to
have a very significant anticonvulsant action37,38 and
certainly is not a standard anticonvulsant for treatment
of epileptic seizures. A closer look at available recent
literature would however suggest, that more emphasis
has been given to prevention of seizures in pre-
132
Neurology of Eclampsia
eclampsia, with MgSO4, than actual rapid seizure
control and preventation of recurrent seizures. There
are also problems associated with MgSO4 including
local irritant effect, loss of patellar reflex (a common
sign used by many obstericians in this country to
check for toxicity), lethargy and depression in mother
and newborn and recurrence/refractoriness of seizures
in many.39,40 From neurologists stand point, rapid
control of seizures and preventing its recurrence needs
to be achieved with a time tested proper
anticonvulsant e.g. phenytoin. This view is gradually
being shared by obstetricians as well. The efficacy of
PHT in controlling eclamptic seizure was probably
first reported by Slater et al in 198741 and
subsequently several reports were published in the
first half of 1990s.42 Some early reports on breakthrough seizures were probably related to low
therapeutic level achieved. The dosage schedule is
similar to that used in status epilepticus, but given at a
slower rate, preferably with checking of serum levels
from time to time (difficult to perform in our setting
and not done in any case in the present series). Other
dosage regimes have also been suggested. A single
high dose intravenous PHT (900 mg) had been used
with success in patients with eclampsia, given on
admission, with no untoward effects in the mother and
the neonate.43 A study in a premier obstetric hospital
in U.K. compared different loading doses of PHT and
preferred a dosage of 15 mg/kg and stressed the need
to follow it up with boosting doses to maintain
therapeutic plasma level.44 Occasionally, however,
seizures recurred even with serum PHT level well
within the therapeutic range.
Quite a few controlled trials in recent times have tried
to establish the efficacy MgSO4 over PHT. Lucas et
al45 randomised over 1000 patients with pregnancy
induced hypertension and pre-eclampsia to receive
either drug and concluded that MgSO4 is superior to
PHT in preventing eclampsia (the two groups being
comparable regarding the risk). Naidu et al46 used
transcranial doppler (TCD) to assess degree of
cerebral vasospasm in eclamptic patients receiving
either MgSO4 or PHT. MgSO4 significantly reduced
the pulsatality index and mean flow velocity
compared to PHT in the middle cerebral artery and
thus appeared to be a better drug to reverse cerebral
vasospasm, a major factor implicated in the
pathogenesis of eclampsia. Chien et al47 tried to
establish the efficacy of MgSO4 from retrospective
analysis of randomised trials through online search of
Medline database between 1966-1995. 1743 patients
133
with pre-eclampsia included in nine randomised trials
were assessed for seizure activity and maternal death.
On both the scores, MgSO4 was favoured as compared
to PHT and the authors concluded MgSO4 to be a
superior drug compared to PHT in preventing seizures
in eclampsia and seizure prophylaxis in preeclampsia. A recent Indian study48 randomly
allocated 50 pregnant women with eclampsia to
receive either MgSO4 or PHT. Women treated with
PHT had a higher incidence of recurrent seizures but
perinatal mortality and morbidity and maternal
mortality were comparable in both groups. The most
recent data is available from the Cochrane database
system review.49 Four well controlled trials
encompassing 823 women were included. MgSO4 (as
compared with PHT) was associated with a substantial
reduction of recurrence of seizures, a favourable trend
in maternal mortality, reduction in risk of pneumonia,
ventilation and admission in ICU of mother and
admission of babies in special care units and reduction
in perinatal mortality. All these favoured use of
MgSO4 compared to PHT.
A close look at the forementioned trials would bring
forth a few important points. In the eye of a
neurologist, PHT is a proper well-tested anticonvulsant and not MgSO4. But the latter appears to
have some disease modifying effect in the setting of
toxemia of pregnancy. Hence, in a patient admitted
with seizures, ideal treatment would be control of the
seizures first using a loading dose of proper
anticonvulsant like PHT and supplementary therapy
with MgSO4 may provide added benefit in preventing
recurrence of seizures and inproving maternal and
perinatal mortality or morbidity. On the other hand, in
patients admitted with severe pregnancy induced
hypertension and pre-eclampsia, MgSO4 would
undoubtedly be the drug of choice for preventing
onset of seizures and reducing morbidity and
mortality. In the present series, PHT was used in all
patients, as all presented with recurrent seizures and
rapid seizure control deemed mandatory.
Furthermore, as neurologists, we were more
accustomed to use PHT than MgSO4. No clear
guideline is given anywhere for the duration of
anticonvulsant therapy in eclampsia. In the present
series, oral anticonvulsants were continued for 3
months postpartum, based only on the observation
that the radiological resolution of the CNS lesions
(discussed earlier) occured in about 3 months. It is felt
that management of eclampsia and severe preeclampsia must be made through close liaison
between the obstetricians and the neurologists.
Neurology India, 50, June 2002
Chakravarty and Chakrabarti
References
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
17.
18.
19.
American College of Obstetricians and Gynaecologists
management of pre-eclampsia. Technical Bulletin number
91, Washington DC. Feb 1991.
Bannerjee S, Chakravarty A : Reversible brain lesions on
MR imaging in a patient with eclampsia. Journal of
Association of Neuroscientists of Eastern India 1997; 2 :
146-152.
Roberts JM, Redman CWG : Pre-eclampsia - more than
pregnancy induced hypertension. Lancet 1993; 341 : 14471451.
Cockell AP, Learmont JG, Smaradon AK et al : Human
placental syncytiotrophoblast microvillous membranes
impair maternal vascular endothelial function. Br J Obstet
Gynaecol 1997; 104 : 235- 240.
Chen G, Wilson R, Mackillop JH et al : Tumor-necrosis-factor
alpha (TNF-) gene polymorphism and expression in preeclampsia. Clin Exp Immunol 1996; 104 : 154-159.
Arngrimsson R, Purandare S, Connor M et al :
Angiotesinogena candidate gene involved in pre-eclampsia.
Nat Genet 1993; 4 : 114-115.
Dizatownson DS, Nelson LM, Easton K et al : The factor V
Leiden mutation may predispose women to severe preeclampsia. Am J Obstet Gynecol 1996; 175 : 902-905.
Arngrimsson R, Hayward C, Naudad S et al : Evidence for a
familial pregnancy induced hypertension locus in the e-NOSgene region. Am J Hum Genet 1997; 61 : 354-362.
Sibai BM : Immunologic aspects of pre-eclampsia. Clin
Obstet Gynecol 1991; 34 : 27.
Lyall F, Greer IA, Boswell F, et al : The cell-adhesion
molecule VCAM-1 is selectively elevated in serum in preeclampsia-does this indicate mechanism of leukocyte
activation ? Br J Obstet Gynecol 1994; 101 : 485-487.
Wisdom SJ, Wilson R, Mckillop JH et al : Antioxidant
systems in normal pregnancy and in pregnancy induced
hhypertension. Am J Obstet Gynecol 1991; 165 : 1701-1704.
Roberts JM, Hubel CA, Taylor RN : Endothelial dysfunction
yes, cytotoxicity no. Am J Obstet Gynaecol 1995; 173 : 978979.
Shibai BM : Eclampsia. In : Neurological disorders of
pregnancy. Goldstein PJ, Stern BJ (eds). Futura Publishing
Co Mount kisco, New York. 1992; P1-24.
Mckay DG : Chronic intravascular coagulation in normal
pregnancy and pre-eclampsia. Contrib Nephrol 1981; 25 :
108.
Leitch CR, Cameron AD, Walker JJ : The changing pattern
of eclampsia over a 60 years period. Br J Obstet Gynecol
1997; 104 : 917- 922.
Strandgaard S : The lower and upper limits for
autoregulation of cerebral blood flow. Stroke 1973; 4 : 323.
Benedetti TJ, Quilligan EJ : Cerebral edema in severe
pregnancy- induced hypertension. Am J Obstet Gynecol
1980; 137 : 860.
Will AD, Lewis KL, Hinshaw DB et al : Cerebral
vasoconstriction in toxaemia. Neurology 1987; 37 : 15551557.
Qurashi AI, Frankel MR, Ottenlips JR et al : Cerebral
haemodynamics in pre-eclampsia and eclampsia. Arch
Neurol 1996; 53 : 1226-1231.
Neurology India, 50, June 2002
20. Gant NF. Daley GL, Chand S et al : A study of angiotensin II.
Pressure response throughout primigravida pregnancy.
J Clin Invest 1973; 52 : 2682.
21. Sibai BM, Schnneider JM, Morrison JC et al : The late postpartum eclampsia controversy. Obstet Gynaecol 1980; 55 :
75-78.
22. Donaldson JO : Eclampsia. In : Neurology of pregnancy. WB
Saunders. Philadelphia. 1989; 269-310.
23. Gyr T, Tamzin MS, Zimmerli W : Postpartum amaurosis in
patients with pre-eclampsia. Z Geburzhilfe. Perinatal 1983;
187 : 293-295.
24. Hauswald M : Cortical blindness and late postpartum
eclampsia. Am J Emerg Med 1987; 5 : 130-132.
25. Beal MF, Chapman PH : Cortical blindness and
homonymous hemianopia in postpartum period. JAMA 1980;
244 : 2085-2087.
26. Arulkumaran S, Gibb DMF, Rouff M et al : Transient
blindness associated with pregnancy induced hypertension.
Br J Obstet Gynaecol 1985; 92 : 847-849.
27. Beeson JH, Dudo EE : Computed axial tomography scan
demonstration of cerebral oedema in eclampsia preceded by
blindness. Obstet Gynecol 1982; 60 : 529-532.
28. Becl DW, Menezes AH : Intracerebral haemorrhage in a
patient with eclampsia. JAMA 1981; 246 : 1442-1443.
29. Raroque HG, Orrison WW, Rosenberg GA : Neurologic
involvement in toxaemia of pregnancy; Reversible MRI
lesions. Neurology 1990; 40 : 167-169.
30. Manfredi M : Reversibility of brain lesions in eclampsia.
J Neurol Neurosurg Psychiatry 1997; 62 (1) : 9.
31. Felz MW, Barnes DB, Figueroa RE : Late postpartum
eclampsia 16 days after delivery : case report with clinical,
radiological and pathophysiologic correlations. J Am Board
Fam Pract 2000; 13 (1) : 39-46.
32. Ito T, Sakai T, Inagawa S et al : MR Angiography of cerebral
vasospasm in pre-eclampsia. AJNR 1995; 16 : 1344-1346.
33. Akutsu T, Sakai F, Hata T et al : Neurological and
neuroimaging studies of eclampsia. Rinsho Shinkeigaku
1992; 32 : 701-707.
34. Cree JE, Meyer J, Hailey DM : Diazepam in labour. Its
metabolism and effect on the clinical condition and
thermogenesis of the newborn. BMJ 1973; 4 : 251-255.
35. Coan EJ, Collingridge GL : Magnesium ions block an Nmethyl-D-Aspartate receptor mediated component of
synaptic transmission in rat hippocampus. Neurosci Lett
1985; 53 : 21-26.
36. Will AD, Lewis KL, Hinshaw DB et al : Cerebral
vasoconstriction in toxaemia. Neurology 1987; 32 : 15551557.
37. Koontz WL, Reid KH : Effect of parenteral magnesium
sulfate on penicillin-induced seizure foci in anaesthesided
cats. Am J Obstet Gynecol 1985; 153 : 96.
38. Standley CA, Irtenkauf SM, Steivart L et al : Magenesium
sulfate versus phenytoin for seizure prevention in amygdalakindled rats. Am J Obstet Gynecol 1994; 171 : 948-951.
39. Lipsitz PJ : The clinical and biochemical effects of excess
magnesium in the newborn. Paediatrics 1971; 47 : 501-509.
40. Dunn R, Lee W, Cotton DB : Evaluation by computerised
axial tomography of eclamptic women with seizure refractory
to magnesium sulfate therapy. Am J Obstet Gynecol 1986;
155 : 267.
134
Neurology of Eclampsia
41. Slater RM, Wilcox FL, Smith WD et al : Phenytoin infusion in
severe pre-eclampsia. Lancet 1987; 1 : 1417-1421.
42. Sibai BM, Eclampsia VI : Maternal - Perinatal outcome in
254 consecutive cases. Am J Obstet Gynecol 1990; 163 :
1049-1055.
43. Coyaji KJ, Otiv SR : Single high dose or intravenous
phenytoin sodium for the treatment of eclampsia. Acta
Obstet Gynecol Scand 1990; 69 : 115-118.
44. Robson SC, Redfern N, Seviour J et al : Phenytoin
prophylaxis in severe pre-eclampsia and eclampsia. Br J
Obstet Gynecol 1993; 100 : 623-628.
45. Lucas MJ, Leveno KJ, Cunninghom FG : A comparison of
magnesium sulfate with phenytoin for the prevention of
eclampsia. N Engl J Med 1995; 333 : 201-205.
46. Naidu S, Payne AJ, Moodley J et al : Randomised trial
assessing the effect on phenytoin and magnesium sulfate on
maternal cerebral circulation in eclampsia using transcranial
doppler ultrasound. Br J Obstet Gynecol 1996; 103 : 111116.
47. Chien PF, Khan KS, Arnott N : Magnesium sulfate in the
treatment of eclampsia and pre-eclampsia : an overview of
the evidence from randomised trials. Br J Obstet Gynaeco
1996; 103 : 1085-1091.
48. Sawhney H, Sawhney IM, Mandal R et al : Efficacy of
magnesium sulfate and phenytoin in the management of
eclampsia. J Obstet Gynecol Res 1999; 25 : 333-338.
49. Duley L, Henderson - Smart D. Magnesium sulfate versus
phenytoin for eclampsia. Cochrane Database Syst Rev
(CD). 2000; 2 : 128.
Accepted for publication : 13th September, 2000.
VISIT
NEUROLOGY INDIA
at
http://www.neurologyindia.com
Features :
!"
Latest issue
Complete contents
Abstracts
Full Text of Articles in pdf format
!"
Archives
Since Vol.45, No.1, March 1997
Complete contents
Abstracts of all articles
Since Vol.47, No.1, March 1999
Full Text of Articles
!"
Search available
On the name of authors
Keywords
Subject headings
135
!"
Feed Back
!"
Editorial Board
!"
Executive committee N.S.I.
For direct message to Editor
Neurology India, 50, June 2002
Anda mungkin juga menyukai
- Anti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaDokumen6 halamanAnti-N-methyl-D-aspartate Receptor (NMDAR) Encephalitis: A Series of Ten Cases From A University Hospital in MalaysiaAbhishek KayalBelum ada peringkat
- The NORSE (New-Onset Refractory Status Epileptic Us) SyndromeDokumen4 halamanThe NORSE (New-Onset Refractory Status Epileptic Us) Syndromebenghooi75Belum ada peringkat
- Tumefactive Demyelinating Lesions: Nine Cases and A Review of The LiteratureDokumen9 halamanTumefactive Demyelinating Lesions: Nine Cases and A Review of The LiteraturePS NeuroBelum ada peringkat
- Acute and Long-Term Neurological Complications in Children With Acute Lymphoblastic LeukemiaDokumen4 halamanAcute and Long-Term Neurological Complications in Children With Acute Lymphoblastic LeukemiahawinnurdianaBelum ada peringkat
- 2009 8 Focus09158Dokumen5 halaman2009 8 Focus09158ninostranoBelum ada peringkat
- Clinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945Dokumen13 halamanClinical Reasoning: A Young Man With Recurrent Paralysis, Revisable White Matter Lesions and Peripheral Neuropathy Word Count: 945aileenzhongBelum ada peringkat
- Evolution of Neurological, Neuropsychological and Sleep-Wake Disturbances After Paramedian Thalamic StrokeDokumen8 halamanEvolution of Neurological, Neuropsychological and Sleep-Wake Disturbances After Paramedian Thalamic Strokepokharelriwaj82Belum ada peringkat
- P ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Dokumen6 halamanP ('t':3) Var B Location Settimeout (Function (If (Typeof Window - Iframe 'Undefined') (B.href B.href ) ), 15000)Riri KumalaBelum ada peringkat
- Posterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsDokumen7 halamanPosterior Reversible Encephalopathy Syndrome (PRES) : Electroencephalographic Findings and Seizure PatternsJasper CubiasBelum ada peringkat
- The Syndrome of Normal-Pressure Hydrocephalus VassilioutisDokumen9 halamanThe Syndrome of Normal-Pressure Hydrocephalus VassilioutisPablo Sousa CasasnovasBelum ada peringkat
- Warumpornlimuntachai Medical Student in Emergency Department, Ramathibodi Hospital, Bangkok, ThailandDokumen13 halamanWarumpornlimuntachai Medical Student in Emergency Department, Ramathibodi Hospital, Bangkok, ThailandAanam FathimaBelum ada peringkat
- NCC Case StudyDokumen4 halamanNCC Case StudyMasego MekgoeBelum ada peringkat
- Case Study StrokeDokumen5 halamanCase Study StrokelilutzuBelum ada peringkat
- Sturge-Weber Syndrome. Study of 55 PatientsDokumen7 halamanSturge-Weber Syndrome. Study of 55 PatientsdzhzrnBelum ada peringkat
- Vestibular Neuritis Affects Both Superior and Inferior Vestibular NervesDokumen10 halamanVestibular Neuritis Affects Both Superior and Inferior Vestibular NervesSabina BădilăBelum ada peringkat
- BMC NeurologyDokumen8 halamanBMC NeurologyTarun MathurBelum ada peringkat
- 104.full AdemDokumen6 halaman104.full AdemayubahriBelum ada peringkat
- Idiopathic Intracranial Hypertension: A Retrospective Clinical StudyDokumen4 halamanIdiopathic Intracranial Hypertension: A Retrospective Clinical StudyNouchan NoupBelum ada peringkat
- TB Meningitis Case Report: Isolated Oculomotor Nerve PalsyDokumen3 halamanTB Meningitis Case Report: Isolated Oculomotor Nerve PalsyRakasiwi GalihBelum ada peringkat
- Long-Term Outcomes After Surgical Treatment of Pediatric Neurogenic Thoracic Outlet SyndromeDokumen11 halamanLong-Term Outcomes After Surgical Treatment of Pediatric Neurogenic Thoracic Outlet Syndromewalter fire 0057Belum ada peringkat
- Posterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlDokumen2 halamanPosterior Reversible Encephalopathy Syndrome Secondary To Acute Post-Streptococcal Glomerulonephritis in A 12-Year-Old GirlwawaningBelum ada peringkat
- Spectrum of Neurological Manifestations in Scorpion StingDokumen6 halamanSpectrum of Neurological Manifestations in Scorpion Stingiaset123Belum ada peringkat
- Clinical Neurology and Neurosurgery: E. Ferrante, F. Rubino, M. Mongelli, I. ArpinoDokumen4 halamanClinical Neurology and Neurosurgery: E. Ferrante, F. Rubino, M. Mongelli, I. ArpinoMikael AngelooBelum ada peringkat
- Basal Gangila CahngesDokumen12 halamanBasal Gangila CahngesJakkani RavikanthBelum ada peringkat
- Acute Multifocal Placoid Pigment EpitheliopathyDokumen8 halamanAcute Multifocal Placoid Pigment EpitheliopathyremotmBelum ada peringkat
- Epilepsy and Brain Abscess: Christine Kilpatrick MD FRACPDokumen3 halamanEpilepsy and Brain Abscess: Christine Kilpatrick MD FRACPnisaBelum ada peringkat
- Clinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old ManDokumen8 halamanClinical Reasoning: Rare Cause of Hemiparesis and Ataxia in A 36-Year-Old MansamuelBelum ada peringkat
- Dysphagia in Lateral Medullary Infarction (Wallenberg's Syndrome)Dokumen8 halamanDysphagia in Lateral Medullary Infarction (Wallenberg's Syndrome)Dani PiñaBelum ada peringkat
- CLL Case PresentationDokumen17 halamanCLL Case PresentationAhmed EshraBelum ada peringkat
- Paediatric Tolosa-Hunt Syndrome: The Need For Treatment Guidelines and Renewed CriteriaDokumen2 halamanPaediatric Tolosa-Hunt Syndrome: The Need For Treatment Guidelines and Renewed CriteriaRakasiwi GalihBelum ada peringkat
- (Journal of Neurosurgery) Pathophysiology of Long-Standing Overt Ventriculomegaly in Adults PDFDokumen8 halaman(Journal of Neurosurgery) Pathophysiology of Long-Standing Overt Ventriculomegaly in Adults PDFKusumaningrum WijayantiBelum ada peringkat
- Idiopathic First Seizure in Adult Life: Who Be Treated?: ShouldDokumen4 halamanIdiopathic First Seizure in Adult Life: Who Be Treated?: ShouldJessica HueichiBelum ada peringkat
- Tratamiento Quirúrgico Efectivo de Las Malformaciones Cavernosas Cerebrales - Un Estudio Multicéntrico de 79 Pacientes PediátricosDokumen4 halamanTratamiento Quirúrgico Efectivo de Las Malformaciones Cavernosas Cerebrales - Un Estudio Multicéntrico de 79 Pacientes PediátricosGonzalo Leonidas Rojas DelgadoBelum ada peringkat
- Rare Spinal InfectionDokumen3 halamanRare Spinal InfectionAlam MartadipuraBelum ada peringkat
- D Evaluation For Resective Epilepsy SurgeryDokumen104 halamanD Evaluation For Resective Epilepsy SurgeryYusuf BrilliantBelum ada peringkat
- Proceedings of The 2014 Spring Meeting of The Society of British 2014Dokumen40 halamanProceedings of The 2014 Spring Meeting of The Society of British 2014rabiatul adawiyahBelum ada peringkat
- Posterior Reversible Encephalopathy Syndrome (Pres) in A Patient With Late Postpartum Eclampsia: Case ReportDokumen4 halamanPosterior Reversible Encephalopathy Syndrome (Pres) in A Patient With Late Postpartum Eclampsia: Case ReportIJAR JOURNALBelum ada peringkat
- Pan 1Dokumen3 halamanPan 1Mithun CbBelum ada peringkat
- Decompressive Hemicraniectomy and DuroplastyDokumen5 halamanDecompressive Hemicraniectomy and DuroplastyAmy NilifdaBelum ada peringkat
- Cost of BC CareDokumen7 halamanCost of BC CareErwin Chiquete, MD, PhDBelum ada peringkat
- Clinical Outcomes of Neonatal Hypoxic Ischemic Encephalopathy Evaluated With Diffusion-Weighted Magnetic Resonance ImagingDokumen6 halamanClinical Outcomes of Neonatal Hypoxic Ischemic Encephalopathy Evaluated With Diffusion-Weighted Magnetic Resonance ImagingDrsandy SandyBelum ada peringkat
- Hemimegalencephaly: A Study of Abnormalities Occurring Outside The Involved HemisphereDokumen5 halamanHemimegalencephaly: A Study of Abnormalities Occurring Outside The Involved Hemisphereapi-148100258Belum ada peringkat
- Neurol J Southeast Asia 1999 4: 83 - 88: LCS Tan, YY Sitoh, HTL TjiaDokumen6 halamanNeurol J Southeast Asia 1999 4: 83 - 88: LCS Tan, YY Sitoh, HTL Tjiarani suwadjiBelum ada peringkat
- Psychiatry Aod Neurological Sciences: Clinical Picture, CT Findings and PrognosisDokumen5 halamanPsychiatry Aod Neurological Sciences: Clinical Picture, CT Findings and PrognosisdamarispaliBelum ada peringkat
- Clinical and Imaging Outcomes After Stroke With Normal AngiogramsDokumen4 halamanClinical and Imaging Outcomes After Stroke With Normal AngiogramsAgustina 'tina' HartutiBelum ada peringkat
- MRI SeizuresDokumen7 halamanMRI SeizuresMarco MalagaBelum ada peringkat
- Seronegative Myasthenia Gravis Presenting With PneumoniaDokumen4 halamanSeronegative Myasthenia Gravis Presenting With PneumoniaJ. Ruben HermannBelum ada peringkat
- Acute Necrotizing Encephalopathy of Childhood: Correlation of MR Findings and Clinical OutcomeDokumen5 halamanAcute Necrotizing Encephalopathy of Childhood: Correlation of MR Findings and Clinical OutcomeayubahriBelum ada peringkat
- PNH Classification Links Anatomy to Epilepsy OutcomesDokumen12 halamanPNH Classification Links Anatomy to Epilepsy OutcomesJuan D. HoyosBelum ada peringkat
- Thrombolysis With Alteplase 3 To 4.5 Hours After Acute Ischemic StrokeDokumen13 halamanThrombolysis With Alteplase 3 To 4.5 Hours After Acute Ischemic StrokeParvathy R NairBelum ada peringkat
- 4 2010 5 b0c82248Dokumen4 halaman4 2010 5 b0c82248Sashi MehBelum ada peringkat
- Idiopathic Intracranial Hypertension (By Dr. Rakshay)Dokumen51 halamanIdiopathic Intracranial Hypertension (By Dr. Rakshay)Rakshay KaulBelum ada peringkat
- Electrocorticographic Monitoring As An Alternative Tool For The Pre-Surgical Evaluation of Patients With Bi-Temporal EpilepsyDokumen7 halamanElectrocorticographic Monitoring As An Alternative Tool For The Pre-Surgical Evaluation of Patients With Bi-Temporal Epilepsydavidmy227464Belum ada peringkat
- Nor (2018) Ischaemic Haemorrhagic Stroke in A ChildDokumen3 halamanNor (2018) Ischaemic Haemorrhagic Stroke in A ChildFelicia HarsonoBelum ada peringkat
- Rabin Stein 2010Dokumen6 halamanRabin Stein 2010luis fermin paredesBelum ada peringkat
- MRI aids diagnosis of tuberculous meningitisDokumen6 halamanMRI aids diagnosis of tuberculous meningitisyayaBelum ada peringkat
- Resultados de Callosotomía en Adultos Con Diagnóstico de Epilepsia Refractaria y Drop-AttackDokumen7 halamanResultados de Callosotomía en Adultos Con Diagnóstico de Epilepsia Refractaria y Drop-AttackIsa PsicogBelum ada peringkat
- Medicine: Refractory Hyponatremia in Neuromyelitis Optica in A Pediatric PatientDokumen4 halamanMedicine: Refractory Hyponatremia in Neuromyelitis Optica in A Pediatric PatientMesan KoBelum ada peringkat
- Cerebral Herniation Syndromes and Intracranial HypertensionDari EverandCerebral Herniation Syndromes and Intracranial HypertensionMatthew KoenigBelum ada peringkat
- Ovid - Virology, Immunology and Pathology of Human Rabies During TreatmentDokumen10 halamanOvid - Virology, Immunology and Pathology of Human Rabies During TreatmentFajar Rudy QimindraBelum ada peringkat
- 6.3 Area Under Any Normal Curve: Normal Curves and Sampling DistributionsDokumen15 halaman6.3 Area Under Any Normal Curve: Normal Curves and Sampling DistributionsFajar Rudy QimindraBelum ada peringkat
- MRS NotesDokumen9 halamanMRS NotesFajar Rudy QimindraBelum ada peringkat
- Cognitive HivDokumen14 halamanCognitive HivFajar Rudy QimindraBelum ada peringkat
- Steyerberg Prediction Modeling 7 Steps Jan10Dokumen45 halamanSteyerberg Prediction Modeling 7 Steps Jan10Fajar Rudy QimindraBelum ada peringkat
- B4 HAND DefinitionDokumen13 halamanB4 HAND DefinitionFajar Rudy QimindraBelum ada peringkat
- NIH Public AccessDokumen12 halamanNIH Public AccessFajar Rudy QimindraBelum ada peringkat
- HIV-associated Neurocognitive Disorders: Review Open AccessDokumen10 halamanHIV-associated Neurocognitive Disorders: Review Open AccessFajar Rudy QimindraBelum ada peringkat
- Penelitian DR Lilik - JoiDokumen16 halamanPenelitian DR Lilik - JoiFajar Rudy QimindraBelum ada peringkat
- S Pss Moving From ExcelDokumen2 halamanS Pss Moving From ExcelAnggah KhanBelum ada peringkat
- Arv HivDokumen5 halamanArv HivFajar Rudy QimindraBelum ada peringkat
- Word Academician Thesis 1.1Dokumen30 halamanWord Academician Thesis 1.1khairul_aBelum ada peringkat
- PDF Callosal Disconnection J StrokeDokumen5 halamanPDF Callosal Disconnection J StrokeFajar Rudy QimindraBelum ada peringkat
- Neurogenic Pulmonary EdemaDokumen12 halamanNeurogenic Pulmonary EdemaFajar Rudy QimindraBelum ada peringkat
- Vancouver Style PDFDokumen25 halamanVancouver Style PDFwewwewBelum ada peringkat
- Aphasia TableDokumen2 halamanAphasia TableFajar Rudy QimindraBelum ada peringkat
- Journal Pone HivDokumen9 halamanJournal Pone HivFajar Rudy QimindraBelum ada peringkat
- Revue Neurologique Volume 169 Issue 10 2013 (Doi 10.1016/j.neurol.2013.07.022) Debette, S. - Vascular Risk Factors and Cognitive DisordersDokumen8 halamanRevue Neurologique Volume 169 Issue 10 2013 (Doi 10.1016/j.neurol.2013.07.022) Debette, S. - Vascular Risk Factors and Cognitive DisordersFajar Rudy QimindraBelum ada peringkat
- Role of Clinical and Paraclinical Manifestations of Methanol Poisoning in Outcome PredictionDokumen6 halamanRole of Clinical and Paraclinical Manifestations of Methanol Poisoning in Outcome PredictionFajar Rudy QimindraBelum ada peringkat
- Hav Steen 2014Dokumen7 halamanHav Steen 2014Fajar Rudy QimindraBelum ada peringkat
- WNL204606Dokumen9 halamanWNL204606Fajar Rudy QimindraBelum ada peringkat
- Seizure Prophylaxis Guidelines for Traumatic Brain InjuryDokumen8 halamanSeizure Prophylaxis Guidelines for Traumatic Brain InjuryvinodksahuBelum ada peringkat
- Gio G Karaki 2013Dokumen13 halamanGio G Karaki 2013Fajar Rudy QimindraBelum ada peringkat
- Auto Testing PatientsDokumen2 halamanAuto Testing PatientsFajar Rudy QimindraBelum ada peringkat
- Picone 2015Dokumen14 halamanPicone 2015Fajar Rudy QimindraBelum ada peringkat
- Guns Tad 2008Dokumen6 halamanGuns Tad 2008Fajar Rudy QimindraBelum ada peringkat
- SDTM Qs - Mhis-Nacc v1 Public DomainDokumen7 halamanSDTM Qs - Mhis-Nacc v1 Public DomainFajar Rudy QimindraBelum ada peringkat
- The effect of risk factors on the duration of cognitive impairmentDokumen28 halamanThe effect of risk factors on the duration of cognitive impairmentFajar Rudy QimindraBelum ada peringkat
- Syrgery Mock 2Dokumen8 halamanSyrgery Mock 2aa.Belum ada peringkat
- Sexual Precocity PDFDokumen5 halamanSexual Precocity PDFmist73Belum ada peringkat
- Cysteine, Methionine, ProlineDokumen3 halamanCysteine, Methionine, ProlineRio BurlazaBelum ada peringkat
- Biederman I Perceptual Pleasure and The Brain A Novel Theory Explains Why The Brain Craves Information and Seeks It Through The SensesDokumen10 halamanBiederman I Perceptual Pleasure and The Brain A Novel Theory Explains Why The Brain Craves Information and Seeks It Through The SensesKwong Gueng ToBelum ada peringkat
- How The Circadian Rhythm Affects Sleep, Wakefulness, and Overall HealthDokumen62 halamanHow The Circadian Rhythm Affects Sleep, Wakefulness, and Overall HealthRosemarie Fritsch100% (1)
- 1.13.2 Clinical Localization and History in NeurologyDokumen42 halaman1.13.2 Clinical Localization and History in Neurologyfikrah sharifBelum ada peringkat
- New European Guidelines Address Hyponatremia ManagementDokumen5 halamanNew European Guidelines Address Hyponatremia ManagementGherciuChirilaLarisaBelum ada peringkat
- Ventilation Perfusion RatiosDokumen22 halamanVentilation Perfusion Ratiosنمر نصارBelum ada peringkat
- Performed Structural DesignDokumen93 halamanPerformed Structural DesignSaiful IslamBelum ada peringkat
- Kurukshetra University Date-Sheets for BA/BSc Part ExamsDokumen12 halamanKurukshetra University Date-Sheets for BA/BSc Part ExamsabhishekBelum ada peringkat
- Asthma Care Quick Reference - Diagnosing and Managing AsthmaDokumen18 halamanAsthma Care Quick Reference - Diagnosing and Managing AsthmaMarizka Putri AftriaBelum ada peringkat
- Seed Maturation Process Explained in DetailDokumen22 halamanSeed Maturation Process Explained in DetailHitsugaya AbdullahBelum ada peringkat
- Interval Training For PerformanceDokumen20 halamanInterval Training For PerformancePaulo Tsuneta100% (1)
- Types of Plant HormonesDokumen6 halamanTypes of Plant HormonesKarren ReyesBelum ada peringkat
- MCQs in AnatomyDokumen29 halamanMCQs in Anatomyusha harithwaalBelum ada peringkat
- Euglena BioDokumen30 halamanEuglena BioMaddie KeatingBelum ada peringkat
- Hypertension ControlDokumen7 halamanHypertension ControlXyla CullenBelum ada peringkat
- ADokumen2 halamanAイ ロBelum ada peringkat
- Optimizing Ba and Iba Concentrations For Micro Propagation of Spineless Yucca Yucca Elephantipes IJERTV8IS010004Dokumen5 halamanOptimizing Ba and Iba Concentrations For Micro Propagation of Spineless Yucca Yucca Elephantipes IJERTV8IS010004SHUBHAM JIBHAKATEBelum ada peringkat
- Regenesis 1Dokumen14 halamanRegenesis 1White Light100% (3)
- Ineffective Airway ClearanceDokumen1 halamanIneffective Airway ClearanceFreisanChenMandumotanBelum ada peringkat
- CH 3 - Study GuideDokumen13 halamanCH 3 - Study Guide2688gieBelum ada peringkat
- IGCSE Biology 2015 Paper 21Dokumen20 halamanIGCSE Biology 2015 Paper 21VeronicaAndrianBelum ada peringkat
- Biology 2000 Paper I Marking SchemeDokumen4 halamanBiology 2000 Paper I Marking Schemeapi-2642329075% (4)
- Essencial Phrasal VerbsDokumen4 halamanEssencial Phrasal VerbsalvereBelum ada peringkat
- HemophiliaDokumen27 halamanHemophiliaDorothy Pearl Loyola Palabrica100% (1)
- Worksheet Central DogmaDokumen3 halamanWorksheet Central DogmaRein Jhonnaley Dioso100% (1)
- Root Resorption: Challenge To The Endodontist: Acta Scientific Dental SciencesDokumen11 halamanRoot Resorption: Challenge To The Endodontist: Acta Scientific Dental SciencesMuhammadDeniRahmanBelum ada peringkat
- Insulin Regulation of Gluconeogenesis 2018Dokumen25 halamanInsulin Regulation of Gluconeogenesis 2018Владимир ДружининBelum ada peringkat
- Pharmaceutics 09 00041 v2Dokumen14 halamanPharmaceutics 09 00041 v2thasyaBelum ada peringkat