Material
Diunggah oleh
Narasimha Murthy InampudiJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Material
Diunggah oleh
Narasimha Murthy InampudiHak Cipta:
Format Tersedia
GEOPHYSICAL RESEARCH LETTERS, VOL. 28, NO.
13, PAGES 2517-2520, JULY 1, 2001
Vitreous forsterite (MgzSiO4):Synthesis,structure, and
thermochemistry
JeanA. Tangeman
l, BrianL. Phillips,andAlexandra
Navrotsky
Department
of Chemical
Engineering
andMaterialsScience,
University
of California
at Davis
J. K. RichardWeber,April D. Hixson,andThomasS. Key
Containerless
Research,Inc., Evanston,Illinois
Abstract. Here we reportthe first synthesis
of a forsterite
(Mg2SiO4)
composition
glassasanessentially
phase-pure
bulk
material. Under containerless
conditions,with heterogeneous
nucleationsitesminimized,glassformsby coolingca. 1 mm
coolingratesof 10L108K/sec)but the resultingmaterial
consisted
of onlyapproximately
10%glassamongthequench
crystals.In the presentstudy,containerless
techniques
were
usedto synthesizeseveralgramsof Mg2SiO4 glasswith a
liquidMg2SiO
4 droplets
in oxygen
at 700K/s. 29SiNMR negligible proportionof crystals (-1%). Elimination of
spectroscopic
dataindicatethattheSiOntetrahedra
andMgO6 containersurfacesallowed pure bulk glass formation at
octahedra
existin a comersharingarrangement
in the glass, relativelymoderatecoolingratesof---700K/sec.
but uponcrystallization
the polyhedralunitsreorganizeto
form edge-sharing
linkages. Transposed
temperature
drop Synthesismethod and glasscomposition
calorimetry
showsthattheglassis 61.4+ 1.3kJ/molhigherin
Vitrification of forsterite was achieved in an aero-acoustic
enthalpythanthecrystal.
[Weber et al., 1994] or aerodynamiclevitator [Weber and
Nordine, 1995]. Sampleswere levitatedin a streamof pure
oxygen, melted using a continuous-waveCO laser, and
Introduction
cooled by blocking the laser beam. In this manner,both
Forsterite
(Mg2SiO4)is an orthosilicate
andend-member
of contaminationand heterogeneousnucleation by crucible
the ubiquitous(Mg, Fe)2SiO
4 (olivine) solid solution. surfacesare avoided. The coolingrate was controlledvia the
Forsteriticolivine is the primarymineralconstituent
in the samplediameter- smallerspheroids
cooledfasterdueto their
Earth'supper mantle [Ringwood,1975] and in meteorites larger specificsurfacearea. Productionof glassyforsterite
[Hashimoto,1990] and Mg-rich olivine also appearsin requiredcoolingrates of---700 K/s or greater(Fig. 1), and
variousglassyandcrystalline
formsin interplanetary
[Bradley
et al., 1999] and interstellardust[Nuthand $tencel,1986;
Dorschnerand Henning,1995;Molsteret al., 1999], comets
[Crovisieret al., 1997],andthe dustdisksthatencirclestars
[Molsteret al., 1999]. Despiteits importancein natural
systems,
studyof the non-equilibrium
behaviorandrelative
stabilityof phaseswhichcanformfromtheMg-orthosilicate
1800
liquidremaindifficultbecause
of the inabilityto synthesize
bulk olivine-composition
glasses. Synthesisof magnesium
silicateglassesby standardmelt-and-quench
methodsis
1600
............................
typicallylimited to molar SiO2concentrations
of 50% or
greater[Cooneyand $harrna,1990]. Usingrf-sputtering,
amorphous
magnesium
silicatefilmswith SiO:concentrations , 1400
aslow as40 tool% canbe synthesized
[Hanadaet al., 1988].
To producea pure forsteritecomposition
(Mg2SiO4)glass
1200
............
(33.33 tool % SiO:), however,alternativetechniquesare
1100
required.
Jeanlozet al. [ 1977]reported
thefirstobservation
of
I
1.5
2
2.5
3
3.5
4
4.5
5
Mg-richolivine(Mgo.88Feo.02SiO4
glass,whichcomprised
a
Time (sec)
small fraction (1-2%) of a sample producedby shock
pressures
of about56 GPa. Williamset al. [ 1989]synthesized Figure 1. Coolingcurvesfor 3.5 and40 mg liquidMg2SiO4
Mg:SiO4glassby ultra-rapidchilling(splat-quenching
with samples under containerlessconditions. The apparent
Now at Containerless
Research,Inc., 906 UniversityPlace,
Evanston,IL 60201.
Copyright
2001by theAmerican
Geophysical
Union.
Papernumber2000GL012222.
0094-8276/01/2000GL012222505.00
2517
temperaturewas measured with an optical pyrometer.
Levitatedspecimenswere heatedto 2270 q- 20 K (---100K
abovethe meltingpointof Mg2SiO4)andheldfor---5seconds
prior to cooling the liquid to ensure completemelting.
Coolingrates determinedat the 1350 K crystalnucleation
temperaturewere 250-750 K/sec; smaller samplescooled
faster. Crystalsnucleatedin largersamplesat a temperature
of 1350 K. Smaller samplesformed glass. Inset showsa
photograph
of glassspheroids.
2518
TANGEMAN ET AL.' VITREOUS FORSTERITE
Table 1. Composition
andThermochemistry
of ForsteriteGlass
MeasuredComposition
of Glass:ElectronMicroprobe
Analyses
Sample MgO (wt%)
SiO2(wt%)
Total
MgO (mol%)
SiO2(mol%)
Fooglass 56.74
44.33
101.07
65.61
34.39
(0.99)
(0.61)
(0.50)
(1.44)
(0.47)
*Averageof 35 analyses;
7 spheroids
analyzed,5 spotanalyses
on each;Coveredtherangeiri
sizeandbubblecontent;Errorsin ( ) are2 standarddeviationsof themean.
Transposed
Temperature
Drop Calorimetry
Thermochemical
cycle:
(1) Mg2SiO4(glass,298 K) = Mg2SiO4(xl, 1075K)
(2) Mg2SiO4(xl, 298 K) = Mg2SiO4(xl, 1075K)
(3) Mg2SiO4(glass,298 K) = Mg2SiO4(xl, 298 K)
Avg.
Avg. ofi Std.Dev. Err.
AH(1) kJ/mol =
59.84
20
1.20
0.99
AH(2) kJ/mol 121.28 14
0.34
0.57
AH(3) = AH(1) - AH(2) = Enthalpyof Vitrificationof Mg2SiO4Glassat 298 K
= 61.44 + 1.25 kJ/mol
Calculated
Valuesfor theAverageHeatCapacityof theSupercooled
LiquidandtheEnthalpy
andEntropyof Fusionof Forsteriteat 2163 K
Avg.Cp,liq(1040-1773
K) = 225.0J/mol'K
AHfusion(2163
K) = 102.8kJ/mol
ASfusion(2163
K) - 47.5 J/mol.K
The C,of the crystalis fromRobieandHemingway[ 1995]. The AHfu.ion
usedto calculatethe
averageC,of the supercooled
liquidis fromNavrotskyet al. [ 1989],andwasobtainedfrom
solutioncalorimetryin the Mg2SiO4-CaMgSi206-CaA12Si208
systemat 1773K.
hence a samplediameterof 0.1 cm (Fig. 1, inset). The
resultingsmooth,transparent,colorlessglassspheroidseach
weigh 2-4 mg and containone or more small (ca. 5% total
volume) internal shrinkagevoids. At coolingrates slower
than 700 K/sec, crystalsnucleatedat about 1350 K (-800 K
below the meltingpoint of Mg2SiO4 crystal). Crystallization
occurredrapidly and was accompanied
by a "thermalevent"
duringwhich the sampletemperatureincreasedby about300
K (Fig. 1).
The glasswas synthesized
from high purityMgO and SiO2
powders,mixedandfusedinto-0.5 gramboulesof crystalline
Mg2SiO4 which were crushedto make samplesfor levitation.
Electronmicroprobeanalysesof initial batchesof the meltprocessedMg2SiO4 showedslight preferentialloss of MgO.
Forsterite-composition
glass(Table 1) wasmadefromstarting
materialcontaining1.25 molar % excessMgO.
scanningcalorimetric(DSC) scanshowedthat only forsterite
waspresent;no otherphaseswere detected.
29SiNMR results' Glass structure
Comparison
of 298iNMR spectra
of glassyandcrystalline
forsterite (Fig. 2) indicated significant structural
dissimilarities.The narrowpeak at -61.8 ppm was due to a
small amount of crystallineforsterite[Magi et al., 1984],
whereas most of the intensity, correspondingto the
amorphous
phase,occurredin a broad,slightlyasymmetrical
peak (7.5 ppm full-width-at-half maximum) having an
average
chemical
shiftof-70.4+ 0.2ppm.Thefull298iNMR
peakwidth for the glassincludesthe chemicalshiftrangefor
dimericgroups([8i207]
6', Q species)
in crystalline
Mg-
silicates(-72 ppm for akermanite[Merwin et al., 1989];-79.0
Most of the glassspheroids
showedno cracksor surface ppm for wadsleyite[Phillips et al., 1997]) and is consistent
crystalsat up to 60X magnification.X-ray diffraction(XRD)
with the presenceof a smallconcentration
of this species,as
patternsfrom a few milligramsof the crushedspheroids
were inferredfrom IR and Raman spectrafor Mg-silicateglasses
characteristicof amorphousmaterial and crystalswere not [McMillan, 1984; Williamset al., 1989].
observedin samplesimagedusing back-scattered
electrons.
The chemical shift differencebetween crystallineand
NMR measurements
confirmedthat the sampleswere -99%
amorphousforsteritecan be relatedto a changein the spatial
amorphous(Fig. 2). The -1% crystallineforsteritedetected relationship
betweenthe Si and Mg bondingpolyhedra. For
by 298iNMR is mostlikely dueto boththe presence
of
Mg-orthosilicates,
the298ichemical
shiftfallsin two distinct
crystals disseminatedthroughoutthe spheroidsand small
crystallizedportionson the surfacesof a few spheroids.The
NMR analysiswas performedon a sampleof-200 glass
spheroids,
a few of whichwere later foundto havetiny areas
of surfacecrystals,but this small volume fraction cannot
accountfor the entire 1% crystallinity. The surfacecrystals
probablyformedin atypicaleventswherethe levitatingbead
cameinto contactwith the sidesof the aerodynamic
levitator.
rangescorrespondingstructurallyto forsterite-likesites in
A, B, and superhydrousB) [Phillips et al., 1997]. The
averagechemicalshift for the forsteriteglassis nearestto that
of the Si-sitein phaseA (MgsSiO4(OH)6)
that sharesonly
comerswith nineMg-octahedra,
soassuming
thatmostof the
XRD and298iNMR analyses
(Fig.2, inset)of a portion
of the
Si-sites
areQ0,thenthoseQ0sitesareprimarilycomer-linked
whichthe [SiO4]
4' tetrahedra
shareedgesandcomerswith
[MgO6]
m'octahedra
(-61.8to -64 ppm)andsitesthatshare
onlycomers
with[MgO6]
'units(-70.6to -75.8ppm;phases
glass sample crystallizedfrom a preliminary differential to Mg-octahedra,rather than both comer- and edge-linked.
Si MAS-NMRSpectrum
of Mg2SiO
4 Glass
.............
.............
Forsterite
=-61.8
ppm
I............................
(,,o)
=-70.4
ppm1
I......
'
........
Amorphous
phase:
-30
-40
-50
-60
and Johari, 1990] despite the absenceof any crystalline
phasesin the glass. Suchliquidsare poorglass-formers
and
considered
extremely'fragile' [Angell, 1995;Ito et al., 1999].
Annealingof thesetruly amorphous
materialsat temperatures
justbelowT canremove
theexothermic
effect,whichis due
-10
-80
-30
-50
-90
-70
-90
-100
-110
-130
-110
ChemicalShift(ppm)
Figure2. 29SiMAS-NMRspectrum
of forsterite
glass.The
smallpeak arisesfrom a small amount(-*1%) of crystalline
forsterite(15= -61.8 ppm) present in the sample. The
amorphous
phaseexhibitsan averagechemicalshift of-70.4
ppm andpeakwidth 7.5 ppm (full-widthat half-maximum).
Inset showsthe spectrumof a portionof the sampletaken
after crystallization. Spectrawere obtainedat 79.5 MHz
using standarddirect-polarization
techniqueswith 3-4 gs
pulses(g/4 tip angle) and 200 s relaxationdelays. Glass:
spinningrate 4.5 kHz, for 1720 acquisitions;
no changewas
observedupon increasingrelaxationdelay to 1000s. Inset:
spinningrate 7.0 kHz, 1164 acquisitions.Frequencies
are
referencedexternallyto tetramethylsilane.
to enthalpy or structural relaxation, and reveal a glass
transitionendothermupon reheating. Preliminaryannealing
experimentscoupledwith DSC scanson Mg2SiOn did not
reveal such an endotherm,suggestingthat nanocrystalline
phasesareindeedpresent.
The fragility of Mg2SiOnliquid cannot be determined
quantitativelyfrom the DSC trace, but qualitativeviscosity
measurements
provide evidenceof the 'extremely fragile'
natureof Mg2SiOn liquid. Great difficulty was encountered
when attemptingto pull fibers from the levitatingmolten
dropletusinga tungstenstinger[Weberet al., 1998]. Such
difficulty suggeststhat the temperaturewindow for fiber
pullingis very narrowandthatthe risein log viscosityvs. 1/T
is steep,a feature characteristicof fragile liquids [Angell,
1995].
Although the absenceof a glass transition precluded
determination
of theconfigurational
heatcapacity
at T and
the heat capacityof the supercooled
liquid, the enthalpyof
vitrification at 298 K, was measured (Table 1). The
transposed
temperaturedrop calorimetricmeasurements
were
performedin a twin Tian-Calvetmicrocalorimeter
at 1075 K
[Navrotsky,1997]. Powderedglasssamples(5 mg pellets)
were droppedinto emptyPt crucibleswithin the calorimeter
andthe heateffectsresultingfrom crystallization
of the glass
The breadth of the peak for forsteriteglass suggestsa weremeasured.Usingthe appropriatethermodynamic
cycle,a
continuous distribution of local structural environments that
valueof 61.4 + 1.3 kJ/molfor the enthalpyof vitrificationwas
spans
forsterite-like
sitesto Q-typespecies
butis dominatedobtained. An independentvalue of 60.1 + 2.0 kJ/molfor the
by Si-sites in a comer-sharingarrangementwith Mg- enthalpyof vitrificationwas determinedfrom integrationof
octahedra.
Thus, nucleation of forsterite requires the crystallization
exothermon the Cptrace. The two values
reorganization,
or a simplerotationof thepolyhedral
units,to agree. Moreover, a value of 61.1 + 4.0 kJ/mol for the
form edge-sharing
linkagesbetweenthe SiO4tetrahedra
and enthalpyof vitrificationof forsteriteglasswas estimatedby
MgO6octahedra.
Navrotskyet al. [1990] andis in excellentagreement
with the
For crystallinephases,the changefrom forsterite-likeSi results of this study. For comparison,the enthalpy of
sitesto comer-shared
sitesis accompanied
by a smallincrease
in theaverage
Si-Obonddistance
of about
0.02A.Molecular
200
dynamicscalculations[Tosselland Lazzeretti,1986] and
experimental
datafor Ca-orthosilicates
[Skibsted
et al., 1990]
suggest
a decrease
in 298ichemical
shiftwith increasing
averageSi-O bonddistance,
thelatterstudygivinga slopeof
about-350ppm/A. Thesetrendsare consistent
with the
presentdataandstructural
interpretations.
100........................ Onset
=1040
K
ofcrystallization
" -100
.........................
Calorimetric results:Relative stability of phases
In additionto the structural
analyses,
successful
production
-300
of essentiallysingle-phaseforsterite compositionglass
enableda complementary
thermochemical
study. The heat
,-400
capacityof the samplefrom400-1450K wasmeasured
using
a Netzschdifferentialscanningcalorimeter(DSC-404). No
-500
glasstransition
wasdiscernible
in thecalorimetric
trace(Fig.
Temperature(K)
3) priorto theonsetof thecrystallization
exotherm
at 1040K.
The absence
of a glasstransition
is attributed
to nucleation
of Figure 3. Heat capacitytraceshowingcrystallizationof a 12
on a
crystals
immediately
uponenteringthe.glass
transition
region. mg sampleof powderedMg2SiOnglass. Measurements
Netzsch DSC-404 were performedunder flowing Ar (60
Thusweestimate
T - 1040K.
If crystalsare dispersedthroughoutthe as-synthesized ml/min), using a scan rate of 10 K/min. The onset of
glass,nucleationsitesare alreadypresentand virtuallyno crystallizationoccurred at 1040 K. Integration of the
crystallization
exothermresultedin a valuefor theenthalpyof
barrierto crystallization
existsonceT is reached.On the
vitrification
at 1040 K of 60.1
+ 2.0 kJ/mol.
The heat
other hand, Mg2SiO
4 liquid may exhibit glasstransition capacityresultsfor the glass and crystal are only semiphenomenologysimilar to that observed in some quantitativebecauseof the small sample mass. X-ray
of thecrystallized
productshowed
thatthematerial
hyperquenched
melts,e.g.glassy
metalalloys,whichshownc diffraction
glass-liquid
transition
endotherm
priorto crystallization
[Ram wasphasepurecrystallineforsterite.
2520
TANGEMAN
ET AL.: VITREOUS
FORSTERITE
vitrificationof an enstatite(MgSiO3) compositionglassis Jeanloz,R., T. J. Ahrens,J. S. Lally, G. L. Nord Jr., J. M. Christie,
andA. H. Heuer,Shock-produced
olivineglass:first observation,
42.2 kJ/mol [Herrig et al., 1985]. The enthalpy of
Science197, 457, 1977.
vitrificationof Mg2SiO4 on a kJ/molbasisis thussignificantly
Magi, M., E. Lippmaa, A. Samoson,G. Engelhardt,and A. -R.
more endothermic
thanthat of MgSiO3 andprovidesthe first
Grimmer,Solid-statehigh-resolution
silicon-29chemicalshiftsin
directquantitativemeasureof the relativestabilityof the end
silicates,
J. Phys.Chem.88, 1518, 1984.
McMillan, P. F., A Raman spectroscopic
studyof glassesin the
memberorthosilicate
glass.
systemCaO-MgO-SiO2,Am. Min. 69, 645-659, 1984.
Merwin, L. H., A. Sebald, and F. Seifert, The incommensurate-
Conclusionsand applications
The measuredenthalpyof vitrification can be used to
constrainthe averageheat capacityof the supercooled
liquid
(between 1040 and 1773 K) as well as the enthalpyand
entropyof fusionof forsteriteat 2163 K, its normalmelting
point. Thesenew estimates(Table 1) can in tum be usedto
constrainmelting and crystallizationprocesses
occurringin
magmaticsystemswithin the Earth and the energeticsof
chondruleformationin the earlysolarsystem[Wasson,1996].
Moreover,
theglassstructure,
asdeduced
fromthe29SiNMR
spectra,and the measuredenthalpy of vitrification, when
commensuratephase transition in akermanite,CaMgSi207,
observed
by in-situ29SiMAS NMR Spectroscopy,
Phys.Chem.
Mineral. 16, 752, 1989.
Molster, F. J., I. Yamamura, L. B. F. M. Waters, A. G. G. M.
Tielens,Th. de Graauw,T. de Jong,A. de Koter,K. Malfait, M.
E. van den Ancker,H. van Winckel, R. H. M. Voors, and C.
Waelkens, Low-temperaturecrystallizationof silicate dust in
circumstellardisks,Nature 401,563, 1999.
Navrotsky,A., ProgreSS
and new directions
in high temperature
calorimetryrevisited,Phys.Chem.Minerals24, 222, 1997.
Navrotsky,
A., P. Maniar,andR. Oestrike,Energetics
of glasses
in
the systemdiopside-anorthite-forsterite,
Contrib.Mineral. Petrol.
105, 81, 1990.
Navrotsky,
A., D. Ziegler,R. Oestrike,
andP. Maniar,Calorimetry
of
combined with additional studies on the kinetics of
silicatemeltsat 1773K: measurement
of enthalpies
of fusionand
crystallizationof forsterite glass under containerless of mixingin the systemsdiopside-anorthite-albite
andanorthite-
conditions may help to elucidate the crystallization forsterite,Contrib.Mineral. Petrol. 101, 122, 1989.
Among
mechanisms
whichoperateas amorphous
magnesium
silicate Nuth, J. A. and R. E. Stencel,Eds., Interrelationships
Circumstellar,Interstellar,and InterplanetaryDust, NASA
dustevolvesfrom precursor
interstellar
cloudsto planetary
ConferencePublication2403, 1986.
systems[Molster et al., 1999]. Finally, the viscosity- Phillips,B. L., P. C. Burnley,K. Worminghaus,
andA. Navrotsky,
29Siand H NMR spectroscopy
of high-pressure
hydrous
temperature
profile(albeitqualitative)
andthereluctant
glassmagnesium
silicates,
Phys.ChemMinerals24 179, 1997.
formingnatureof Mg2SiO
4 suggestthat vitreousforsterite
S. andG. P. Johari,Glass-liquid
transition
in hyperquenched
couldbe themostfragileglass-forming
silicateliquidstudied Ram,
metalalloys,Phil. Mag. 61,299, 1990.
to date. Clearly,containerless
technologywill facilitatethe Ringwood,
A. E. Composition
andPetrology
of theEarth'sMantle,
McGraw-Hill, New York, 1975.
studyof glasses
andsupercooled
liquidsin otherwisedifficult
properties
of
to vitrify systems
andwill providean environment,
parallelto Robie,R. A. andB. S. Hemingway,Thermodynamic
at 298.15 K and 1 bar (105
that in outerspace,which is appropriate
for investigating mineralsand relatedsubstances
Pascals)pressureand at higher temperatures,
US Geololgical
materialsprocessing
in ourgalaxy.
SurveyBulletin2131, 1995.
Skibsted,J., J. Hjorth,andH. J. Jakobsen,
Correlationbetween9Si
Acknowledgments.Experiments
at UC Daviswere supported
by
NMR chemicalshiftsand meanSi-O bondlengthsfor calcium
the Center for High PressureResearch,an NSF Science and
silicates,Chem.Phys.Lett., 172, 279 1990.
TechnologyCenter. Materialssynthesisat Containerless
Research, Stebbins,J. F. and M. Kanzaki, Local structureand chemicalshifts
Inc. wassupported
by NASA.
for six-coordinated
silicon in high-pressure
mantle phases,
Science251,294, 1991.
Tossell,J. A. and P. Lazzeretti,Ab initio calculations
of 9SiNMR
References
chemicalshifts for some gas phase and solid-statesilicon
fluoridesandoxides,J. Chem.Phys.84, 369, 1986.
Angell, C. A. Formationof glassesfrom liquidsand bipolymers,
Wasson,J. T., Chondrule
formation:energetics
andlengthscales,in
Science267, 1924, 1995.
Chondrulesand the Protoplanetary
Disk, R. H. Hewins, Ed.,
Bradley,J.P., T. P. Snow, D. E. Brownlee,and M. S. Hanner,in
CambridgeUniversityPress,45, 1996.
Solid InterstellarMatter: The ISO Revolution,L. d'Hendecourt,
Weber,J. K. R., J. J. Felten,B. Cho, andP. C. Nordine,Glassfibres
C. Joblin,A. Jones,Eds.,Springer,Berlin,297-315,1999.
of pure and erbium- or neodymium-doped
yttria-alumina
Cooney,T. F. andS. K. Sharma,Structureof glasses
in the systems
compositions,
Nature 393, 769, 1998.
Mg2SiOn-Fe2SiO4,
Mn2SiOn-Fe2SiO4,
Mg2SiOn-CaMgSiO4,
and Weber,J. K. R., D. S. Hampton,D. R. Merkley,C. A. Rey, M. M.
Mn2SiOn-CaMnSiO4,
J. Non-Cryst.Sol. 122, 10, 1990.
Zatarski, and P. C. Nordine, Aero-acousticlevitation: a method
Crovisier,J., K. Leech,D. Bocke16e-Morvan,
T. Y. Brooke,M. S.
for containerless
liquid-phase
processing
at high temperatures,
Rev. Sci.Instrum. 65(2), 456, 1994.
Hanner,B. Altieri,H. U. Keller,andE. Lellouch,Thespectrum
of
liquid-phase
cometHale-Bopp
(C/199501) observed
withtheInfraredSpace Weber,J. K. R. and P. C. Nordine,Containerless
Observatoryat 2.9 astronomical
unitsfrom the Sun,Science275,
1904, 1997.
Dorschner,
J. and T. Henning,Dust metamorphosis
in the galaxy,
Astron.Astrophys.
Rev. 6, 271, 1995.
Hanada,T., N. Soga, and T. Tachibana,Coordinationstate of
magnesium
ions in rf-sputtered
amorphous
films in the system
MgO-SiO2,J. Non-Cryst.Sol. 105, 39, 1988.
processingat high temperatures,Microgravity Sci. and
Technology,VII, 279, 1995.
Williams,Q., P. McMillan,andT. F. Cooney,Vibrationalspectraof
olivine compositionglasses:the Mg-Mn join, Phys. Chem.
Minerals 16, 352, 1989.
B. Phillips,A. Navrotsky,and J. Tangeman,Departmentof
Hashimoto,
A.,Evaporation
kinetics
offorsterite
andimplications
for Chemical Engineeringand Materials Science, University of
the earlysolarnebula,Nature 347, 53, 1990.
Californiaat Davis,DavisCA 95616,USA. (email:blphillipsand
Hervig, R. L., D. Scott,and A. Navrotsky,Thermochemistry
of
anavrotsky@ucdavis.
edu,tangeman@containerless.com)
A. Hixson,T. Key, and R. Weber, Containerless
Research,Inc.,
glassesalongjoins of pyroxenestoichiometry
in the system
CaSiO6-Mg:Si:O6-A1406,
Geochim.Cosmochim.
Acta 49, 1497,
906 University Place, Evanston, IL 60201, USA. (email:
1985.
weber@containerless.com)
Ito, K., C. T. Moynihan, and C. A. Angell, Thermodynamic
determination
of fragilityin liquidsanda fragile-to-strong
liquid (ReceivedAugust17,2000;revisedJanuary12,2001;
transitionin water, Nature 398, 492, 1999.
accepted
January18, 2001.)
Anda mungkin juga menyukai
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- LNG An IntroductionDokumen60 halamanLNG An Introductionsyafiq86% (7)
- Lecture 28 PDFDokumen11 halamanLecture 28 PDFBhavesh Dilip Chanchlani100% (1)
- SCFM To AcfmDokumen6 halamanSCFM To AcfmFaizanBelum ada peringkat
- Introduction To Offshore Petroleum Production SystemDokumen55 halamanIntroduction To Offshore Petroleum Production SystemJoaoCOS100% (1)
- Oxyfuel Cutting - Process and Fuel GasesDokumen6 halamanOxyfuel Cutting - Process and Fuel Gasesunknown8787Belum ada peringkat
- Nozzles and Diffusers: A Comprehensive GuideDokumen13 halamanNozzles and Diffusers: A Comprehensive GuideBhuvnesh Singh50% (2)
- Separator CalcsDokumen20 halamanSeparator CalcsHoney Tiwari100% (3)
- Open Cycle Powe PlantDokumen31 halamanOpen Cycle Powe PlantherdanandiBelum ada peringkat
- Material Testing Lab ManualDokumen58 halamanMaterial Testing Lab Manualrammohan reddyBelum ada peringkat
- Faculty Adversitement 2021Dokumen1 halamanFaculty Adversitement 2021Narasimha Murthy InampudiBelum ada peringkat
- 02 03 BalomenosDokumen16 halaman02 03 BalomenosmiladrahimianBelum ada peringkat
- Transport Phenomena-Gaskell PDFDokumen687 halamanTransport Phenomena-Gaskell PDFmirandaBelum ada peringkat
- Slaeg Vaeloriza Id 6928Dokumen29 halamanSlaeg Vaeloriza Id 6928Narasimha Murthy InampudiBelum ada peringkat
- Microstructure, SDAS and Mechanical Properties of A356 Alloy Castings Made in Sand and Granulated Blast Furnace Slag MouldsDokumen13 halamanMicrostructure, SDAS and Mechanical Properties of A356 Alloy Castings Made in Sand and Granulated Blast Furnace Slag MouldsNarasimha Murthy InampudiBelum ada peringkat
- Effect of Surface Treatment On Wear Behavior of Magnesium Alloy AZ31Dokumen4 halamanEffect of Surface Treatment On Wear Behavior of Magnesium Alloy AZ31Narasimha Murthy InampudiBelum ada peringkat
- Faculty Adversitement 2021Dokumen1 halamanFaculty Adversitement 2021Narasimha Murthy InampudiBelum ada peringkat
- GO Ms No 38Dokumen92 halamanGO Ms No 38Narasimha Murthy InampudiBelum ada peringkat
- Chromite-A Cost-Effective Refractory Raw Material For Refractories in Various Metallurgical ApplicationsDokumen14 halamanChromite-A Cost-Effective Refractory Raw Material For Refractories in Various Metallurgical ApplicationsNarasimha Murthy InampudiBelum ada peringkat
- ISSN 2277 - 7164 Original Article: Advances in Polymer Science and Technology: An International JournalDokumen7 halamanISSN 2277 - 7164 Original Article: Advances in Polymer Science and Technology: An International JournalNarasimha Murthy InampudiBelum ada peringkat
- Fabrication and Characterization of 2024 Aluminum-High Entropy Alloy CompositesDokumen7 halamanFabrication and Characterization of 2024 Aluminum-High Entropy Alloy CompositesNarasimha Murthy InampudiBelum ada peringkat
- Guidelines and Instructions To CandidatesDokumen3 halamanGuidelines and Instructions To CandidatesNarasimha Murthy InampudiBelum ada peringkat
- Characterization of Zircon-Based Slurries For Investment CastingDokumen10 halamanCharacterization of Zircon-Based Slurries For Investment CastingNarasimha Murthy InampudiBelum ada peringkat
- Fracture and Fracture Toughness of Cast Irons: W. L. Bradley and M. N. SrinivasanDokumen33 halamanFracture and Fracture Toughness of Cast Irons: W. L. Bradley and M. N. SrinivasanNarasimha Murthy InampudiBelum ada peringkat
- RKGUT Powder Metallurgy CourseDokumen2 halamanRKGUT Powder Metallurgy CourseNarasimha Murthy InampudiBelum ada peringkat
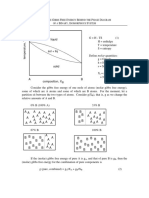
- Gibbs Free Energy Behind The Phase Diagram of A Binary, Isomorphous SystemDokumen8 halamanGibbs Free Energy Behind The Phase Diagram of A Binary, Isomorphous Systemkgupta27Belum ada peringkat
- Gomsno380301 PDFDokumen126 halamanGomsno380301 PDFNarasimha Murthy InampudiBelum ada peringkat
- Mechanical Alloying of Cu-Al O NanoparticlesDokumen4 halamanMechanical Alloying of Cu-Al O NanoparticlesNarasimha Murthy InampudiBelum ada peringkat
- Characterizing and Improving The Thermal Conductivity of Engineered Clay Barriers For Sealing A Deep Geological Repository Alex Man, Jason Martino, Chang-Seok Kim and Deni PriyantoDokumen18 halamanCharacterizing and Improving The Thermal Conductivity of Engineered Clay Barriers For Sealing A Deep Geological Repository Alex Man, Jason Martino, Chang-Seok Kim and Deni PriyantoNarasimha Murthy InampudiBelum ada peringkat
- Characterization of Zircon-Based Slurries For Investment CastingDokumen10 halamanCharacterization of Zircon-Based Slurries For Investment CastingNarasimha Murthy InampudiBelum ada peringkat
- 1 JMES 607 2014 Karzazi PDFDokumen12 halaman1 JMES 607 2014 Karzazi PDFVirgiBelum ada peringkat
- Gibbs Free Energy Behind The Phase Diagram of A Binary, Isomorphous SystemDokumen8 halamanGibbs Free Energy Behind The Phase Diagram of A Binary, Isomorphous Systemkgupta27Belum ada peringkat
- 7 MetallurgyDokumen2 halaman7 MetallurgyNarasimha Murthy InampudiBelum ada peringkat
- 1 Manufacturing TechnologyDokumen2 halaman1 Manufacturing TechnologyNarasimha Murthy InampudiBelum ada peringkat
- High Entropy Alloys: A Renaissance in Physical Metallurgy : Meeting ReportDokumen3 halamanHigh Entropy Alloys: A Renaissance in Physical Metallurgy : Meeting ReportNarasimha Murthy InampudiBelum ada peringkat
- METAL CUTTING AND FORMING PROCESSESDokumen1 halamanMETAL CUTTING AND FORMING PROCESSESNarasimha Murthy InampudiBelum ada peringkat
- 16 10 06recentprogressinhigh-Entropyalloys-1Dokumen19 halaman16 10 06recentprogressinhigh-Entropyalloys-1Narasimha Murthy InampudiBelum ada peringkat
- Foundry Technology - II Students Handbook: Class XIIDokumen135 halamanFoundry Technology - II Students Handbook: Class XIINarasimha Murthy InampudiBelum ada peringkat
- Solution Manual For Chemistry An Atoms Focused Approach Second Edition Second EditionDokumen37 halamanSolution Manual For Chemistry An Atoms Focused Approach Second Edition Second EditionAndrewMartinezjrqo100% (44)
- HF 570 / HF 575 / HF 578 Series: Tank Mounted Return Line FiltersDokumen36 halamanHF 570 / HF 575 / HF 578 Series: Tank Mounted Return Line FilterssitnikovsBelum ada peringkat
- Crafft Floor Standing Catalog 2020 - CBU ChinaDokumen2 halamanCrafft Floor Standing Catalog 2020 - CBU ChinaMohanad ElgayarBelum ada peringkat
- EXPERIMENT 2-Purification and Melting Point DeterminationDokumen3 halamanEXPERIMENT 2-Purification and Melting Point Determinationjune100% (1)
- LCHS 4000 LNG Description of System EngDokumen34 halamanLCHS 4000 LNG Description of System EngAhmad ImranBelum ada peringkat
- Process Instrumentation AssignmentDokumen1 halamanProcess Instrumentation AssignmentLaaptu MailBelum ada peringkat
- Measuring Absolute Viscosity Using Ostwald ViscometerDokumen6 halamanMeasuring Absolute Viscosity Using Ostwald ViscometerFlorecita CabañogBelum ada peringkat
- Srinivas Garimella Et Al. - Condensation of Zeotropic Mixtures of Low-Pressure Hydrocarbons and Synthetic RefrigerantsDokumen10 halamanSrinivas Garimella Et Al. - Condensation of Zeotropic Mixtures of Low-Pressure Hydrocarbons and Synthetic RefrigerantsGonzalo Vicencio FuentesBelum ada peringkat
- 07HEAT - WS 03: Solve The Crossword On "Heat" Within 10 MinutesDokumen2 halaman07HEAT - WS 03: Solve The Crossword On "Heat" Within 10 MinutesSHUBHAM KUMARBelum ada peringkat
- UoD Fluid Mechanics Tutorial on U-tube & Inclined-tube ManometersDokumen3 halamanUoD Fluid Mechanics Tutorial on U-tube & Inclined-tube ManometersGabrielBelum ada peringkat
- Thermo Physical Properties of FluidsDokumen24 halamanThermo Physical Properties of FluidsPipeline EngineerBelum ada peringkat
- GATE Chemical Engineering 1995Dokumen7 halamanGATE Chemical Engineering 1995Kubra ĖdrisBelum ada peringkat
- Class 11 Physics Notes Chapter 9 Studyguide360Dokumen19 halamanClass 11 Physics Notes Chapter 9 Studyguide360Astrid RedBelum ada peringkat
- Fluid Clasisfication and PropertiesDokumen19 halamanFluid Clasisfication and PropertiesQueen SuaBelum ada peringkat
- Gas Chlorine StationsDokumen46 halamanGas Chlorine StationsakramhomriBelum ada peringkat
- Enhanced Hybrid Science 6 Q1 M4 W4Dokumen13 halamanEnhanced Hybrid Science 6 Q1 M4 W4Mariel SalazarBelum ada peringkat
- Thesis Presentation HollyDokumen41 halamanThesis Presentation HollyОльга Владимировна МинаковаBelum ada peringkat
- AerosolsDokumen29 halamanAerosolsKulbhushan SharmaBelum ada peringkat
- Spe Distinguished Lecturer Series Spe FoundationDokumen37 halamanSpe Distinguished Lecturer Series Spe FoundationaidanBelum ada peringkat
- Science - Lesson On States of Matter For Pre K StudentsDokumen30 halamanScience - Lesson On States of Matter For Pre K StudentsHồng Ngát PhíBelum ada peringkat
- Breakdown in Electronegative Gases, V-T Characteristics & Post Breakdown Phenomena Post Breakdown PhenomenaDokumen22 halamanBreakdown in Electronegative Gases, V-T Characteristics & Post Breakdown Phenomena Post Breakdown PhenomenaAjeng PratiwiBelum ada peringkat
- Earth Subsystems LessonDokumen21 halamanEarth Subsystems Lessonangel romiscalBelum ada peringkat
- Semiconductor SpintronicsDokumen343 halamanSemiconductor Spintronicsmayankbehl100% (1)