Answer Scheme Term 2 Trial
Diunggah oleh
Ting TCHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Answer Scheme Term 2 Trial
Diunggah oleh
Ting TCHak Cipta:
Format Tersedia
Answer Scheme Term 2 Trial Exam 2015
No
Description
Marks
15
Section A
1 A
C3H4 + O2 3CO2 + 2H2O
+184
3(-394) 2(-286)
Hc = [3(-394)+2(-286)] [+184+0] = -1938 kJ mol-1
2H+ + 2e- H2 ; I mol of H2 needs 2F electric current
Q = It = 8 x 100 x 60 = 48,000 C
No. of moles of H2 = 48,000 9.65 x 104 x 2 = 0.25 mol
Volume of H2 = 0.25 x 24.4 = 6.1 dm3 + O2 = 9.15
2 C
3 D
4 C
Since both electrodes are the same metal, Eo = 0 V
Ecell =Eo +
=0+
5
6
7
8
9
10
B
D
D
B
B
C
+0.028 V
MgSO4.xH2O MgSO4 + xH2O
Mass (g)
2.1
MgSO4
H2O
4.3 2.1 = 2.2
No of moles
2.1 120 = 0.0175
1
2.2 18 = 0.122
7
Ratio
11
12
13
14
15
D
B
A
D
D
Section B
15
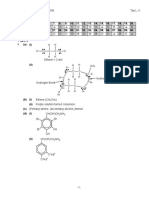
16. (a) Lattice energy is the energy released when one mole of an ionic compound
is formed from its gaseous ions.
+
Energy
Na (g) + Br (g) + e
-1
(b)
+ 112 kJ mol-1
Na+ (g) + Br2(g)
Na(g) + Br2(g)
Na(s) + Br2(g)
-325 kJ mol-1
Na+ (g) + Br (g)
+ 495 kJ mol1
+ 108 kJ mol1
-329 kJ mol-1
NaBr (s)
Lattice energy
(c) 108 + 495 + 112 + (-325) + lattice energy = 329 ..
Lattice energy = 719 kJ mol-1
17. (a)
Eo = 1.36 1.07
= + 0.29 V .
A positive Eo value means Cl2 is able to oxidize Br to Br2.
Cl2 + 2Br 2Cl + Br2
(b) Chlorine reacts with water to form HOCl and HCl that are colourless.
Cl2(aq) + H2O(l) HCl(aq) + HOCl(aq)
When exposed to sunlight, HOCl undergoes decomposition to form HCl
and O2.
2HOCl(aq) 2HCl(aq) + O2(g)
This will shift the above equilibrium to the right causing more Cl 2 to react
with water and its colour fades.
17. (c)
3Cl2(aq) + 6OH(aq) 5Cl(aq) + ClO3(aq) + 3H2O(l)
Chlorine undergoes disproportionation in hot alkali to form chlorate(I) and
chlorate(V).
Section C
18.(a)(i) The potential difference obtained when a half-cell of an element is
connected to a hydrogen standard electrode at 298K and 1 atm.
(ii)
(b)
K+(aq) + e K(s) ;
Eo = - 2.92 V
Cu2+(aq) + 2e Cu(s) ; Eo = + 0.34V
Br2 (g) + 2e 2Br(aq) ; Eo = + 1.07 V
The strength of the reducing power in descending order is K, Cu, Br
(OR K is a stronger reducing agent than Cu & Cu is a stronger reducing agent than
Br ion)
The strength of the reducing agent depends on the ability to release e-.
The more negative the standard electrode potential (E), the higher the
tendency of the metals or the anions to release electrons.
The strength of an oxidising agent depends on the ability to accept e-.
The more positive the standard electrode potential (Eo), the higher the
tendency of the cations or the non-metals to accept electrons. .
The strength of the oxidising power in descending order is Br 2, Cu2+, K+.
(OR Br2 is a stronger oxidising agent than Cu2+ ion while Cu2+ ion is a stronger
oxidising agent than K+ ion).
NaCl(aq) Na+(aq) + Cl(aq)
In dilute sodium chloride solution :
At the anode, two possible reactions that can take place are :
2Cl(aq) Cl2(aq) + 2eE0 = - 1.36 V
+
2H2O(l) O2(g) + 4H (aq) + 4e
E0 = - 1.23 V
Since the Eo for the second reaction is less negative, it will occur in
preference to the first reaction. Thus, water is oxidised to oxygen gas.
In concentrated soium chloride solution:
In this case, Cl is oxidised in preference to H2O. This is because the ease of
a species to be discharged at an electrode increases with concentration.
Thus, chlorine is produced as it is more concentrated.
30
1
1
1
1
1
1
1
1
(c)(i) 2Zn + O2 2ZnO / 2Zn + O2 + 2H2O 2Zn(OH)2 ............................................
5
5
(ii) Q = It = 2.1 x 10 x 1 = 2.1 x 10 C ......................................................................
O2 + 2H2O +4e 4OH
4F = 4(96500) C = 1 mol
2.1 x 105 C = (2.1 x 105) (4 x 96500)
= 0.54 mol ......................................................................
(iii) Aluminium-air battery
(iv) The cell can be recharged / No air pollutants are produced.
1
1
19. (a) The melting point increases in the order of P, Na, Al, Si.
P exists as P4 molecules, in a simple covalent molecular structure.
The bonds between the molecules are weak van der Waals forces.
Na and Al have giant metallic lattice structures.
The bonds are strong metallic bonds hence more energy is needed to
break the bonds.
The metallic bond in Al is stronger than in Na because Al has 3 valence
electrons compared to only 1 in Na.
Si has a giant covalent structure bonded by strong covalent bonds.
19. (b) Sodium oxide is a basic oxide, thus reacts with acid.
Na2O(s) + 2H+ (aq) 2Na+ (aq) + H2O (l)
Aluminium oxide is an amphoteric oxide which can react with both acid
and alkali. .
Al2O3 (s) + 6H+ (aq) 2Al3+ (aq) + 3H2O (l)
[accept using HCl /H2SO4 for equation, must be balanced]
Silicon dioxide, SiO2 and phosphorus pentoxide, P2O5 are acidic oxides,
thus no reaction with acid.
1
1
1
1
1
(c) Sodium carbonate .
2NaOH(aq) + CO2 (g) Na2CO3(s) + H2O (l)
1
1
20. (a) The reaction proceeds as following :
R-CH=CH2 + H2 R-CH2CH3
Nickel used in this reaction acts as a heterogeneous catalyst. .
Nickel is a transition element that provides a suitable surface for the
adsorption of the reacting molecules, RCH=CH2 and H2 by making use of its
outermost d orbitals.
The adsorption brings the reacting molecules closer to each other and at
the same time weakens the intramolecular covalent bonds.
On top of that, it also holds the molecules in the correct orientation for
new bonds to be formed. All these cause the rate of reaction to increase.
When the bonds are totally broken & the new bonds formed, the products
are released from the surface of nickel to provide a new surface for the
adsorption of other reacting molecules.
(b)(i) Complex ion is an ion formed when a central metal ion is bonded to a
group of ions or molecules through coordinate bonds.
A ligand is an ion or molecule that has lone-pair electrons that can be
donated to a metal central ion to form coordinate bonds. .
(ii) Name : Ethylenediaminetetraacetatocuperate(II) ion / (EDTA)copper(II)
Shape : Octahedral
It is hexadentate ligand that forms six coordinate bonds per molecule with
the central Cu2+ ion.
1
1
1
1
1+1
1
1
1
1
1
1
1
1
1
1
1+1
1+1
1
2
1
1+1
Anda mungkin juga menyukai
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDari EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionPenilaian: 5 dari 5 bintang5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersDari EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersBelum ada peringkat
- Solutions To Problem Set 2Dokumen5 halamanSolutions To Problem Set 2Andy Nguyen100% (1)
- CH 18Dokumen6 halamanCH 18France Mico SobrevegaBelum ada peringkat
- Topic 9.4 2009 Transition Elements Prelim SolnDokumen17 halamanTopic 9.4 2009 Transition Elements Prelim SolndeadbeanBelum ada peringkat
- Tutorial 20: Electrochemistry - Suggested Solutions 1 (A) (1) (I)Dokumen7 halamanTutorial 20: Electrochemistry - Suggested Solutions 1 (A) (1) (I)DomBelum ada peringkat
- Soal ElectrochemistryDokumen3 halamanSoal ElectrochemistryHerlinda OktaBelum ada peringkat
- Electrolysis HLDokumen34 halamanElectrolysis HLRyan BoukaaBelum ada peringkat
- Chem F4 PP1 MSDokumen5 halamanChem F4 PP1 MSandy gideonBelum ada peringkat
- ChemDokumen10 halamanChemAnshika singh sisodiyaBelum ada peringkat
- Chapter9 AnswersDokumen5 halamanChapter9 AnswersedytfuyBelum ada peringkat
- Electrochemistry Worksheet 2: Done in FigDokumen8 halamanElectrochemistry Worksheet 2: Done in Figrezwanur rahmanBelum ada peringkat
- IJC H2 Paper 1 and 2 Answers (For Sharing)Dokumen9 halamanIJC H2 Paper 1 and 2 Answers (For Sharing)Sharon HowBelum ada peringkat
- Reduction-Oxidation Reactions and ElectrochemistryDokumen14 halamanReduction-Oxidation Reactions and Electrochemistrykaushi123Belum ada peringkat
- Answers To Examination Style QuestionsDokumen5 halamanAnswers To Examination Style QuestionsClayanne KnottBelum ada peringkat
- The Transition Elements: Practice ExamplesDokumen15 halamanThe Transition Elements: Practice Exampleskennethleo69Belum ada peringkat
- Nov 2008Dokumen13 halamanNov 2008dharshanaabBelum ada peringkat
- Ujian 1 STPM t2.2015Dokumen6 halamanUjian 1 STPM t2.2015Bbiesoth AJBelum ada peringkat
- Lec 1 QuestıonsDokumen2 halamanLec 1 QuestıonsJumper- VitaBelum ada peringkat
- HKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng AnsDokumen10 halamanHKDSE Chem FX Mock Exam Paper 1 2012 Set 1 Eng Ansleung_ting_2Belum ada peringkat
- Tutorial 9 - Level 2 Worked SolutionsDokumen11 halamanTutorial 9 - Level 2 Worked SolutionsBloodCypherBelum ada peringkat
- Class 11 Chemistry Topperlearning Sample Paper3Dokumen23 halamanClass 11 Chemistry Topperlearning Sample Paper3phultushiblsBelum ada peringkat
- Problem7 PDFDokumen5 halamanProblem7 PDFJunyuanSongBelum ada peringkat
- CH 26Dokumen8 halamanCH 26LilyBelum ada peringkat
- Stoichiometry: Unit: 3Dokumen5 halamanStoichiometry: Unit: 3Premangshu GhoshalBelum ada peringkat
- Chapter 4 ElectrolysisDokumen8 halamanChapter 4 ElectrolysisPremBelum ada peringkat
- Tugas ElektrokimiaDokumen5 halamanTugas ElektrokimiaYasser PatelBelum ada peringkat
- Cls Jeead-15-16 Xii Che Target-5 Set-2 Chapter-3Dokumen44 halamanCls Jeead-15-16 Xii Che Target-5 Set-2 Chapter-3Kartik67% (6)
- Lesson 8 - Electrolysis Part 3Dokumen16 halamanLesson 8 - Electrolysis Part 3Dishna KarunasekaraBelum ada peringkat
- Tutorial 4 - ElectrochemistryDokumen3 halamanTutorial 4 - ElectrochemistryAnis IssabellaBelum ada peringkat
- Chapter 11Dokumen20 halamanChapter 11helloblarg100% (1)
- Marking Scheme: ChemistryDokumen11 halamanMarking Scheme: ChemistryVinay TyagiBelum ada peringkat
- MCQ Prelims 2006Dokumen12 halamanMCQ Prelims 2006Sherman HoBelum ada peringkat
- Redox Dan Electrochemistry (Kimia)Dokumen65 halamanRedox Dan Electrochemistry (Kimia)Rocky Simon HiaBelum ada peringkat
- Class - Xii Chemistry Sample Paper - 3 Time: Three Hours Max. Marks: 70 General InstructionsDokumen17 halamanClass - Xii Chemistry Sample Paper - 3 Time: Three Hours Max. Marks: 70 General Instructionssoumya mazumdarBelum ada peringkat
- Form Four (4) Chemistry 233/1 Marking SchemeDokumen3 halamanForm Four (4) Chemistry 233/1 Marking SchemeTwinomujuniBelum ada peringkat
- UPSEE Sample Papers 2 (UPSEE Chemistry Questions Paper 2)Dokumen6 halamanUPSEE Sample Papers 2 (UPSEE Chemistry Questions Paper 2)Firdosh KhanBelum ada peringkat
- VJC 2007Dokumen14 halamanVJC 2007sswee_1Belum ada peringkat
- Electrochem Understanding - AnswersDokumen11 halamanElectrochem Understanding - AnswersSiva NeshBelum ada peringkat
- Electrolysis: Electrolysis of Molten SaltsDokumen2 halamanElectrolysis: Electrolysis of Molten SaltsSunnyBelum ada peringkat
- SMK Dato Jaafar, JohorDokumen8 halamanSMK Dato Jaafar, JohorJun Hao ChongBelum ada peringkat
- Eamcet 2008 EnggDokumen15 halamanEamcet 2008 EnggjanmanchiBelum ada peringkat
- Electrochem Tutorial SolutionsDokumen30 halamanElectrochem Tutorial SolutionsDarren LimBelum ada peringkat
- RedOx ChemDokumen36 halamanRedOx ChemMary Ann DimacaliBelum ada peringkat
- Chapter 10 ElectrochemistryDokumen76 halamanChapter 10 ElectrochemistryPatrickBelum ada peringkat
- Lesson 8 - Electrolysis Part 2Dokumen16 halamanLesson 8 - Electrolysis Part 2Dishna KarunasekaraBelum ada peringkat
- Solutions of ElectrolytesDokumen22 halamanSolutions of Electrolytesrara_park270% (1)
- RJC 2011 Chem Prelim Paper3ANSDokumen12 halamanRJC 2011 Chem Prelim Paper3ANSJean HomeBelum ada peringkat
- Electrochemistry MCQ SendDokumen7 halamanElectrochemistry MCQ SendRajendra ChikkamathBelum ada peringkat
- Kcet 2014 Chemistryr1 PDFDokumen14 halamanKcet 2014 Chemistryr1 PDFAnweshaBose80% (20)
- 2010 A Level H2 P3 Suggested AnswersDokumen10 halaman2010 A Level H2 P3 Suggested AnswersMichelle LimBelum ada peringkat
- Tenkasi District Schools .Qu - KeyDokumen16 halamanTenkasi District Schools .Qu - Keydevilssworld143Belum ada peringkat
- CHAPTER 9 Electrochemistry Structure and Essay 13-19Dokumen5 halamanCHAPTER 9 Electrochemistry Structure and Essay 13-19peter edwardBelum ada peringkat
- Revision Chapter 9-13Dokumen123 halamanRevision Chapter 9-13Ummul-KBelum ada peringkat
- Cls Jeead-16-17 Xii Che Target-5 Set-2 Chapter-3Dokumen48 halamanCls Jeead-16-17 Xii Che Target-5 Set-2 Chapter-3ArijitSherlockMudiBelum ada peringkat
- Electrochemistry - 23-11-2023Dokumen92 halamanElectrochemistry - 23-11-2023Krish RawatBelum ada peringkat
- 10 ElectrochemistryDokumen77 halaman10 ElectrochemistrySyamil Adzman100% (1)
- Homework 9 SolutionsDokumen6 halamanHomework 9 Solutionsgary_cantuBelum ada peringkat
- Topic 9 (Galvanic Cell) - Tutorial - Level 2 AnswerDokumen7 halamanTopic 9 (Galvanic Cell) - Tutorial - Level 2 AnswerCheng Xun LeeBelum ada peringkat
- EssayDokumen9 halamanEssayTing TCBelum ada peringkat
- Periodic TableDokumen8 halamanPeriodic TableKhairiyah AbdullahBelum ada peringkat
- Kalendar STPM 2018Dokumen2 halamanKalendar STPM 2018Ting TCBelum ada peringkat
- Analysis Past Year Chemistry SPM Question (2003-2017)Dokumen7 halamanAnalysis Past Year Chemistry SPM Question (2003-2017)Ting TCBelum ada peringkat
- Answer, Pre-Exam Practice Chem Sem 3 EssayDokumen29 halamanAnswer, Pre-Exam Practice Chem Sem 3 EssayTing TCBelum ada peringkat
- TEMPLET ReBeST SEBENAR 2019 PDFDokumen1 halamanTEMPLET ReBeST SEBENAR 2019 PDFTing TCBelum ada peringkat
- Kelantan Kim K1 PDFDokumen22 halamanKelantan Kim K1 PDFTing TCBelum ada peringkat
- New Doc 2017-07-31Dokumen2 halamanNew Doc 2017-07-31Ting TCBelum ada peringkat
- Answer For Objective QuestionsDokumen20 halamanAnswer For Objective QuestionsTing TCBelum ada peringkat
- Skema Penang Trial SPM 2014Dokumen21 halamanSkema Penang Trial SPM 2014Ting TCBelum ada peringkat
- Skema Penang Trial SPM 2014Dokumen21 halamanSkema Penang Trial SPM 2014Ting TCBelum ada peringkat
- Break SheetDokumen1 halamanBreak SheetTing TCBelum ada peringkat
- Shape of O3Dokumen2 halamanShape of O3Ting TCBelum ada peringkat
- Important Diagrams LatestDokumen5 halamanImportant Diagrams LatestTing TCBelum ada peringkat
- Break ExsheetDokumen1 halamanBreak ExsheetTing TCBelum ada peringkat
- A4 Sheet Labels TemplateDokumen3 halamanA4 Sheet Labels TemplateEk AjnabiBelum ada peringkat
- Chapter 2 Chemistry Form 6Dokumen10 halamanChapter 2 Chemistry Form 6Ting TCBelum ada peringkat
- SPM Physics Formula List Form 5Dokumen0 halamanSPM Physics Formula List Form 5HaziAmiBelum ada peringkat
- New Document ScanDokumen6 halamanNew Document ScanTing TCBelum ada peringkat
- Answer Chapter 1Dokumen3 halamanAnswer Chapter 1Ting TCBelum ada peringkat
- Break SheetDokumen1 halamanBreak SheetTing TCBelum ada peringkat
- Break SheetDokumen1 halamanBreak SheetTing TCBelum ada peringkat
- Break SheetDokumen1 halamanBreak SheetTing TCBelum ada peringkat
- Answer Scheme Term 2 TrialDokumen3 halamanAnswer Scheme Term 2 TrialTing TCBelum ada peringkat
- Physical Chemistry Lab ManualDokumen36 halamanPhysical Chemistry Lab ManualTing TC100% (1)
- Exp10 PDFDokumen17 halamanExp10 PDFAnurag BajpaiBelum ada peringkat
- Exp10 PDFDokumen17 halamanExp10 PDFAnurag BajpaiBelum ada peringkat
- How To Fix Number Formatting in Mail Merge Greenwich - Computer - Help PDFDokumen5 halamanHow To Fix Number Formatting in Mail Merge Greenwich - Computer - Help PDFTing TCBelum ada peringkat
- Phase DiagramDokumen3 halamanPhase DiagramTing TCBelum ada peringkat
- Topic 2.3 FormativeDokumen2 halamanTopic 2.3 Formativeishaan50% (2)
- Basic of Fluid FlowDokumen51 halamanBasic of Fluid FlowIqra Zulfiqar100% (1)
- Catabolism of Proteins and Amino AcidsDokumen63 halamanCatabolism of Proteins and Amino Acidsflairtique shopBelum ada peringkat
- Fly Ash: Safety Data SheetDokumen5 halamanFly Ash: Safety Data SheetGaluh AlmasBelum ada peringkat
- Exam Sample QuestionsDokumen9 halamanExam Sample QuestionsYousab JacobBelum ada peringkat
- Recent Enhancements in Pulsed Eddy Current Analysis: Improving The Sizing of Small FlawsDokumen4 halamanRecent Enhancements in Pulsed Eddy Current Analysis: Improving The Sizing of Small Flawsafiqhashim89Belum ada peringkat
- Literature Review On Pervious Concrete Literature Review On Pervious ConcreteDokumen23 halamanLiterature Review On Pervious Concrete Literature Review On Pervious ConcreteVivek KumarBelum ada peringkat
- Ce6411 Strength of Materials Laboratory (Civil)Dokumen32 halamanCe6411 Strength of Materials Laboratory (Civil)KishanKanhaiyaBelum ada peringkat
- C 1087 00 Sellantes Compatibilidad EstructurasDokumen4 halamanC 1087 00 Sellantes Compatibilidad Estructurasadrianhhhh1984Belum ada peringkat
- Auxetic MaterialsDokumen65 halamanAuxetic MaterialsSubramani PichandiBelum ada peringkat
- Asme B16.34Dokumen15 halamanAsme B16.34Achraf Ismail100% (1)
- Basics of Cleaning & Cleaning ValidationDokumen6 halamanBasics of Cleaning & Cleaning Validationjhpjayant100% (1)
- Quantitative JDokumen4 halamanQuantitative Jryan caballerobatuangBelum ada peringkat
- PSP CastingDokumen7 halamanPSP CastingalexisBelum ada peringkat
- 4HB0 01 Que 20140108Dokumen32 halaman4HB0 01 Que 20140108Lalith77Belum ada peringkat
- Freezing Point Depression & Boiling Point Elevation-ExploreLearning SimulationDokumen9 halamanFreezing Point Depression & Boiling Point Elevation-ExploreLearning SimulationSunita BiswalBelum ada peringkat
- Appendix 7.: Nutritional Goals For Age-Sex Groups Based On Dietary Reference Intakes &Dokumen2 halamanAppendix 7.: Nutritional Goals For Age-Sex Groups Based On Dietary Reference Intakes &GloryJaneBelum ada peringkat
- H. Schubert-Wet Classification and Wet Screening of Fine ParticlesDokumen17 halamanH. Schubert-Wet Classification and Wet Screening of Fine Particlesxiaochi1989Belum ada peringkat
- Saso 2203 - DSDokumen11 halamanSaso 2203 - DSAhmedBelum ada peringkat
- Osmometry ElectrochemistryDokumen25 halamanOsmometry ElectrochemistryMohamed MidoBelum ada peringkat
- Answers Bahan Pelajar Bintang Carbon Compound: Quiz 1 1 (A)Dokumen5 halamanAnswers Bahan Pelajar Bintang Carbon Compound: Quiz 1 1 (A)airinBelum ada peringkat
- Worksheet14 HybridDokumen5 halamanWorksheet14 HybridRAGINI AGARWALBelum ada peringkat
- DOC316.52.93094 - 3ed - Boric AcidDokumen6 halamanDOC316.52.93094 - 3ed - Boric AcidLim CalcynBelum ada peringkat
- Seaflo Neo SL Z (1606) PDFDokumen2 halamanSeaflo Neo SL Z (1606) PDFTrịnh Minh KhoaBelum ada peringkat
- Russell J. Donnelly - Fifty-Five Years of Taylor - Couette FlowDokumen35 halamanRussell J. Donnelly - Fifty-Five Years of Taylor - Couette FlowQMDhidnwBelum ada peringkat
- Assigment PlantDokumen2 halamanAssigment PlantAra Lee100% (1)
- The One Dimensional Heat Equation 1 Informal Derivation in One DimensionDokumen6 halamanThe One Dimensional Heat Equation 1 Informal Derivation in One DimensionThulasi RamBelum ada peringkat
- E Clips CurvesDokumen78 halamanE Clips Curvesner68Belum ada peringkat
- Advanced Engineering, Worldwide Facilities & Comprehensive Technical SupportDokumen12 halamanAdvanced Engineering, Worldwide Facilities & Comprehensive Technical SupportCenon MalabananBelum ada peringkat
- Class03 ChemistryG12 Notes and HomeworkDokumen68 halamanClass03 ChemistryG12 Notes and HomeworkAndy Rei KouBelum ada peringkat