Use of Steam and Co2 As Activating Agents
Diunggah oleh
vinodJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Use of Steam and Co2 As Activating Agents
Diunggah oleh
vinodHak Cipta:
Format Tersedia
Carbon, Vol. 33, No. 1, pp.
15-23, 1995
Copyright 0 1995 Elsevier Science Ltd
Pergamon
Printed in Great Britain. All rights reserved
0008.6223/95$9.50 + .OO
0008-6223(94)00100-6
THE USE OF STEAM AND CO2 AS ACTIVATING AGENTS
IN THE PREPARATION OF ACTIVATED CARBONS
F. RODRIGUEZ-REINOSO, M. MOLINA-SABIO and M. T. GONZALEZ
Departamento de Quimica InorgAnica, Universidad de Alicante, Apartado 99, E-03080 Alicante, Spain
(Received 22 February 1994; accepted in revised form 11 July 1994)
Abstract-Four
series of activated carbon have been prepared from carbonized olive stones. One of them,
series D, was prepared using carbon dioxide as activating agent, and the other three, series AV, W, and
H, with water vapor under different experimental conditions. Two of the series, D and H, were prepared
in such a way that the gasification rate for both reactants was identical, in an attempt to reduce the effect
of the relative differences in diffusion and accessibility of both gases to the interior of the particles. The
changes in porosity of the original char during activation have been studied by adsorption of N, at 77 K
and CO, at 273 K, as well as by mercury porosimetry. The results obtained show that carbon dioxide produces an opening, followed by widening, of narrow microporosity, whereas water vapor widens the microporosity from the early stages of the process, the resulting activated carbon exhibiting lower micropore
volume. However, dilution of water vapor and high activation temperatures approach the development
of total microporosity by steam to that of carbon dioxide, although there is a more important role of narrow microporosity widening in the former.
Key Words-Activated
carbon, CO, activation, steam activation, porosity development.
1. INTRODUCTION
the micropore volume of fibers activated with carbon
dioxide was larger than when activated with steam;
they claimed that steam produces a larger widening of
the micropores
(which become mesopores) than carbon dioxide.
In principle,
one could expect, following Wigmans[9], that the larger molecular dimensions of the
CO2 molecule would make the diffusion of this molecule through the pore system of the carbon particle
more difficult and slower, with subsequent more limited accessibility to the micropores and slower gasification rate. Although there seems to be no controversy
on the latter (slower gasification rate for CO& the effect on the development of porosity, especially that of
microporosity, does not seem to follow a clear tendency.
The general objective of this work is to study the
development
of porosity in a char subjected to gasification with steam and carbon dioxide. Since the
porosity is also dependent upon the experimental conditions used during activation, several series have been
prepared with steam by modifying the partial pressure
and the activation temperature. Two of the series were
prepared in such a way that the gasification rate with
both activating agents was identical, in an attempt to
reduce the relative differences in diffusion and accessibility of both gases.
The porosity of activated carbons is conditioned,
among other factors, by the carbonaceous
precursor
and the activation method used. In physical activation,
the gasification of a char with gases, the char is reacted
at high temperature (usually above 800C) with steam,
carbon dioxide, or a mixture of these. Both carbon dioxide and steam are mild oxidant at NO-95OC, and
eliminate carbon atoms from the char particle via CO
and/or CO + Hz, in such a way as to favor the selective burning of the interior of the particle, with the
subsequent creation of porosity[ 11.
The experimental
information
described in the literature on the gasification rate of different forms of
carbon, such as graphite[2,3],
coke[4], chars[5-71, or
carbon fibers@], show that, for a given temperature,
the reactivity with steam is larger than with carbon dioxide. However,
when the effect of the activating
agent on the development of porosity is analyzed, the
results do not show a clear tendency. Thus, Tomkov
et a/.[6], when activating brown coal cokes with carbon dioxide and steam to common degrees of burnoff, found that the latter produced activated carbons
with larger adsorptive capacity and wider pore size distribution, with a larger contribution
of mesoporosity.
This tendency is opposite to that described by Wigmans[9], who deduced that carbon dioxide widens the
microporosity
and leads to a larger mesopores/micropores ratio than steam. Kiilh et af.[4], for different
cokes activated in a range of temperatures (900-l lOOC),
showed that the BET surface area of cokes activated
with steam is, in general, larger than when using carbon dioxide in the preparation
of the activated carbons. However,
Ryu et a/.[8], found the opposite
effect when activating pitch-based carbon fibers, since
2. EXPERIMENTAL
All series of activated carbons were prepared from
a common precursor, a char obtained from olive stones
by carbonization
in nitrogen at 850C for 2 hours.
This char was activated in a horizontal furnace under
a constant flow of activating agent, using different
gases (steam and carbon dioxide), activation temper15
16
F.
RODRiGUEZ-REINOSO
ature, and residence time. In this way, four different
series were prepared:
1. Series D: CO* flow (80 ml/min) at 825C for different periods of time, to obtain activated carbons
in the burn-off range 8%80%.
2. Series AV: N,/water
vapor flow (80 ml/min), at
800C for different periods of time to cover the
burn-off range 9-74070.
3. Series W: 100% water vapor flow (100 ml/min,
provided by injection of 0.08 ml/min of liquid
water from a peristaltic pump) at 800C for different periods of time to cover the burn-off
range
lo-71%.
4. Series H: 100% water vapor flow (100 ml/min) at
750C for different periods of time to cover the
burn-off range 8-74%.
A more detailed description of experimental details
for the preparation
of series D, AV, and W can be
found in previous reports[lO-131.
The nomenclature
of all activated carbons includes the burn-off reached
in its preparation.
The comparison
of the changes in porosity of the
char produced by the different experimental
conditions has been based on some parameters
which,
though they do not completely characterize
the carbon, provide very useful information on the evolution
of the different pore size ranges. The parameters chosen are as follows:
1. Micropore volume deduced from the application of
the Dubinin-Raduskhevich
(DR) equation to the
adsorption
isotherms of Nz at 77 K and COZ at
273 K, I/,(N,) and Ve(CO,), respectively. Previous
work[14,15] has shown that the linear part of the
DR plot including experimental
data up to a relative pressure of P/P0 = 0.3, provides the total
volume of micropores,
including
the so-called
supermicropores[l6],
whereas the &(COz) value
(given the very low relative pressure range covered
at the temperature
of adsorption,
273 K) corresponds to the volume of narrow micropores (up to
about 0.7 nm).
2. Mesopore
volume deduced from the adsorption
isotherm of N, (77 K), calculated by subtracting
the value of V,(N,) from the amount adsorbed at
P/P0 = 0.95, Qss(N2) - V,(N,). In type I isotherms[l6], the amount adsorbed at pressures near
unity (0.95 in this case) corresponds
to the total
amount adsorbed in both the micropores (filled at
low relative pressures) and the mesopores (filled by
capillary condensation at pressures above 0.2), and
consequently
the subtraction
of V, from the total
amount will provide the volume of mesopores. This
parameter is very useful when comparing the porous texture of activated carbons.
3. Pore volume deduced from mercury porosimetry,
covering the pore size range 7.5-15000 nm, I$,,.
This value is sub-divided into Vmeao(pore size up
ef
d.
to 50 nm) and I,,,,,, (pore size larger than 50 nm),
following the recommendations
of the IUPAC[16].
This permits a more detailed comparison
of pore
structures in the activated carbons.
3. RESULTS
The weight losses (burn-off)
experienced
by the
char as a function of residence time for the four series
of activated carbon prepared are plotted in Fig. 1. It
is clearly shown that the gasification rate is constant
in all series except in series AV, activated with diluted
steam at 8OOC, for which the gasification
rate in
the first stages of the process is about 1% h- but it
increases from 30% burn-off to 1.7% h-. On the
other hand, the reactivity of the char with pure steam
at 800C (series W) is almost double that of series D
(activated with carbon dioxide), even though the activation temperature
for the latter is higher (825C).
In order to obtain activated carbons with steam having comparable gasification rates to those of series D,
the temperature
of gasification had to be lowered to
750C (series H).
The four series of activated carbons prepared will
show if the porosity developed by steam activation is:
1) a function of the gasification rate originated by the
changes in gasification temperature (series W and H);
2) a function of the activation temperature combined
with the dilution of water vapor, keeping to some extent the gasification rate (series AV and H), and 3) dif-
Burn-off
(%)
loo2
20
40
60
80
Time (h)
Fig. 1. Evolution of burn-off versus activation
bons gasified in CO2 and steam.
time for car-
Steam and COz as activating agents
n (mmol/g)
17
VHs@m3/s)
35
1.4
A74 \
meoo macro
\ -\
25
0.6-
0
0.0
0.2
0.4
0.6
0.6
1.0
P/P,
Fig. 2. Adsorption of N, at 77 K for some carbons of series
D, H, W, and AV.
Fig. 3. Cumulative pore volumes (mercury porosimetry) for
some carbons of series D, H, W, and AV (R, in nm).
ferent from the porosity developed by carbon dioxide
activation,
even though the gasification
rate is similar (series AV, H, and D).
Figure 2 includes some examples of N2 (77 K) adsorption isotherms and Fig. 3 some cumulative pore
volume plots deduced from mercury porosimetry,
in
all cases for activated carbons with comparable burnoff, but prepared using the different experimental conditions of the four series. On the other hand, Fig. 4
includes the plots for the evolution of the volume of
different pore size ranges as a function of burn-off,
deduced from the adsorption
of N, at 77 K and mercury porosimetry,
all data being referred to one gram
of activated carbon (see Table 1).
activation is simultaneously to enhance and widen narrow micropores, the latter effect predominating
above
30-40% burn-off, as denoted by the increasing value
of Vo(N,) with respect to Vo(C02). This widening of
microporosity
does not correspond with a remarkable
increase in the volume of mesopores (Fig. 4a), in contrast to the evolution of the volume of macropores,
which reaches values as high as the volume of micropores (compare Figs. 4a and 4~). Although the volumes
of Fig. 4c correspond to pore sizes larger than 7.5 nm,
and consequently
they include part of mesoporosity,
the proportion
of mesoporosity
is relatively small, as
deduced from Fig. 3 and Table 1.
3.1 Series D
In general terms, activation with carbon dioxide
uniformly develops all the pore size ranges of the carbon, in the sense that increasing activation produces
a continuous increase in the volume of micropores and
mesopores (Figs. 4a and 4b) and macropores (Fig. 4c).
Furthermore,
there is a linear relationship between the
different pore volumes and burn-off, with the exception of V,(N,). There is for Vo(N2) a more important
development
of microporosity
for burn-offs
above
40%. On the other hand, V,(N,) and ve(CO,) are coincident only up to about 20% burn-off (see Table I),
the difference in favor of Vo(N,) increasing with activation, especially above 40% burn-off. Consequently,
it is possible to state that the effect of carbon dioxide
3.2 Series AV
Activation with diluted steam at 800C produces,
as in the case of carbon dioxide, a development of all
ranges of porosity in the carbon. However, there are
some clear differences with respect to series D, which
are not important in the first stages of the process, but
become very important
at medium-high
burn-offs.
Thus, the development
of narrow microporosity
is
hindered in the activation with diluted steam, since
Ve(CO,) is almost constant with increasing activation
(Fig. 4b). This means that only widening of existing
narrow micropores occurs during activation, the value
of &(N,) being similar to series D. The difference is
more marked for the values of I&(N2)
- V,(N,),
mesoporosity
(Fig. 4a). In fact, the linear portions
of the adsorption
isotherm at medium-high
relative
F. RODRIGUEZ-REINOSO et al.
18
V (cm3 lg)
0.81
dA
q iW
OAV
AD
OH
qW
OH
b)
0.6
0
Burn-off
IfiD
Fig. 4. Evolution
20
40
60
80
Burn-off
(%)
OAV
o.oc-,
0
(%)
V,,(cm3/9)
T----
20
40
60
80
Burn-off
(%)
of pore volume (per gram of activated carbon) as a functions of burn-off:
micropores
N2, and -----mesopores;
b) micropores
CO,; c) V,,.
pressures (see Fig. 2) for carbons AV74 and D70 are
very different; the former exhibit a larger slope. This
difference means that activation with diluted steam essentially produces a widening of the micropores
to
meso and macropores,
the proportion
of mesopores
a) ~
being more important in series AV than in D, as shown
in Figs. 3 and 4a. Macropore size distribution
is also
different in the two series; the development
of largesize macroporosity
in series D constitutes an important contribution
to the total pore volume and it is
19
Steam and CO, as activating agents
Table 1. Pore volumes (cm3/g) for activated carbons series
CO, (273 K)
N, (77 K)
Carbon
Vo 0%)
vO.95
Vo
v,
(CO,)
VHg
Vmacro
VmeSO
D-8
D-19
D-34
D-52
D-70
D-80
0.26
0.31
0.39
0.55
0.67
0.78
0.03
0.03
0.04
0.03
0.05
0.05
0.26
0.30
0.36
0.41
0.47
0.51
0.21
0.33
0.47
0.64
0.78
0.93
0.10
0.23
0.40
0.57
0.70
0.81
0.11
0.10
0.07
0.07
0.08
0.12
AV-9
AV-17
AV-37
AV-53
AV-63
AV-74
0.26
0.28
0.41
0.52
0.65
0.77
0.01
0.03
0.11
0.17
0.18
0.31
0.27
0.28
0.31
0.33
0.33
0.34
0.21
0.24
0.47
0.66
0.83
1.33
0.08
0.10
0.32
0.49
0.59
0.93
0.13
0.14
0.15
0.17
0.24
0.40
w-10
W-23
w-41
W-58
w-71
0.24
0.32
0.47
0.62
0.73
0.01
0.03
0.06
0.08
0.12
0.23
0.27
0.30
0.32
0.31
0.22
0.39
0.57
0.66
1.15
0.14
0.30
0.45
0.50
0.90
0.08
0.09
0.12
0.16
0.25
H-8
H-22
H-37
H-52
H-74
0.24
0.33
0.39
0.46
0.55
0.01
0.04
0.04
0.06
0.08
0.21
0.25
0.28
0.30
0.29
0.47
0.53
0.51
0.58
0.94
0.19
0.28
0.41
0.45
0.72
0.28
0.25
0.10
0.13
0.22
uniformly
distributed up to log R = 2.0 (pore width =
200 nm), whereas the largest development in series AV
is localized in the pore size range log R = 2.5-3.0 (pore
width = 600-2000 nm).
size distributions (except in the mesoporosity),
but different from those obtained when activating with CO1
(series D).
3.4
3.3
Mercury porosimetry
Series W
Although series W has also been prepared by activation with steam, it is different from series AV in that
activation takes place in the former with 100% steam,
leading to a larger reactivity (Fig. 1). However, the development of porosity with increasing activation takes
place, in general terms, in a similar fashion to series
AV. In fact, only slight differences are observed in the
values of V,(N,), V,(CO,) and I&, the differences in
&,,(Nz) - Ve(N,) being larger. Such differences may
be observed in more detail in Fig. 2. Thus, the isotherms for AV74 and W71 exhibit a linear portion
of P/P0 = 0.3-0.8, that of the former having a larger
slope, thus indicating a larger development
of mesoporosity with diluted steam activation. Similar conclusions may be reached when comparing the cumulative
plots deduced from mercury porosimetry (Fig. 3). Although the general shape of the plots are similar, the
more marked differences are found in the pore volume
of the smallest pore widths (7.5 nm, log R = 0.57) and
for macropores
of around 1300 nm (log R = 2.8). It
seems then that activation with steam (both pure and
diluted) proceeds in a similar way, namely, widening
of the microporosity,
without appreciable creation of
new micropores.
It is true, however, that the widening is more important when the steam is diluted with
nitrogen. In any case, activation with the two modes
of steam produces activated carbons with similar pore
Series H
Carbons of series H, though prepared with steam
at the same gasification rate (by adjusting the activation temperature)
as series D with carbon dioxide, exhibit a porosity which in general terms is closer to
carbons of the other series prepared by activation with
steam (series AV and W) than to carbons of series D.
This is clearly shown by the similarity in the shape of
the adsorption isotherm of N2 (77 K) in Fig. 2 and the
cumulative pore volume plots deduced from mercury
porosimetry
(Fig. 3). Furthermore,
the evolution of
Ve(CO,) with burn-off for series H is similar to those
of series AV and W. However, the lower activation
temperature
develops to a lower extent all ranges of
porosity. In particular, the micropore and mesopore
volumes are lower than in the other two series, the volume of large size pores being rather similar at low and
medium burn-offs.
Consequently,
it seems as if the
pore size distribution
of the activated carbon is conditioned, in general terms, by the activating agent (carbon dioxide or steam) and not by the experimental
conditions used (temperature,
dilution), which have a
direct influence on the final volume of pores developed, at least in the cases under study here.
3.5
Evolution of porosity during activation
In order to better analyze the creation or destruction of pores during activation, it is much more convenient to use the results expressed per unit mass of
F. RODRIGUEZ-REINOSO et al.
20
the starting char[9,12,17],
as in Figs. 5a, 5b, and 5c.
There is in almost all plots an initial increase in adsorbed volume, followed by a decrease at higher
burn-offs. Therefore, only in the first stages of the ac-
tivation process (low burn-off)
is there creation of
porosity, the widening of which and burning of the exterior of the particle becoming significant at burn-offs
larger than 40 to 50%.
(cm3 /gchar)
AD
OAV
V,(C0,)(cm3
qW
a)
OH
/gchar)
AD
OAV
qW
b)
OH
0.3
0.0
0.0
20
40
60
80
20
40
60
Burn-off (%)
0.41
AD
OAV
Burn-off (96)
OW
cl
OH
0.3
0.2
0.1
0.0
0
20
40
60
80
Burn-off (%)
Fig. 5. Evolution of pore volume (per gram of starting char) as a function of burn-off.
pores N,, and ------
80
mesopores;
b) micropores
CO*; c) vHs.
a) _
micro-
Steam and CO2 as activating agents
It is important to note that the maximum development of micro and macroporosity
in series H (prepared at lower temperature)
takes place at around
20% burn-off, a value lower than for the maxima of
the other series, around 40%-50% burn-off. It is also
observed that for activation with water vapor (series H,
W, and AV) the increase in activation temperature and
the use of diluted steam favor the development
of
mesoporosity measured as V& - Vo(NZ), and of macroporosity (the latter at high burn-offs).
This development of porosity differs from the one observed for
carbon dioxide activation (series D) because, in this
case, activation produces a continuous destruction of
mesoporosity
(Fig. 5a), and there is a marked loss of
macroporosity
from 50% burn off (Fig. SC).
A more in-depth examination
of Fig. 5 permits a
better differentiation
among the series, since it reveals
details of the evolution of porosity in the char particle during activation. This evolution may be summarized for the four series as follows:
1. Series D: there is an opening of microporosity
(V,(CO,) and V,(N2) increase) up to about 20%
burn-off, followed by a widening (V,(NJ increases
and VO(COz) decreases) up to about 40%-50%
burn-off, the microporosity
decreasing thereafter.
A similar evolution is observed for VHgrwhich does
not correspond
to the continuous
decrease in the
mesoporosity
measured by adsorption,
V0,95(N2) b(N2).
2. Series AV: the evolution of microporosity
is similar to series D, the difference being that the widening of narrow micropores beings at lower burn-off
(~20%).
However,
the evolution of meso- and
macroporosity
is completely different, since it increases continually with burn-off.
3. Series W: the evolution of total microporosity,
Vo(N2), is similar to the two previous series, but
the narrow microporosity,
V,(CO,), is not developed and decreases after 20% burn-off at the same
rate as in series AV. The mesoporosity,
V0,&N2) Vo(N2), increases up to 40% burn-off, remaining
constant thereafter.
VHgfollows a similar pattern
to series AV, but the development
of porosity is
larger for series W at low-medium
burn-offs.
4. Series H: the evolution of narrow microporosity
is
different to the other series, since it is destroyed
from the first stages of activation. However, V,(N,)
increases as in the other series up to 20% burn-off,
the decrease at larger burn-offs taking place at a
higher rate than in any of the other series. Meso
and macroporosity
follow the same sequence as in
series D, but the destruction
of large-size porosity
takes place at lower rate in series H.
4. DISCUSSION
From the results presented above, one can deduce
that there are several factors (activation temperature,
21
steam dilution, and activating agent) conditioning the
porosity of the final activated carbon. In order to facilitate the discussion, it is better to differentiate
the
process for steam activation (influence of temperature
and dilution) and then to compare it with carbon dioxide activation.
4.1
Series H and W
When the activating gas comes in contact with
the char, it will react with both the exterior and the
interior of the particle, the latter requiring previous
diffusion of the activating gas. At the activation temperature used in the preparation
of series H, 75OC,
the diffusion rate of water molecules to the interior of
the particle is sufficiently high to expect a development
of the whole range of porosity, at least in the early
stages of activation. However, the progress of the reaction brings about the production
of CO and H-Z,
which are inhibitors of the reaction (especially H2).
The increase in the concentration
of inhibitors in the
interior of the particle will favor the reaction of steam
at the exterior, with the subsequent destruction of porosity. The results of Fig. 5 for series H show the predominance of external burning upon the reaction in
the interior of the particle at burn-offs higher than
20%. Up to 20% burn-off there is a widening of the
microporosity,
since V,(N,) increases and Vo(C02)
remains almost constant, with a simultaneous
slight
increase in Vo.,,(N2) - V,(N,) and V&. There is destruction of porosity for burn-offs larger than 20%,
especially in the range of microporosity,
since the volume of meso- and macroporosity
decreases slowly (the
widening of porosity produced by steam continues until the end of the process).
The increase in activation temperature
to 800C
(series W) makes the inhibiting effect of hydrogen less
effective[l8,19]
and, consequently,
one could expect
a larger development
of micro-, meso-, and macroporosity than in series H, with the maximum development of porosity displaced to larger burn-offs (around
50%, as shown in Fig. 5). Further increases in activation temperature
do not necessarily imply larger
development
of porosity because the rate for the carbon-Hz0
increases rapidly and the control of the reaction is more influenced by pore and bulk diffusion
effects. Under these conditions, the reaction would take
place predominantly
on the exterior of the particle
[2,20]. For this precursor, the maximum development
of porosity has been found to be in the 800-850C
range[21]. It is noteworthy that for both series W and
H the development
of micro- and macroporosity
is
high in comparison with the increase in mesoporosity
measured by Vo.ss(N2) - Vo(Nz). In fact, the changes
produced in the latter range of pore size are very small
in both series. It seems as though the development
of
macropores produced during activation is due mainly
to direct creation by action of the activating agent on
the exterior of the particles. Thus, the proportion due
to the widening of smaller pores is negligible, except
for carbons obtained at large burn-offs; the widening
F.
22
RODRiCLJEZ-REINOSO
of porosity compensating
the loss of macroporosity,
with a net creation in carbon W-71.
4.2
Series Hand
AV
When water vapor is diluted with nitrogen (series
AV), there is a decrease in gasification rate and, consequently, an increase in activation temperature is convenient. Under these conditions (inhibition by CO and
HZ is less effective due to the increase in temperature),
the reaction of the activating agent in the interior of
the particle is easier, with subsequent development of
micro and mesoporosity
in a wide range of burn-off,
and a simultaneous
retarded development
of macroporosity. Furthermore,
since the reaction in the interior of the particle is more favored than in series H,
there is (see Fig. 5) a creation of microporosity
(initial increase in the volume of narrow micropores) and
further widening of this porosity in a wide range of
burn-off.
The total volume of micropores,
V,(N,),
increases up to about 40% burn-off. This increase in
pore width means a net creation of mesoporosity
and
macroporosity,
which continues up to the end of the
process.
4.3
Series H, AV, and D
The comparison of activation with steam or carbon
dioxide is more complex since, 1) they have different
molecular dimensions, and 2) the reactivity, for a common temperature, is different. For a given porosity the
effective diffusivity of water vapor, with smaller molecular weight and dimensions, is estimated to be around
50% larger than that of carbon dioxide. Such ratio is
maintained approximately
constant with temperature
(in the 750~825C range), since the activation energy
value for the effective diffusivity is very low[20,22].
Consequently,
water vapor would be expected to develop the narrower microporosity
to a larger extent
than carbon dioxide. For series W and D, prepared at
similar temperature
and with a gas flow of 100% for
both activating agents, the results of Figs. 5a and 5b
do not show this expected behavior. This is because
steam produces widening of existing narrow micropores, increase in V,(N,) and decrease in l+,(CO,),
whereas carbon dioxide permits the creation of narrow
micropores, increase in ~&COz), in the first stages of
the process.
It could be argued that the differences found in series W and D are due to the larger experienced reactivity for steam and, consequently,
the results would
not be comparable,
since an increase in reactivity
could mean a larger contribution of mass transport effects over the chemical reaction[2]. A more appropriate comparison
would be to condition the activation
with carbon dioxide and steam in such a way that the
steam reaction produces the same rate of carbon atom
removal from the char as carbon dioxide. This can be
attained by either diluting the steam flow (first stages
of series AV) or by decreasing the reaction temperature (the whole series H), and comparing with series
D. As shown in Fig. 5a, dilution increases the development of microporosity
in the early stages of the ac-
et ai.
tivation process, though pore widening predominates
at burn-off larger than 10%. This widening is so pronounced that, at high burn-offs, the loss of porosity
of large dimensions by external burning is compensated
for, at least in part, by the widening of micropores.
From the results for series H and D (Figs. 4a, 4b,
5a, 5b), it is deduced that the development
of microporosity, as measured by V,(N,) and V,(CO,), by steam
gasification
is much lower than by carbon dioxide.
Furthermore,
as shown in Fig. 5b, there is a continuous destruction of narrow microporosity
in series H,
in contrast with the creation of this porosity taking
place in early stages of series D. On the other hand,
microporosity
widening predominates
to a larger extent for steam.
All results given above show that microporosity
widening is observed in all series prepared with steam,
and it seems relatively clear that the activating agent
is the parameter controlling to a larger extent the development of porosity. Carbon dioxide produces an
increase in narrow microporosity
in the early stages
of activation, whereas steam is more effective in micropore widening. The explanation
of the different
porous structure of activated carbons prepared by activation with steam and carbon dioxide is not simple,
but two hypotheses could be advanced. Considering
that activation is taking place in a char with a narrow
microporosity,
of pore width similar to the size of the
nitrogen molecule, as shown[23] by the fact that adsorption is a rate process with a positive temperature
coefficient (the adsorption of 90 K is much larger than
that of N2 at 77 K):
1) for a common overal reactivity, the water molecules within the porous structure react less selectively than carbon dioxide, producing micropore
widening. A previous work[24] has shown the different attack of carbon dioxide and steam on graphite, and it is ascribed to the fact that the carbon
dioxide is linear, with oxygen atoms in the two ends,
whereas the water molecule is angular, with oxygen in the middle.
2) activation with carbon dioxide leaves in the carbon
structure a larger number of oxygen surface groups
than steam[25]; these surface groups, in the activated carbons obtained in the early stages of activation (when porosity is still relatively narrow),
may act as constrictions
of the micropore, reducing the effective pore width. This effect will disappear at larger burn-offs,
when the pore size is
larger. Further work is in progress to determine
if this hypothesis can be proved to be the more
realistic.
5. CONCLUSIONS
The results described in this work are based on an
experimental program designed to elucidate the different effect of the activating agent (carbon dioxide or
steam) in the development
of porosity of a char obtained from olive stones. The four series of activated
Steam and CO, as activating
carbons prepared cover a wide range of experimental
conditions
and burn-off,
and include samples prepared with both activating
agents using the same
activation rate. The results clarify the apparent discrepancies in previous published work[4,6,8,9],
in the
sense that:
1) Carbon dioxide produces an opening, followed later
by widening, of narrow microporosity,
whereas
steam widens the microporosity
from the early
stages of the activation process, even in the case in
which the removal rate of carbon atoms from the
char is the same for the two activating agents.
2) For a common gasification
rate, activation with
pure steam leads to activated carbons with lower
micropore volume than carbon dioxide. However,
dilution of water vapor and high activation temperatures approach the development
of total microporosity by steam to that of carbon dioxide, although there is a more important role of narrow
microporosity
widening in the former.
3) Steam activation produces a larger development of
meso- and macroporosity
than carbon dioxide activation, the resulting activated carbon exhibiting
a wider pore size distribution.
Acknowledgements-This
work was supported
by the
DGICYT
(project
No. PB91-0747).
M.T.G.
thanks the
DGICYT for a fellowship to carry out this work.
REFERENCES
1. R. C. Bansal, J. B. Donnet, and H. F. Stoekcli, Active
Curbon. Marcel Dekker, New York (1988), p. 14.
2. P. L. Walker, Jr., F. Rusinko, Jr., and L. G. Austin, In
Advances in Catalysis (Edited by D. D. Eley, P. W. Selwood, and P. B. Weisz), Vol. XI, p. 133. Academic
Press, New York (1959).
3. G. R. Henning,
In Chemistry and Physics of Carbon
(Edited by P. L. Walker, Jr.), Vol. 2, p. 33. Marcel Dekker, New York (1966).
4. H. Kiihl, M. M. Kashani-Motlagh,
H. J. Miihlen, and
K. H. van Heek, Fuel 71, 879 (1992).
5. G. Q. Lu and D. D. Do, Carbon 30, 21 (1992).
agents
23
6. K. Tomkov, T. Siemieniewska,
F. Czechowski,
and A.
Jankowska,
Fuel 56, 121 (1977).
7. W. F. DeGroot and G. N. Richards,
Carbon 27, 247
(1989).
8. S. K. Ryu, H. Jin, D. Gondy, N. Pusset, and P. Ehrburger, Carbon 31, 841 (1993).
9. T. Wigmans, Carbon 27, 13 (1989).
A. Linares-Solano,
J. M. Martin10. J. Garrido-Segovia.
Martinez, M. Molina-Sabio,
F. Rodriguez-Reinoso,
and
R. Torregrosa, J. Chem. Sot., Faraday Trans. I 83, 1081
(1987).
A. Linares-Solano,
M. Molina11. F. Rodriguez-Reinoso,
Sabio, and I. Perez-Lled6,
Extended Abstracts 17th Biennial Conference on Carbon, Lexington,
KY (1985),
p. 239.
In Fundamental Issues in Con12. F. Rodriguez-Reinoso,
trol of Carbon Gasification Reactivity (Edited by J.
Lahaye and P. Ehrburger),
NATO ASI Series E-192,
p. 533. Kluwer Academic Publishers,
Dordrecht,
Netherlands (1991).
and F. Rodriguez13. M. T. GonzBlez, M. Molina-Sabio,
Reinoso, Proceedings Carbon 92, Essen, Germany, Paper P-C5, Deutsche Keramische
Gesellschaft
(1992),
p. 310.
J. M. Martin-Martinez,
14. J. Garrido, A. Linares-Solano,
M. Molina-Sabio,
F. Rodriguez-Reinoso,
and R. Torregrosa, Langmuir 3, 76 (1987).
J. Garrido, J. M. Martin-Martinez,
15. F. Rodriguez-Reinoso,
M. Molina-Sabio,
and R. Torregrosa,
Carbon 27, 23
(1989).
16. S. J. Gregg and K. S. W. Sing, Adsorption, Surface and
Porosity. Academic Press, London (1982).
17. H. Marsh and B. Rand, Carbon 9, 47 (1971).
and M. A. Elliott, In Chemis18. C. G. von Fredersdorff
try of Coal Utilization (Edited by H. H. Lowry), Supplementary
Volume, p. 754. Wiley, New York (1963).
C. Y. Chen, and D. S. Scott, Znd.
19. J. F. Johnstone,
Engng. Chem. 44, 1564 (1952).
Prog. Energy Combust. Sci. 4, 221
20. N. M. Laurendau,
(1978).
M. Molina-Sabio,
and F. Rodriguez21. M. T. Gonzilez,
Reinoso, Carbon 32, 1407 (1994).
22. G. F. Froment and K. B. Bischoff, Chemical Reactor
Anafysis and Design. John Wiley, New York (1979),
p. 163.
J. D. L6pez-Gonzrilez,
and C.
23. F. Rodriguez-Reinoso,
Berenguer,
Carbon 20, 513 (1982).
24. R. T. Yang and C. Wong, J. Catalysis 82, 245 (1983).
and F. Rodriguez25. M. T. Gonzrilez, M. Molina-Sabio,
Reinoso, Extended Abstracts 21st Biennial Conference
on Carbon, Buffalo, NY (1993), p. 416.
Anda mungkin juga menyukai
- Combustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasDari EverandCombustion of Pulverised Coal in a Mixture of Oxygen and Recycled Flue GasBelum ada peringkat
- Development of The "Micro Combustor"Dokumen6 halamanDevelopment of The "Micro Combustor"Haris AbdullaBelum ada peringkat
- Production of Synthesis GasDokumen18 halamanProduction of Synthesis GasGonzalo TitoBelum ada peringkat
- MW 323Dokumen8 halamanMW 323dio prabowoBelum ada peringkat
- 49 1 Anaheim 03-04 0837Dokumen2 halaman49 1 Anaheim 03-04 0837castelo_grandeBelum ada peringkat
- 09 Combustion (Burners, Combustion Systems)Dokumen1 halaman09 Combustion (Burners, Combustion Systems)ahmed1581973Belum ada peringkat
- AC Catalst PTDokumen5 halamanAC Catalst PTJarretBelum ada peringkat
- Cooling Effect by Adsorption DesorptionDokumen5 halamanCooling Effect by Adsorption DesorptionParimal BhambareBelum ada peringkat
- Superficial VelocityDokumen8 halamanSuperficial VelocitybatazivoBelum ada peringkat
- Belghit 2009Dokumen6 halamanBelghit 2009mohammedelamenBelum ada peringkat
- Yeo Il Yoon, Il Hyun Baek, and Sang Do Park: To Whom All Correspondence Should Be Addressed. (E-Mail: Ihbaek@kier - Re.kr)Dokumen8 halamanYeo Il Yoon, Il Hyun Baek, and Sang Do Park: To Whom All Correspondence Should Be Addressed. (E-Mail: Ihbaek@kier - Re.kr)Mohammed AsherBelum ada peringkat
- Lewis Number Effects On Turbulent Burning VelocityDokumen8 halamanLewis Number Effects On Turbulent Burning VelocitycoccoBelum ada peringkat
- Production of Synthesis Gas: Caalysis Today, 18 (1993) 305-324Dokumen20 halamanProduction of Synthesis Gas: Caalysis Today, 18 (1993) 305-324ainmnrhBelum ada peringkat
- Gasification of Selected Woody PlantsDokumen8 halamanGasification of Selected Woody PlantsCitra Adelina SitorusBelum ada peringkat
- Effects of Wet CO Oxidation On The Operation of Engines and Power GeneratorsDokumen6 halamanEffects of Wet CO Oxidation On The Operation of Engines and Power GeneratorsEduardoBelum ada peringkat
- Unique Gasifier For Hydrogen Prod 2Dokumen15 halamanUnique Gasifier For Hydrogen Prod 2Arumugam RamalingamBelum ada peringkat
- Development of A Zero-Emissions Sulfur-Recovery Process. 1.Dokumen12 halamanDevelopment of A Zero-Emissions Sulfur-Recovery Process. 1.Soroosh ZareBelum ada peringkat
- Comparative Study Between Fluidized Bed and Fixed Bed ReactorsDokumen14 halamanComparative Study Between Fluidized Bed and Fixed Bed Reactorsanon_982022273Belum ada peringkat
- Applied Thermal Engineering: W.S. Loh, I.I. El-Sharkawy, K.C. NG, B.B. SahaDokumen6 halamanApplied Thermal Engineering: W.S. Loh, I.I. El-Sharkawy, K.C. NG, B.B. SahaMarijke HeggerBelum ada peringkat
- Feasibility of In-Situ Combustion of Tar From A Tarmat ReservoirDokumen16 halamanFeasibility of In-Situ Combustion of Tar From A Tarmat ReservoirReservorio UagrmBelum ada peringkat
- Optimisation of SRUDokumen12 halamanOptimisation of SRUHuzefaFDBelum ada peringkat
- Lopes2009 (Adsorber) PDFDokumen30 halamanLopes2009 (Adsorber) PDFmauraBelum ada peringkat
- P01 19 PDFDokumen0 halamanP01 19 PDFgarridolopezBelum ada peringkat
- Ni Catalysts With Mo Promoter For Methane Steam ReformingDokumen7 halamanNi Catalysts With Mo Promoter For Methane Steam ReformingGeorge CojocaruBelum ada peringkat
- Alvarez Galvan 2011 Applied Catalysis B EnvironmentalDokumen11 halamanAlvarez Galvan 2011 Applied Catalysis B EnvironmentalBabak RouhiBelum ada peringkat
- International Journal of Heat and Mass Transfer: Kuniyasu Ogawa, Yosuke Inagaki, Akio OhnoDokumen17 halamanInternational Journal of Heat and Mass Transfer: Kuniyasu Ogawa, Yosuke Inagaki, Akio OhnodzakyBelum ada peringkat
- Sadooghi PaperDokumen5 halamanSadooghi PaperKarlaqd CaramónBelum ada peringkat
- Hydrogen From SMR 1Dokumen2 halamanHydrogen From SMR 1Pramanshu RajputBelum ada peringkat
- MW 12Dokumen8 halamanMW 12dio prabowoBelum ada peringkat
- Research Article: Parametric Study of High-Efficiency and Low-Emission Gas BurnersDokumen8 halamanResearch Article: Parametric Study of High-Efficiency and Low-Emission Gas BurnersRolando PradaBelum ada peringkat
- Decrease in Carbonyl Sulfide in The Feed To Claus Converters Shift CatalystsDokumen3 halamanDecrease in Carbonyl Sulfide in The Feed To Claus Converters Shift CatalystsJoel OngBelum ada peringkat
- Preparation of Sorbents. The PotassiumDokumen5 halamanPreparation of Sorbents. The PotassiumFarah TalibBelum ada peringkat
- Get FileDokumen9 halamanGet FileAzza M. ElnenaeyBelum ada peringkat
- Co-Gasification of Colombian CoalDokumen7 halamanCo-Gasification of Colombian Coalwcamilo015Belum ada peringkat
- An Investigation of Reaction Furnace Temperatures and Sulfur RecoveryDokumen10 halamanAn Investigation of Reaction Furnace Temperatures and Sulfur RecoveryIffatBelum ada peringkat
- CO2 AdsorberDokumen5 halamanCO2 Adsorbersmastic8884985Belum ada peringkat
- Activation of Waste Tire Char Upon Cyclic Oxygen Chemisorption#DesorptionDokumen8 halamanActivation of Waste Tire Char Upon Cyclic Oxygen Chemisorption#DesorptionFrancisco HerasBelum ada peringkat
- Spontaneous Combustion Coal Parameters For The Crossing-Point Temperature (CPT) Method in A Temperature-Programmed System (TPS)Dokumen15 halamanSpontaneous Combustion Coal Parameters For The Crossing-Point Temperature (CPT) Method in A Temperature-Programmed System (TPS)lopohi2934Belum ada peringkat
- The Oxidation of Soot A Review of Experiments, Mechanisms and Modesl - Stanmore-Etal - 2001Dokumen22 halamanThe Oxidation of Soot A Review of Experiments, Mechanisms and Modesl - Stanmore-Etal - 2001Hemanth KappannaBelum ada peringkat
- Energy Efficiency in Building Using Co2 Heat Pump Water HeatingDokumen7 halamanEnergy Efficiency in Building Using Co2 Heat Pump Water HeatingMarclauryn AdewaleBelum ada peringkat
- Hydrogen As Future Energy SourceDokumen8 halamanHydrogen As Future Energy SourceWilliam ChangBelum ada peringkat
- Design, Process Simulation and Construction of An Atmospheric Dual Fluidized Bed CombustionDokumen9 halamanDesign, Process Simulation and Construction of An Atmospheric Dual Fluidized Bed Combustionapi-3799861Belum ada peringkat
- My Awma Paper VersionsDokumen97 halamanMy Awma Paper VersionsOμάδα 5Belum ada peringkat
- Hydrogen Production From Natural Gas Thermal Cracking: Design and Test of A Pilot-Scale Solar Chemical ReactorDokumen7 halamanHydrogen Production From Natural Gas Thermal Cracking: Design and Test of A Pilot-Scale Solar Chemical ReactorChandarshekar SwaminathanBelum ada peringkat
- Evaluation of The Co2 Re Activity of Chars Obtained Under Conventional and Oxyfuel AtmospheresDokumen5 halamanEvaluation of The Co2 Re Activity of Chars Obtained Under Conventional and Oxyfuel AtmospheresjulianapohlmannufrgsBelum ada peringkat
- 1 s2.0 001793108490262X MainDokumen18 halaman1 s2.0 001793108490262X MainReza KH100% (1)
- Chemical-Looping Combustion of Solid Fuels in A 10 KW Pilot - Batch Tests With Five FuelsDokumen8 halamanChemical-Looping Combustion of Solid Fuels in A 10 KW Pilot - Batch Tests With Five FuelsAzharuddin Ehtesham FarooquiBelum ada peringkat
- Ethylene Production Via Partial Oxidation and Pyrolysis of Ethane - M. Dente, A. Berettal, T. Faravelli, E. Ranzi, A. Abbr, M. Notarbartolo PDFDokumen6 halamanEthylene Production Via Partial Oxidation and Pyrolysis of Ethane - M. Dente, A. Berettal, T. Faravelli, E. Ranzi, A. Abbr, M. Notarbartolo PDFAlejandro HernandezBelum ada peringkat
- 1 s2.0 S1540748922002073 MainDokumen10 halaman1 s2.0 S1540748922002073 Main别康民Belum ada peringkat
- An Experimental Investigation of A Flue GasDokumen9 halamanAn Experimental Investigation of A Flue GasmichaelBelum ada peringkat
- Journal of The Energy Institute: Luning Tian, Wei Yang, Zhenhui Chen, Xianhua Wang, Haiping Yang, Hanping ChenDokumen7 halamanJournal of The Energy Institute: Luning Tian, Wei Yang, Zhenhui Chen, Xianhua Wang, Haiping Yang, Hanping ChenBill ChenBelum ada peringkat
- Ignition and Devolatilization of Pulverized Bituminous Coal Particles During Oxygen Carbon Dioxide Coal CombustionDokumen8 halamanIgnition and Devolatilization of Pulverized Bituminous Coal Particles During Oxygen Carbon Dioxide Coal CombustionthinhklBelum ada peringkat
- Report On Sulphur RecoveryDokumen9 halamanReport On Sulphur Recoveryvidit SinghBelum ada peringkat
- Minchao Han, Yuhua Ai, Zheng Chen, Wenjun Kong: A B A C ADokumen7 halamanMinchao Han, Yuhua Ai, Zheng Chen, Wenjun Kong: A B A C AEduardoBelum ada peringkat
- Process Optimization-Carrasquero (OQ8)Dokumen7 halamanProcess Optimization-Carrasquero (OQ8)raktim66Belum ada peringkat
- Activities and Selectivities Temperatures Relevant Chemical Interconversions Copper Metal-Oxide Catalysts at Heat-Pumps Based Isopropanol/ AcetoneDokumen4 halamanActivities and Selectivities Temperatures Relevant Chemical Interconversions Copper Metal-Oxide Catalysts at Heat-Pumps Based Isopropanol/ AcetoneOlga ĆalasanBelum ada peringkat
- Assignment 04,204107027Dokumen14 halamanAssignment 04,204107027Shanku Pratim BorahBelum ada peringkat
- Hong AnalysisDokumen38 halamanHong AnalysisleovenuBelum ada peringkat
- Fateh Enviro Engineers SchemeDokumen1 halamanFateh Enviro Engineers SchemevinodBelum ada peringkat
- Gokul - 8.7.16Dokumen8 halamanGokul - 8.7.16vinodBelum ada peringkat
- Final Theses Sunny M.Ed.Dokumen101 halamanFinal Theses Sunny M.Ed.vinodBelum ada peringkat
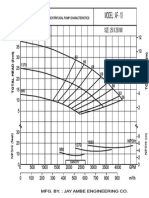
- Jay Ambe Axial Pump AF - 10 CurveDokumen1 halamanJay Ambe Axial Pump AF - 10 CurvevinodBelum ada peringkat
- Artificial IntelligenceDokumen14 halamanArtificial IntelligencevinodBelum ada peringkat
- Jay Ambe AF - 16 CurveDokumen1 halamanJay Ambe AF - 16 CurvevinodBelum ada peringkat
- Kriloskar KSMB Pump SeriesDokumen1 halamanKriloskar KSMB Pump SeriesvinodBelum ada peringkat
- 0.5 MLD PlantDokumen1 halaman0.5 MLD PlantvinodBelum ada peringkat
- Personality AssessmentDokumen69 halamanPersonality AssessmentvinodBelum ada peringkat
- IO Grundfos HEPDokumen100 halamanIO Grundfos HEPDeepikaBelum ada peringkat
- Operation Characteristic of A Mechanical Vapor Recompression Heat Pump Driven by A Centrifugal FanDokumen8 halamanOperation Characteristic of A Mechanical Vapor Recompression Heat Pump Driven by A Centrifugal FanvinodBelum ada peringkat
- Modeling of Ammonia Removal From Wastewater Using Air Stripping/ Modified Clinoptilolite: Reusability, Optimization, Isotherm, Kinetic, and Equilibrium StudiesDokumen22 halamanModeling of Ammonia Removal From Wastewater Using Air Stripping/ Modified Clinoptilolite: Reusability, Optimization, Isotherm, Kinetic, and Equilibrium StudiesvinodBelum ada peringkat
- Water Spray Reactor For Ammonia Removal Via Air Stripping: An Evaluation On Mass Transfer and Process EfficiencyDokumen8 halamanWater Spray Reactor For Ammonia Removal Via Air Stripping: An Evaluation On Mass Transfer and Process EfficiencyvinodBelum ada peringkat
- PT Mass BalanceDokumen3 halamanPT Mass BalancevinodBelum ada peringkat
- Img 0006-1Dokumen1 halamanImg 0006-1vinodBelum ada peringkat
- Chemical For NeutralizationDokumen2 halamanChemical For NeutralizationvinodBelum ada peringkat
- Stripper ColumnDokumen1 halamanStripper ColumnvinodBelum ada peringkat
- Effluent DataDokumen1 halamanEffluent DatavinodBelum ada peringkat
- Manish KumarDokumen4 halamanManish KumarvinodBelum ada peringkat
- Process Biochemistry: Beatriz Veleirinho, J.A. Lopes-da-SilvaDokumen4 halamanProcess Biochemistry: Beatriz Veleirinho, J.A. Lopes-da-SilvavinodBelum ada peringkat
- Solving PH Problems On A SpreadsheetDokumen6 halamanSolving PH Problems On A SpreadsheetvinodBelum ada peringkat
- FSSAIDokumen1 halamanFSSAIvinodBelum ada peringkat
- Evap DDokumen27 halamanEvap DvinodBelum ada peringkat
- Activated Carbons From LignocellulosicDokumen8 halamanActivated Carbons From LignocellulosicvinodBelum ada peringkat
- Operating Cost Analysis and Treatment of Domestic Wastewater by Electrocoagulation Using Aluminum ElectrodesDokumen7 halamanOperating Cost Analysis and Treatment of Domestic Wastewater by Electrocoagulation Using Aluminum ElectrodesvinodBelum ada peringkat
- Removal of Color in Cane Juice ClarificationDokumen8 halamanRemoval of Color in Cane Juice ClarificationFederico LeonBelum ada peringkat
- Sugarcane Material 2520balanceDokumen7 halamanSugarcane Material 2520balanceSagir AdamuBelum ada peringkat
- Crest Cellulose - Heat and Mass AblanceDokumen1 halamanCrest Cellulose - Heat and Mass AblancevinodBelum ada peringkat
- Units 6-10 Review TestDokumen20 halamanUnits 6-10 Review TestCristian Patricio Torres Rojas86% (14)
- Navamsa Karma and GodDokumen9 halamanNavamsa Karma and GodVisti Larsen50% (2)
- A Scenario of Cross-Cultural CommunicationDokumen6 halamanA Scenario of Cross-Cultural CommunicationN Karina HakmanBelum ada peringkat
- Csm6 Ext1y11 BookDokumen955 halamanCsm6 Ext1y11 BookJesse Davis100% (12)
- Diagnosis: Acute GastroenteritisDokumen1 halamanDiagnosis: Acute GastroenteritisSakshi RanabhatBelum ada peringkat
- 1929 Davos DisputationDokumen20 halaman1929 Davos DisputationEstevao Cruz100% (1)
- 9.2 Volumetric Analysis PDFDokumen24 halaman9.2 Volumetric Analysis PDFJoaquinBelum ada peringkat
- TOEIC® Practice OnlineDokumen8 halamanTOEIC® Practice OnlineCarlos Luis GonzalezBelum ada peringkat
- Middle Grades ReportDokumen138 halamanMiddle Grades ReportcraignewmanBelum ada peringkat
- Amtek Auto Analysis AnuragDokumen4 halamanAmtek Auto Analysis AnuraganuragBelum ada peringkat
- Donna Claire B. Cañeza: Central Bicol State University of AgricultureDokumen8 halamanDonna Claire B. Cañeza: Central Bicol State University of AgricultureDanavie AbergosBelum ada peringkat
- (Durt, - Christoph - Fuchs, - Thomas - Tewes, - Christian) Embodiment, Enaction, and Culture PDFDokumen451 halaman(Durt, - Christoph - Fuchs, - Thomas - Tewes, - Christian) Embodiment, Enaction, and Culture PDFnlf2205100% (3)
- Polynomials Level 3Dokumen17 halamanPolynomials Level 3greycouncilBelum ada peringkat
- Unsaturated Polyester Resins: Influence of The Styrene Concentration On The Miscibility and Mechanical PropertiesDokumen5 halamanUnsaturated Polyester Resins: Influence of The Styrene Concentration On The Miscibility and Mechanical PropertiesMamoon ShahidBelum ada peringkat
- Oration For Jon Kyle ValdehuezaDokumen2 halamanOration For Jon Kyle ValdehuezaJakes ValBelum ada peringkat
- Vrushalirhatwal (14 0)Dokumen5 halamanVrushalirhatwal (14 0)GuruRakshithBelum ada peringkat
- Vocabulary Task Harry PotterDokumen3 halamanVocabulary Task Harry PotterBest FriendsBelum ada peringkat
- Angel Number 1208 Meaning Increased FaithDokumen1 halamanAngel Number 1208 Meaning Increased FaithKhally KatieBelum ada peringkat
- Soal Midtest + Kunci JawabanDokumen28 halamanSoal Midtest + Kunci JawabanYuyun RasulongBelum ada peringkat
- 115 FinargDokumen294 halaman115 FinargMelvin GrijalbaBelum ada peringkat
- RPT Form 2 2023Dokumen7 halamanRPT Form 2 2023NOREEN BINTI DOASA KPM-GuruBelum ada peringkat
- Context: Lesson Author Date of DemonstrationDokumen4 halamanContext: Lesson Author Date of DemonstrationAR ManBelum ada peringkat
- Bird Beak ActivityDokumen4 halamanBird Beak Activityapi-314222661Belum ada peringkat
- Baltimore Catechism No. 2 (Of 4)Dokumen64 halamanBaltimore Catechism No. 2 (Of 4)gogelBelum ada peringkat
- Radiopharmaceutical Production: History of Cyclotrons The Early Years at BerkeleyDokumen31 halamanRadiopharmaceutical Production: History of Cyclotrons The Early Years at BerkeleyNguyễnKhươngDuyBelum ada peringkat
- WWW - Ib.academy: Study GuideDokumen122 halamanWWW - Ib.academy: Study GuideHendrikEspinozaLoyola100% (2)
- 38 Page 2046 2159 PDFDokumen114 halaman38 Page 2046 2159 PDFAkansha SharmaBelum ada peringkat
- In Christ Alone My Hope Is FoundDokumen9 halamanIn Christ Alone My Hope Is FoundJessie JessBelum ada peringkat
- Assignment Submission Form: Pgid Name of The MemberDokumen9 halamanAssignment Submission Form: Pgid Name of The MemberNamit GaurBelum ada peringkat
- Broshure JepanDokumen6 halamanBroshure JepanIrwan Mohd YusofBelum ada peringkat