Ionic Equilibrium Guide
Diunggah oleh
Chhavindra TripathiDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Ionic Equilibrium Guide
Diunggah oleh
Chhavindra TripathiHak Cipta:
Format Tersedia
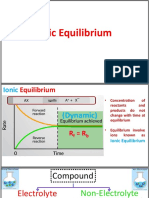
Ionic Equilibrium
In chemical equilibrium we studied reaction involving
molecules only but in ionic equilibrium we will study reversible
reactions involving formation of ions in water. When solute is polar
covalent compound then it reacts with water to form ions.
Electrical conductors
Substances, which allow electric current to pass through them, are
known as conductors or electrical conductors. Conductors can be divided
into two types,
(1) Conductors which conduct electricity without undergoing any
chemical change are known as metallic or electronic conductors.
(2) Conductors which undergo decomposition (a chemical change)
when an electric current is passed through them are known as electrolytic
conductors or electrolytes.
Electrolytes are further divided into two types on the basis of their
strengths,
(i) Substances which almost completely ionize into ions in their
aqueous solution are called strong electrolytes. Degree of ionization for this
type of electrolyte is one i.e., 1 .
Dilution of solution Amount of solvent
(iv) Degree of ionisation of an electrolyte in solution increases with
rise in temperature.
(v) Presence of common ion : The degree of ionisation of an
electrolyte decreases in the presence of a strong electrolyte having a
common ion.
Ostwald's dilution law
The strength of an acid or a bas is experimentally measured by
determining its dissociation or ionisation constant.
When acetic acid (a weak electrolyte) is dissolved in water, it
dissociates partially into H or H 3 O and CH 3 COO ions and the
following equilibrium is obtained,
CH3COOH H 2O CH 3 COO H 3 O
Applying law of chemical equilibrium,
K
[CH 3 COO ] [ H 3 O ]
[CH 3 COOH ] [ H 2 O ]
In dilute solution, [H 2 O] is constant. The product of K and
constant [H 2 O] is denoted as K a , the ionization constant or dissociation
constant of the acid is,
For example : HCl, H 2 SO 4 , NaCl, HNO3 , KOH , NaOH,
HNO3 , AgNO3 , CuSO4 etc. means all strong acids, bases and all types
Ka
of salts.
(ii) Substances which ionize to a small extent in their aqueous
solution are known as weak electrolytes. Degree of ionization for this types
of electrolytes is 1 .
For example : H2O, CH3COOH , NH4 OH , HCN,
Liq. SO 2 ,
HCOOH etc. means all weak acids and bases.
[CH 3 COO ] [H 3 O ]
[CH 3 COOH ]
The fraction of total number of molecules of an electrolyte which ionise
into ions is known as degree of dissociation/ionisation .
If ' C ' represents the initial concentration of the acid in moles L1
and the degree of dissociation, then equilibrium concentration of the
ions (CH 3 COO and H 3 O ) is equal to C and that of the
CH3COOH H 2O CH 3 COO H 3 O
(1) Postulates of Arrhenius theory
(i) In aqueous solution, the molecules of an electrolyte undergo
spontaneous dissociation to form positive and negative ions.
(ii) Degree of ionization ( )
Number of dissociate d molecules
Total number of molecules of electrolyt e before dissociati on
(iii) At moderate concentrations, there exists an equilibrium
between the ions and undissociated molecules, such as, NaOH Na
This equilibrium state is called ionic equilibrium.
(iv) Each ion behaves osmotically as a molecule.
(2) Factors affecting degree of ionisation
(i) At normal dilution, value of is nearly 1 for strong electrolytes,
while it is very less than 1 for weak electrolytes.
(ii) Higher the dielectric constant of a solvent more is its ionising
power. Water is the most powerful ionising solvent as its dielectric constant
is highest.
1
Con. of solution
Initial conc
Conc. at eqb. C(1 )
C
C
Substituting the values of the equilibrium concentrations in equation
(i), we get
Ka
C .C
C 2 2
C 2
C(1 ) C(1 ) 1
In case of weak electrolytes, the value of
neglected in comparison to 1 i.e., 1 1 .
..(ii)
is very small and can be
Hence, we get
OH ; KCl K Cl
(iii)
..(i)
undissociated acetic acid C(1 ) i.e., we have
Arrhenius theory of electrolytic dissociation
1
wt. of solution
Ka C 2 or
Ka
C
..(iii)
The degree of dissociation, can therefore be calcualted at a given
concentration, C if K a is known. Furher, if V is the volume of the
solution in litres containing 1 mole of the electrolyte, C 1 / V . Hence we
have
Ka V
..(iv)
Similarly, for a weak base like NH4 OH , we have
Kb / C Kb V
The above equations lead to the following result
..(v)
Anda mungkin juga menyukai
- Ionic Equilibrium ExplainedDokumen93 halamanIonic Equilibrium ExplainedhappyBelum ada peringkat
- 03 - Ionic Equilibrium (Theory) Module-3-1Dokumen31 halaman03 - Ionic Equilibrium (Theory) Module-3-1Raju SinghBelum ada peringkat
- Electrolysis PDFDokumen37 halamanElectrolysis PDFHarini SridharanBelum ada peringkat
- Chemical Equilibrium 1Dokumen49 halamanChemical Equilibrium 1samarthasai2006Belum ada peringkat
- ElectrochemistryDokumen19 halamanElectrochemistrypriyanshu dhawanBelum ada peringkat
- Unit II Vi Semister Electrchemistry: OelectrolyteDokumen31 halamanUnit II Vi Semister Electrchemistry: OelectrolyteAmber AminBelum ada peringkat
- Water's Role as a SolventDokumen137 halamanWater's Role as a SolventMaheshBelum ada peringkat
- Theory of IonisationDokumen2 halamanTheory of IonisationVIKRAM KUMARBelum ada peringkat
- Chapter # 7Dokumen16 halamanChapter # 7ALINA -Belum ada peringkat
- Types of Solutions and Solubility RulesDokumen18 halamanTypes of Solutions and Solubility RulesZudotaBelum ada peringkat
- Conductivity and Ion Identification ExperimentDokumen5 halamanConductivity and Ion Identification ExperimentdhanielieneBelum ada peringkat
- Determining The Dissociation Products of ElectrolytesDokumen3 halamanDetermining The Dissociation Products of Electrolytesnicole olivaBelum ada peringkat
- Chapter 2Dokumen14 halamanChapter 2Bùi Hữu ĐứcBelum ada peringkat
- Ionic Equilibrium GuideDokumen58 halamanIonic Equilibrium Guidehemant sahooBelum ada peringkat
- Reactions in Aqueous SolutionsDokumen11 halamanReactions in Aqueous SolutionsMilinPatelBelum ada peringkat
- Ionic Equilibrium ExplainedDokumen14 halamanIonic Equilibrium Explained8842 AnuragBelum ada peringkat
- Variation of Conductance With Temperature in Electrolytes: Ankur Roy Chowdhury Roll NumberDokumen14 halamanVariation of Conductance With Temperature in Electrolytes: Ankur Roy Chowdhury Roll NumberAnkur RoychowdhuryBelum ada peringkat
- Electrochemistry for Materials Science SummaryDokumen46 halamanElectrochemistry for Materials Science SummaryGustavo Adolfo Piñero BorgesBelum ada peringkat
- Types of ElectrolytesDokumen24 halamanTypes of ElectrolytesPranoy Baishya100% (1)
- Electrolysis 2022-23Dokumen18 halamanElectrolysis 2022-23Yasha RizviBelum ada peringkat
- Factors Affecting Electrolytic Conductance (Under 40 charsDokumen2 halamanFactors Affecting Electrolytic Conductance (Under 40 charsbewildered-escapist-4301Belum ada peringkat
- 4.0 Reactions in Aqueous SolutionsDokumen19 halaman4.0 Reactions in Aqueous Solutionsparkinsondilys7Belum ada peringkat
- Ionic EquilibriumDokumen13 halamanIonic EquilibriumTrupti ChavanBelum ada peringkat
- Appendix ConductivityDokumen4 halamanAppendix Conductivityfhammad673Belum ada peringkat
- BLB chp4Dokumen88 halamanBLB chp4Nora Zor-elBelum ada peringkat
- KR CHB301B 1Dokumen102 halamanKR CHB301B 1Ankita SinghBelum ada peringkat
- Types of ElectrolytesDokumen95 halamanTypes of ElectrolytesDeepak Sirone100% (4)
- Determination of Dissociation Constant From Conductivity MeasurementsDokumen7 halamanDetermination of Dissociation Constant From Conductivity MeasurementsRaluca IosuBelum ada peringkat
- Physical ChemistryDokumen47 halamanPhysical ChemistryAnshuman JainBelum ada peringkat
- Lecture 12a ChemDokumen7 halamanLecture 12a Chemlldgee33Belum ada peringkat
- Bài 1- Tính chất của dung môi nướcDokumen61 halamanBài 1- Tính chất của dung môi nướcdoannguyenBelum ada peringkat
- IonizationDokumen10 halamanIonizationInk TVBelum ada peringkat
- ELECTROLYSISDokumen29 halamanELECTROLYSISDXN LUDHIANABelum ada peringkat
- Chemistry Content Palm CardsDokumen52 halamanChemistry Content Palm Cardsk.gardnerBelum ada peringkat
- Electrochemistry For Materials Science - BELTOWSKA - 2 PDFDokumen50 halamanElectrochemistry For Materials Science - BELTOWSKA - 2 PDFSumit ShankarBelum ada peringkat
- Chapter 6Dokumen26 halamanChapter 6DXN LUDHIANABelum ada peringkat
- Electrolysis_2015Dokumen13 halamanElectrolysis_2015martinmbondjo062Belum ada peringkat
- Unit 4 Electrochemical EnergyDokumen49 halamanUnit 4 Electrochemical EnergyRitchel Conde BoholBelum ada peringkat
- Chapter 5Dokumen31 halamanChapter 5Mohammad Y Abu AyyashBelum ada peringkat
- 11 ChemistryDokumen20 halaman11 ChemistrykabhiBelum ada peringkat
- Chemistry Content Palm CardsDokumen52 halamanChemistry Content Palm CardsMasonBelum ada peringkat
- Chemical Equilibrium-2Dokumen13 halamanChemical Equilibrium-2MUHAMMAD YASEENBelum ada peringkat
- Electrolyte and NonDokumen7 halamanElectrolyte and NonSuwahono, M.PdBelum ada peringkat
- Ionic Equilibrium (Chem)Dokumen82 halamanIonic Equilibrium (Chem)Draw with Hassan JamalBelum ada peringkat
- Lecture 8Dokumen24 halamanLecture 8Md Al AminBelum ada peringkat
- Electrochemistry in 40 CharactersDokumen60 halamanElectrochemistry in 40 CharactersSamarth GBelum ada peringkat
- Ethanol PrecipitationDokumen12 halamanEthanol PrecipitationАндрей ЛысенкоBelum ada peringkat
- Electrolytic Conductance ExplainedDokumen11 halamanElectrolytic Conductance ExplainedBùi Hữu ĐứcBelum ada peringkat
- Chemistry I: Notes For First Semester College ChemistryDokumen5 halamanChemistry I: Notes For First Semester College ChemistrystudenflBelum ada peringkat
- CHEMISTRY - (With Answers)Dokumen23 halamanCHEMISTRY - (With Answers)Arnold MutasaBelum ada peringkat
- Electrochemistry: ConductorDokumen20 halamanElectrochemistry: ConductorMithun Madhukar MaskeBelum ada peringkat
- Unit-3Electrochemistry 88896Dokumen37 halamanUnit-3Electrochemistry 88896Dhatri SriramBelum ada peringkat
- Kimia Teknik 6Dokumen52 halamanKimia Teknik 6Febry MiftakhulBelum ada peringkat
- Experiment 5 Dissimilarity Between Ionic and Covalent CompoundsDokumen5 halamanExperiment 5 Dissimilarity Between Ionic and Covalent CompoundsNurasyilah YakubBelum ada peringkat
- Water BME-211 (2120)Dokumen50 halamanWater BME-211 (2120)Jack WengroskyBelum ada peringkat
- CH 4 Ism GC6 eDokumen33 halamanCH 4 Ism GC6 eJean Beatriz Sta AnaBelum ada peringkat
- Chapter 6 ElechtrochemistryDokumen23 halamanChapter 6 ElechtrochemistryDannarajen ChandranBelum ada peringkat
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersDari EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersBelum ada peringkat
- GCSE Chemistry Revision: Cheeky Revision ShortcutsDari EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsPenilaian: 4.5 dari 5 bintang4.5/5 (3)
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsDari EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsBelum ada peringkat
- PblockDokumen41 halamanPblockChhavindra TripathiBelum ada peringkat
- Pattern and Series Logic QuestionsDokumen8 halamanPattern and Series Logic QuestionsChhavindra TripathiBelum ada peringkat
- Chemical BondingDokumen2 halamanChemical BondingChhavindra TripathiBelum ada peringkat
- Social Science Class 10th MCQDokumen1 halamanSocial Science Class 10th MCQChhavindra TripathiBelum ada peringkat
- Ionic and Covalent Bonds ExplainedDokumen1 halamanIonic and Covalent Bonds ExplainedChhavindra TripathiBelum ada peringkat
- (A) Sound Travels in A Straight Line (B) Sound Travels As Waves (C) Sound Is A From of Energy (D) Sound Travels Faster in Vacuum That Then in AirDokumen2 halaman(A) Sound Travels in A Straight Line (B) Sound Travels As Waves (C) Sound Is A From of Energy (D) Sound Travels Faster in Vacuum That Then in AirChhavindra TripathiBelum ada peringkat
- Chemical BondingDokumen2 halamanChemical BondingChhavindra TripathiBelum ada peringkat
- Chemical Bonding TestDokumen2 halamanChemical Bonding TestChhavindra TripathiBelum ada peringkat
- New Microsoft Office Word DocumentDokumen1 halamanNew Microsoft Office Word DocumentChhavindra TripathiBelum ada peringkat
- L-1 Mole ConceptDokumen16 halamanL-1 Mole ConceptAkhilesh KumarBelum ada peringkat
- Logical Sequence of Word - Exercis2 Verbal Reasoning - WWW - Joinexam.inDokumen10 halamanLogical Sequence of Word - Exercis2 Verbal Reasoning - WWW - Joinexam.inChhavindra TripathiBelum ada peringkat
- TDDokumen9 halamanTDChhavindra TripathiBelum ada peringkat
- Table 5.6Dokumen1 halamanTable 5.6Chhavindra TripathiBelum ada peringkat
- Stoichiometry: An Introduction to Mole Concepts and CalculationsDokumen39 halamanStoichiometry: An Introduction to Mole Concepts and CalculationsChhavindra TripathiBelum ada peringkat
- Payment ReceiptDokumen1 halamanPayment ReceiptChhavindra TripathiBelum ada peringkat
- About BlankDokumen1 halamanAbout BlankChhavindra TripathiBelum ada peringkat
- Atom and Molecules and STR of AtomDokumen21 halamanAtom and Molecules and STR of AtomChhavindra TripathiBelum ada peringkat
- Payment ReceiptDokumen1 halamanPayment ReceiptChhavindra TripathiBelum ada peringkat
- (289675466) Resume CT @IITRDokumen3 halaman(289675466) Resume CT @IITRChhavindra TripathiBelum ada peringkat
- 5 Electron CountingDokumen12 halaman5 Electron CountingcpunxzatawneyBelum ada peringkat
- Electrical Properties of Materials Mod-1Dokumen18 halamanElectrical Properties of Materials Mod-1Darshan rajBelum ada peringkat
- 018 10Dokumen3 halaman018 10ilkerkozturkBelum ada peringkat
- 6ra 2620 6d v57 1a Z Simoreg d38035 Siemens Manual 02Dokumen18 halaman6ra 2620 6d v57 1a Z Simoreg d38035 Siemens Manual 02Stefan IstratescuBelum ada peringkat
- Bomba Electrica 1500gpm 300HP (Medidas)Dokumen1 halamanBomba Electrica 1500gpm 300HP (Medidas)Fire ChileBelum ada peringkat
- 3/27/2016 Portable AC On Rent Pune - Portable AC Rentals Pune - AC Rentals Pune On SulekhaDokumen3 halaman3/27/2016 Portable AC On Rent Pune - Portable AC Rentals Pune - AC Rentals Pune On SulekhadcoolsamBelum ada peringkat
- RAIS PDA AppDokumen8 halamanRAIS PDA Appzaw lin ooBelum ada peringkat
- Genie GTH 4013Dokumen202 halamanGenie GTH 4013Sam Manutenção100% (2)
- Sungris BrochureDokumen8 halamanSungris Brochurechemasi123Belum ada peringkat
- SGP Chapter-1Dokumen81 halamanSGP Chapter-1Shashank ReddyBelum ada peringkat
- High Voltage Products Reliable Products - EN PDFDokumen102 halamanHigh Voltage Products Reliable Products - EN PDFSiva ReddyBelum ada peringkat
- MeasurementDokumen4 halamanMeasurementJemason100% (1)
- Mayo College: Dining Hall at AjmerDokumen79 halamanMayo College: Dining Hall at AjmerFaquruddinBelum ada peringkat
- Folio SainsDokumen15 halamanFolio SainsMohammad Afifi Rohman80% (5)
- Pump Mechanical Seals GuideDokumen41 halamanPump Mechanical Seals GuideArief Hidayat100% (1)
- Diesel Engines for Unrestricted Continuous OperationDokumen2 halamanDiesel Engines for Unrestricted Continuous OperationJorge Bellido100% (1)
- The Krebs Cycle ExplainedDokumen12 halamanThe Krebs Cycle ExplainedHo Man ChanBelum ada peringkat
- Table 4E4A - Current Carrying Capacity in AmpereDokumen1 halamanTable 4E4A - Current Carrying Capacity in AmperesalvuBelum ada peringkat
- Kvpy Pee PDFDokumen9 halamanKvpy Pee PDFstudysteps.inBelum ada peringkat
- ELECTRONICDokumen13 halamanELECTRONICMahmoued YasinBelum ada peringkat
- Dna60 PDFDokumen15 halamanDna60 PDFAc IdBelum ada peringkat
- RP Manuale D'uso e Manutenzione - CAVALLINO CE PDFDokumen24 halamanRP Manuale D'uso e Manutenzione - CAVALLINO CE PDFMiraBelum ada peringkat
- Overhead Phil MC KeownDokumen30 halamanOverhead Phil MC KeownAditya AoleBelum ada peringkat
- Final Directory Handbook For ADIPEC 2023Dokumen54 halamanFinal Directory Handbook For ADIPEC 2023Zharif ZainiBelum ada peringkat
- Wa500-6 Sen00236-04d PDFDokumen1.705 halamanWa500-6 Sen00236-04d PDFanggie100% (4)
- Report 04.02.20Dokumen4 halamanReport 04.02.20Kartik SoniBelum ada peringkat
- Survey of Tea Vendors 01Dokumen2 halamanSurvey of Tea Vendors 01Sandeep SinghBelum ada peringkat
- Pt6 Fuel Nozzle Exchange Kits: Adapter Assemblies, Fuel ManifoldDokumen1 halamanPt6 Fuel Nozzle Exchange Kits: Adapter Assemblies, Fuel ManifoldBerchBelum ada peringkat
- WM2077CW Service ManualDokumen44 halamanWM2077CW Service ManualMichael David SharkeyBelum ada peringkat
- 568100Dokumen2 halaman568100Talha TariqBelum ada peringkat
- Short and Open Circuit Test On TransformerDokumen1 halamanShort and Open Circuit Test On TransformerRyan DagsilBelum ada peringkat