Diagnosis and Treatment of Allergic Skin Disorders in The Elderly
Diunggah oleh
felipetheJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Diagnosis and Treatment of Allergic Skin Disorders in The Elderly
Diunggah oleh
felipetheHak Cipta:
Format Tersedia
THERAPY IN PRACTICE
Drugs Aging 2001; 18 (11): 827-835
1170-229X/01/0011-0827/$22.00/0
Adis International Limited. All rights reserved.
Diagnosis and Treatment of Allergic
Skin Disorders in the Elderly
Susan T. Nedorost and Seth R. Stevens
Department of Dermatology, Case Western Reserve University and University Hospitals of
Cleveland, Cleveland, Ohio, USA
Contents
Abstract
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
1. Approach to the Management of the Elderly Patient with Pruritic Dermatitis
2. Allergic Eczematous Dermatitis: Contact Dermatitis . . . . . . . . . . . . . .
2.1 Cellular Basis of Allergic Contact Dermatitis . . . . . . . . . . . . . . . .
2.2 Prevalence of Allergic Contact Dermatitis in the Elderly . . . . . . . . .
2.3 Allergens Causing Contact Dermatitis in the Elderly . . . . . . . . . . . .
2.4 Patch Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
2.5 Photoallergens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
3. Eczematous Dermatoses Not Due to Contact Allergy . . . . . . . . . . . . .
4. Management of Eczematous Dermatitis . . . . . . . . . . . . . . . . . . . . .
5. Allergic Noneczematous Dermatosis . . . . . . . . . . . . . . . . . . . . . . .
6. Investigating Rashes Due to Systemic Allergic Reactions . . . . . . . . . . . .
7. Conclusion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Abstract
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
827
828
829
829
829
829
830
830
831
831
832
833
834
Allergic skin disorders in the elderly may arise from contact with or ingestion
of offending allergens. Itching associated with skin allergy must be distinguished
from other causes of itching in the elderly such as xerosis, itching due to systemic
disease and bullous disease. Although elderly people have somewhat decreased
cell-mediated immunity and may be harder to sensitise under experimental conditions, they have had many years to acquire allergic responses, and therefore
develop contact dermatitis frequently.
Patch testing is a valuable tool to diagnose contact allergy and should be used
often in the elderly, particularly in patients at high risk of contact dermatitis, such
as those with chronic lower extremity dermatitis or ulcers due to venous stasis.
When prescribing topical medications to high risk patients, a knowledge of the
common sensitisers is important. In addition to allergy to medicaments and dressings used to treat stasis ulcers, contact allergy to dental prostheses and medications used to treat ocular disease are common in the elderly as a result of increased
usage and exposure.
Rash caused by ingested allergens is much more commonly due to medications
than to food in the elderly. Allergic noneczematous dermatoses in the elderly are
commonly drug-induced. Urticarial skin reactions are often associated with the
administration of antibacterials, nonsteroidal anti-inflammatory drugs (NSAIDs),
antidepressants or opioids. Morbilliform rashes are a common sign of systemic
828
Nedorost & Stevens
reaction to anticonvulsants, gold, allopurinol or diuretics. Phototoxic reactions
may be associated with the administration of tetracyclines, diuretics, NSAIDs
and antihyperglycaemic agents. Patient-specific variables such as HLA type and
concomitant medication may affect the likelihood of an allergic response to medication.
Many elderly patients take multiple medications, which can make diagnosis
of drug allergy difficult because diagnosis is most commonly accomplished by
observing clinical response once the medication is withdrawn. In the case of
lichenoid cutaneous reactions, clinical improvement may take several months
after withdrawal of the offending drug. Laboratory tests to detect drug-induced
allergic skin disorders may be available in the future.
Itching, with or without a rash, is the most common cutaneous complaint in the elderly. The prevalence of itching and of dry skin continues to increase from the young elderly aged in their sixties
to the very elderly aged in their nineties.[1,2] Xerosis is the most common cause of itching in the elderly. Other common causes of itching include allergic and irritant contact dermatitis, seborrhoeic
dermatitis, stasis dermatitis with or without autoeczematisation and medication reactions (table
I). Systemic causes such as thyroid disease, malignancy, renal or biliary disease, or HIV infection are
of particular concern when a patient has generalised itching without a cutaneous rash.
1. Approach to the Management of the
Elderly Patient with Pruritic Dermatitis
Elderly patients with pruritic dermatitis should
be instructed to avoid frequent washing and the use
of skin irritants such as harsh, deodorant-type
soaps, astringents and lotions. Bathing should be
followed immediately by the application of a bland
emollient ointment or a cream emollient, if an oint-
ment is not acceptable to the patient. Lotions are
more popular with patients, but require many more
ingredients to formulate compared with bland
emollients containing only water in petrolatum or
mineral oil. Lotions are less effective as moisturisers
than bland emollients of the cream type and contain
potential contact sensitisers and therefore should
be avoided if possible. Extremely low humidity
should be corrected with environmental controls,
where possible. If stasis oedema of the lower extremities is present, this should be corrected with
compression. Stasis dermatitis can cause generalised dermatitis which is now increasingly understood as a manifestation of immune autoreactivity.[3] If asteatosis and stasis dermatitis are not
present (or have been corrected), and the patient
still has dermatitis, then the possibility of allergic
contact dermatitis should be considered; this is best
investigated by a dermatologist familiar with patch
testing. If there are morbilliform, urticarial or lichenoid lesions present in addition to eczematous
dermatitis, then systemic drug allergy should be
considered.
Table I. Characteristics of the rashes discussed in this article
Type of dermatitis
Morphology
Distribution
Allergic contact dermatitis
Oozing in acute form; red scaly
Pattern corresponds to exposure
Seborrheic dermatitis
Red, dandruff-like scale
Scalp, central face and upper torso
Atopic dermatitis
Like other dermatitis forms above, often
lichenified when chronic
Commonly affects hands, face and neck in adults
Urticaria
Smooth-surfaced welts
Any part of skin or mucous membranes
Morbilliform rash
Red macules and papules
Usually starts on torso and spreads to extremities
Lichenoid rash
Purple, thin plaques with fine scale
Can involve any area but often volar wrist and legs
Cutaneous vasculitis
Purple, non-blanching
Favours gravity-dependent areas such as legs
Adis International Limited. All rights reserved.
Drugs Aging 2001; 18 (11)
Allergic Skin Disorders
829
2. Allergic Eczematous Dermatitis:
Contact Dermatitis
2.1 Cellular Basis of Allergic
Contact Dermatitis
Elderly patients can develop allergic contact
dermatitis despite the fact that cell-mediated immunity is decreased as a result of unknown mechanisms. Absolute T and B cell counts are not reduced with age.[4] Age does not seem to affect the
number of Langerhans cells in the elderly skin
compared with the nonelderly skin,[5] although
there are reports to the contrary.[6] However, allergic contact sensitivity to poison ivy appears to decrease with age[7] and elderly men are harder to
sensitise with dinitrochlorobenzene compared with
younger men.[8] Susceptibility to some,[9] but not
all,[10] cutaneous irritants decreases with age. It is
possible that this decreased irritation reduces the
production of cytokine danger signals that are necessary for the cutaneous sensitisation process. Because sensitisation, once acquired, is long-lasting,
and years of potential exposure and cumulative acquisition of allergens produces a high incidence of
contact dermatitis in the elderly.
2.2 Prevalence of Allergic Contact
Dermatitis in the Elderly
In a group of healthy volunteers aged 68 to 87
years and without skin disease, the prevalence of
positive patch test reactions to a standard screening
tray was 37% compared with a prevalence of 15%
in a young comparator population with a mean age
of 24 years.[11] This is consistent with the persistence of acquired allergies over many years. In patients aged >60 years and referred for evaluation
of dermatitis, patch test reactions have been reported to be positive in as many as 75% of patients,
with the highest percentage of positive reactions
reported in patients aged >70 years.[12] However,
other studies have shown a peak prevalence of positive patch test reactions in young women (due to
nickel sensitivity in jewellery) and in adults aged
40 to 50 years (due to occupational sensitisation)
Adis International Limited. All rights reserved.
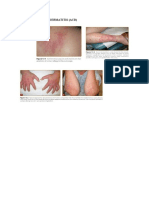
Fig. 1. A patient with venous stasis ulcers of the lower limb who
has multiple contact allergies resulting in dermatitis.
with a decline in the prevalence of positive patch
reactions in patients >60 years of age.[13]
This variation in the prevalence of positive
patch test reactions among the young and the elderly patients may be a function of study methodology. Variables include number and type of allergens patch tested and the time interval between the
application of the patch test and the final reading.
Other contributory factors include differences in
the study population including regional variation
in occupation and the use of personal care items
and topical medication, and inclusion or exclusion
of patients with high risk diseases such as venous
stasis ulcers.
2.3 Allergens Causing Contact Dermatitis in
the Elderly
Nickel and fragrance/Balsam of Peru are the
two most common contact allergens detected with
patch testing in the elderly. Nickel sensitivity
peaks in youth and declines with age, while allergy
to fragrance gradually increases with age.[14] Fragrance allergy may become more common with increasing use of moisturisers to treat xerosis associated with aging; the majority of elderly Americans
use such moisturisers.[2]
Many elderly people dye their hair. Allergy to
paraphenylenediamine used in dark hair dyes gives
a severe reaction with swelling of the face and periDrugs Aging 2001; 18 (11)
830
orbital region beginning hours to days after the dye
exposure. Patients with this allergy must avoid permanent hair dyes. They may, however, tolerate
temporary (rinse out) dyes or metallic dyes that are
applied daily.[15]
Venous stasis ulcers are associated with a high
incidence of multiple contact allergens.[16] Figure
1 shows a woman with venous stasis and superficial lymphocytic vasculitic ulcers who developed
redness and itching of the skin surrounding the ulcers. Patch testing revealed reactions to fragrance,
the preservatives DMDM hydantoin and imidazolidinyl urea, and the topical antibacterials neomycin and bacitracin, all of which were currently or
previously applied to her leg ulcers. Avoidance of
these ingredients resulted in resolution of her dermatitis.
In addition to the allergens noted in the case
described above, patients with venous stasis ulcers
may also become allergic to materials used to provide compression. Allergy to rubber chemicals
(thiurams, carba, mercaptobenzothiazole, black
rubber) were found in 15% of the patients with venous stasis ulcers who were patch tested.[17] Allergy to preservatives in Unna boots has also been
reported.[18]
Elderly patients who have undergone ostomies
for gastrointestinal or urinary disorders often develop irritant or allergic stomal dermatitis. Allergens causing ostomy dermatitis include adhesives,
fragrances, rubber chemicals and acrylates.[15]
Chronic conjunctivitis may be due to or complicated by contact allergy to topical medications or
preservatives in topical medications in about onethird of the patients.[19] When the offending ingredient is an antibacterial or preservative, the patch
tests are readily positive, but patch testing with
ophthalmic -blockers may give false negative results even when higher than usual concentrations
of the= -blockers and larger patch sizes are used.
In these cases, use testing or withdrawal of the
medication may be necessary to confirm diagnosis.[20]
Cheilitis and stomatitis in the elderly may be
due to contact dermatitis to flavourants in denti Adis International Limited. All rights reserved.
Nedorost & Stevens
frices or to sunscreens. Less commonly, these
symptoms may be due to denture materials; acrylic
allergy is much more common in workers with occupational exposure to uncured acrylics than in
denture wearers exposed only to the final product.[21]
2.4 Patch Testing
Patch testing in the elderly does not usually differ from that in younger patients. Elderly patients
may require assistance to remove their patches at
48 hours if they are not returning to the clinic at
that time; many patients lack the flexibility to reach
the patches on their back. Many elderly patients
prefer sponge bathing to full baths or showers and
are less inconvenienced by the bathing restrictions
inherent in the patch test process than are younger
patients. Topical medications are a common cause
of allergic contact dermatitis in the elderly and several of these medications, including neomycin and
the corticosteroids, often give late positive reactions. It is therefore important to perform delayed
readings at 7 to 10 days in patients tested for allergy
to these materials. Corticosteroid allergy, in particular, may be missed if a late delayed reading is not
performed.[22]
2.5 Photoallergens
Photopatch testing should be carried out whenever there is a photodistributed eczematous rash.
Photopatch test allergens include plant derivatives
that are often photoactive, as well as sunscreens
and other allergens from the standard tray such as
fragrance, some of which may cause a rash and give
positive patch test results only after exposure to
ultraviolet light.
Chronic actinic dermatitis is a condition seen
primarily in elderly dark-skinned men who have a
hypersensitivity response to ultraviolet and visible
light. These patients almost always have positive
patch tests to airborne allergens or photoallergens,
which in some way must stimulate subsequent development of sensitivity to light even in the absence of the allergen. Immunosuppressants are ofDrugs Aging 2001; 18 (11)
Allergic Skin Disorders
831
ten needed to control this severely debilitating disease.[23]
3. Eczematous Dermatoses Not Due to
Contact Allergy
Seborrhoeic, nummular and atopic dermatitis
occur in the elderly and can mimic allergic disease.
Seborrhoeic dermatitis may affect a more widespread area of skin in the elderly patient compared
with younger adults. Figure 2 shows seborrhoeic
dermatitis of the back and neck in an elderly
woman patient who also had more classic scalp
dermatitis and blepharitis and who had negative
patch testing to rule out contact dermatitis to personal care products including shampoo.
Atopic dermatitis is much less common in the
elderly compared with children and young adults.[24]
Atopy is associated with seasonal mucosal allergies, asthma, positive prick tests to various protein
allergens and positive atopy patch tests to aeroallergens such as dust mite. Food allergy is far
less common in adults than in children with atopic
dermatitis. Some adults, however, may benefit by
avoiding pseudoallergens which release histamine such as salicylates and benzoates which occur
naturally and as additives in the diet.[25] Interestingly, elderly patients with nummular eczema are
more likely than age-matched controls to show
positive atopy patch tests to dust mite. [26] Lateonset atopic dermatitis, without the usual history
of atopy, may explain the occurrence of dermatitis
in some adult patients referred for patch testing
who have negative patch test results and appear to
have endogenous eczema of unknown origin.[27]
Scabies should enter the differential diagnosis
in generalised dermatitis: institutional acquisition
of scabies is common in the elderly.[28]
4. Management of
Eczematous Dermatitis
A thorough history and physical examination,
with patch testing if indicated, will allow classification of the type of eczema.
Adis International Limited. All rights reserved.
Fig. 2. A patient with seborrhoeic dermatitis involving the back
and neck. Patch tests were negative.
Allergic contact dermatitis is best treated by patient education and avoidance of identified allergens.
Stasis dermatitis is treated with compression of
the oedematous lower extremity.
Seborrhoeic dermatitis is treated with anti-yeast
medications.
Asteatotic dermatitis is treated with emollients.
Atopic dermatitis, and all of the other forms of
dermatitis that are recalcitrant to other therapies, can be treated with topical or systemic corticosteroids.
Topical corticosteroids should not be used continuously, as they often cause tachyphylaxis as well
as adverse effects such as atrophy and striae. Topical corticosteroids, when applied on the face, may
cause steroid rosacea. Cataracts or glaucoma can
occur through application of topical corticosteroids in the periorbital area. Systemic corticosteroids contribute to osteoporosis and weight gain,
and can aggravate peptic ulcer disease, hypertension and diabetes mellitus. If adverse effects preclude the use of corticosteroids, or if they are
ineffective, treatment with the new nonsteroidal
topical immunosuppressive drug tacrolimus, phototherapy, or systemic immunosuppressives such
as cyclosporin may be required.
The desire to prevent allergic contact dermatitis
in the elderly patient does have implications for
prescribing. Potent sensitisers like neomycin
Drugs Aging 2001; 18 (11)
832
should be avoided in high-risk patients such as
those with stasis ulcers. In fact, some researchers
have suggested avoiding the routine use of topical
antibacterials even after surgical procedures, as the
reduced risk of contact allergy with white petrolatum alone compared with use of bactitracin may
outweigh the risk of wound infection.[29] When
prescribing topical corticosteroids for patients at
high risk of allergic contact dermatitis, it should be
noted that fluorinated corticosteroids are less likely
to sensitise, and are preferred to non-fluorinated
corticosteroids such as hydrocortisone and hydrocortisone-17-butyrate.[30]
5. Allergic Noneczematous Dermatosis
Sensitisation by the oral route is less common
than topical sensitisation, and more likely to produce drug-specific antibodies rather than delayed-
Fig. 3. Erythroderma and scales in a patient with a severe
morbilliform drug eruption associated with the administration of
carbamazepine.
Adis International Limited. All rights reserved.
Nedorost & Stevens
type hypersensitivity.[31] When urticarial, lichenoid or morbilliform lesions are present with or
without eczema in an elderly person with a pruritic
rash, a reaction to systemic medication is the likely
diagnosis.
Urticarial skin reactions are often caused by antibacterials, nonsteroidal anti-inflammatory drugs
(NSAIDs), antidepressants or opioids.[32] The hives
often resolve promptly after discontinuing the medication, but occasionally may last for weeks. Urticaria may also be caused by contact with rather
than ingestion of the allergen. Common causes of
contact urticaria include latex, as well as proteins
in foods.[33] Histamine H1 receptor antagonists (antihistamines) are very useful in managing the pruritus
of urticaria, in contrast to their limited usefulness
in eczematous pruritus.[34] The dosage of antihistamines may be increased until limited by sedation.
Caution should be exercised to avoid respiratory
suppression in patients also receiving other sedating medications.
Morbilliform (maculopapular) rashes are the
most common sign of systemic reaction to medication (figure 3). Such rashes are often associated
with the administration of antibacterials, anticonvulsants, gold, allopurinol or diuretics,[32] and are
more common in patients with concomitant viral
infection.[31] In the case of Stevens-Johnson syndrome or toxic epidermal necrolysis, suspected
drugs should be withdrawn as soon as possible.
These severe reactions with mucosal involvement
can be fatal, and have an improved prognosis if the
offending drug is withdrawn early in the course[35]
(figure 4).
Lichenoid drug reactions have clinical and histological features of lichen planus, and are often
associated with the administration of quinidine, blockers or thiazides.[36] These reactions may take
months to resolve after discontinuation of the causative drug.
Cutaneous vasculitis may be caused by diuretics, antibacterials and other medications.[32,36]
Phototoxic reaction may be associated with the
administration of tetracyclines, diuretics, NSAIDs
and antihyperglycaemic drugs.[37]
Drugs Aging 2001; 18 (11)
Allergic Skin Disorders
Fig. 4. A patient with toxic epidermal necrolysis who had widespread epidermal loss requiring endotracheal intubation went
on to die from complications of his drug reaction.
Fixed drug eruptions occur in limited areas, often on mucous membranes, as red or vesicular lesions that flare after each ingestion of the causative
medication, with progressive hyperpigmentation
between active episodes. They may be caused by
antibacterials such as cotrimoxazole (trimethoprim-sulfamethoxazole) or analgesics, particularly
NSAIDs. The tendency to develop this eruption
may be linked to the presence of HLA-B22.[38]
6. Investigating Rashes Due to Systemic
Allergic Reactions
There is currently no reliable, routine test to determine the cause of a drug-induced rash when the
patient is receiving multiple medications, as is the
case with many elderly patients. If the reaction is
mild, the most likely medication (often the medication most recently introduced) should be discontinued and substituted with a structurally unrelated
medication. The patient should be observed and if
no clinical improvement is noted after several
weeks, the next most likely medication should be
discontinued and so on until improvement is noted.
If the reaction is severe, all medications should be
substituted with structurally unrelated alternatives.[31]
Prick testing can be used to document some
drug allergies, such as penicillin,[31] and patch testing is sometimes useful when performed on lesio Adis International Limited. All rights reserved.
833
nal skin to document the cause of fixed drug eruption.[39] Oral provocation testing may be useful
when the reaction has not been severe, but generally should not be used to document severe reactions.
In vitro tests to determine susceptibility to a
drug reaction are being investigated. One severe
drug-induced adverse reaction is the anticonvulsant hypersensitivity syndrome, characterised by a
skin rash, lymphadenopathy and fever and at times
hepatitis, nephritis, pneumonitis or haematological
abnormalities. This rare condition has a delayed
onset after initiation of therapy with aromatic anticonvulsants such as phenytoin, carbamazepine or
phenobarbital (phenobarbitone). The role of the
immune system in this condition remains controversial. However, Shear and colleagues[40] have
developed an in vitro sensitivity test in which murine hepatic microsomal enzymes (e.g. cytochrome
p450) are incubated with the test drug, in order to
convert it to potentially toxic metabolites, and lymphocytes from the patient. Lymphocytes are used
because they are conveniently obtained cells and
they normally contain epoxide hydrolases, not because they are immunocytes. These hydrolases
normally detoxify the toxic areneoxide intermediate metabolite of the drugs. If the patient is deficient in these enzymes, these toxic intermediates
build up and the test lymphocytes die, yielding a
positive test. These authors have shown that a very
similar test can also be used to detect some adverse
reactions to sulphonamides.[41]
In addition to cytotoxicity assays, such as those
described by Shear, Spielberg and colleagues,[40,41]
tests for the in vitro assessment of the true immunological hypersensitivity risk are also available.
The latter tests are largely experimental and incompletely validated. Lymphocyte transformation
assays combine mononuclear leucocytes from the
patient with the suspected allergenic drug.[42] In
the event of a positive reaction, allergen-specific T
cells will undergo activation and growth. Tritiated
thymidine, a radiolabelled DNA precursor, is also
added to the assay, and will be taken up and incorporated into the DNA of daughter cells generated
Drugs Aging 2001; 18 (11)
834
Nedorost & Stevens
in the assay. Reactivity to the test compound is detected as an increased radioactivity in the tested
cells, reflecting antigen-specific T cell growth.
This test, like the cytotoxicity assay, is still developmental and requires specialised laboratory techniques and is subject to difficulty in interpretation.
Another related approach has been recently described by Yawalker et al.[43] In this report, T cells
derived from positive skin tests were shown to
demonstrate drug specificity manifested as increased tritiated thymidine incorporation, cytokine
production and cytolytic activity.
The tests described above have not yet been validated to the same extent as in vivo skin testing or
tests of humoral immunity such as radioallergosorbent tests. However, they do provide hope that
in the future we will develop the capacity to identify individuals who are at increased risk of developing severe drug eruptions. Alternatively, the antigen specificity testing described by Yawalker et
al.[43] may eventually lead to advanced tests capable of identifying which of the several drugs that
an individual patient may be taking is the cause of
a drug eruption.
7. Conclusion
Allergic skin disease in the elderly patient may
be eczematous if the cause is an environmental
contactant and urticarial or polymorphous if the
cause is an ingested allergen. Patch testing should
be utilised to determine the cause of allergic contact dermatitis. Patient history and discontinuation
of medications may be required to determine the
cause of skin reactions due to systemic allergens.
There are in vitro tests on the horizon that may help
to more rapidly determine the cause of a cutaneous
systemic drug reaction.
References
1. Thaipisuttikul Y. Pruritic skin diseases in the elderly. J Dermatol
1998; 25 (3): 153-7
2. Beauregard S, Gilchrest BA. A survey of skin problems and skin
care regimens in the elderly. Arch Dermatol 1987; 123 (12):
1638-43
3. Fehr BS, Takashima A, Bergstresser PARR, et al. T cells reactive to keratinocyte antigens are generated during induction of
contact hypersensitivity in mice: a model for autoeczematization in humans? Am J Contact Derm 2000; 11 (3): 145-54
Adis International Limited. All rights reserved.
4. Thivolet J, Nicolas JF. Skin ageing and immune competence.
Br J Dermatol 1990; 122 Suppl. 35: 77-81
5. Czernielewski JM, Masouye I, Pisani A, et al. Effect of chronic
sun exposure on human Langerhans cell densities. Photodermatol 1988; 5 (3): 116-20
6. Gilchrest BA, Murphy GF, Soter NA. Effect of chronologic
aging and ultraviolet irradiation on Langerhans cells in human
epidermis. J Investig Dermatol 1982; 79 (2): 85-8
7. Smith JG, Kiem IM. Allergic contact sensitivity in the aged. J
Gerontol 1961; 16: 118-9
8. Schwartz M. Eczematous sensitization in various age groups. J
Allergy 1953; 24: 143-8
9. Cua AB, Wilhelm KP, Maibach HI. Cutaneous sodium lauryl
sulphate irritation potential: age and regional variability. Br J
Dermatol 1990; 123 (5): 607-13
10. Coenraads PJ, Bleumink E, Nater JP. Susceptibility to primary
irritants: age dependence and relation to contact allergic reactions. Contact Derm 1975; 1 (6): 377-81
11. Mangelsdorf HC, Fleischer AB, Sherertz EF. Patch testing in an
aged population without dermatitis: high prevalence of patch
test positivity. Am J Contact Derm 1996; 7 (3): 155-7
12. Goh CL, Ling R. A retrospective epidemiology study of contact
eczema among the elderly attending a tertiary dermatology
referral centre in Singapore. Singapore Med J 1998; 39 (10):
442-6
13. Walton S, Nayagam AT, Keczkes K. Age and sex incidence of
allergic contact dermatitis. Contact Derm 1986; 15 (3): 136-9
14. Wantke F, Hemmer W, Jarisch R, et al. Patch test reactions in
children, adults and the elderly: a comparative study in patients with suspected allergic contact dermatitis. Contact
Derm 1996; 34 (5): 316-9
15. Reitschel RL, Fowler JF, editors. Fishers contact dermatitis.
5th ed. Philadelphia: Lippincott, Williams and Wilkins, 2001
16. Marasovic D, Vuksic I. Allergic contact dermatitis in patients
with leg ulcers. Contact Derm 1999; 41 (2): 107-9
17. Gooptu C, Powell SM. The problems of rubber hypersensitivity
(Types I and IV) in chronic leg ulcer and stasis eczema patients. Contact Derm 1999; 41 (2): 89-93
18. Praditsuwan P, Taylor JS, Roenigk Jr HH. Allergy to Unna boots
in four patients. J Am Acad Dermatol 1995; 33 (5 Pt 2): 906-8
19. Rudzki E, Kecik T, Rebandel P, et al. Frequency of contact
sensitivity to drugs and preservatives in patients with conjunctivitis [letter]. Contact Derm 1995; 33 (4): 270
20. Carriere M, Giordano-Labadie F, Schwarze HP, et al. Difficulties in the interpretation of patch test reactions to ophthalmic
beta-blockers. Contact Derm 1998; 39 (6): 319-20
21. Gebhardt M, Geier J. Evaluation of patch test results with denture material series. Contact Derm 1996; 34: 191-5
22. Isaksson M, Bruze M, Bjorkner B, et al. The benefit of patch
testing with a corticosteroid at a low concentration. Am J
Contact Derm 1999; 10 (1): 31-3
23. Hawk JL. Photosensitivity in the elderly. Br J Dermatol 1990;
122 Suppl. 35: 29-36
24. Hill LW, Sulzberger MB. Evolution of atopic dermatitis. Arch
Dermatol Syphilology 1935; 32: 451-63
25. Worm M, Ehlers I, Sterry W, et al. Clinical relevance of food
additives in adult patients with atopic dermatitis. Clin Exp
Allergy 2000; 30 (3): 407-14
26. Aoyama H, Tanaka M, Hara M, et al. Nummular eczema: an
addition of senile xerosis and unique cutaneous reactivities to
environmental aeroallergens. Dermatology 1999; 199 (2):
135-9
27. MacKenzie-Wood AR, Freeman S. Unclassified endogenous
eczema. Contact Derm 1999; 41 (1): 18-21
Drugs Aging 2001; 18 (11)
Allergic Skin Disorders
28. Yap KB, Siew MG, Goh CL. Pattern of skin diseases in the
elderly seen at the National Skin Centre (Singapore) 1990
[see comments]. Singapore Med J 1994; 35 (2): 147-50
29. Smack DP, Harrington AC, Dunn C, et al. Infection and allergy
incidence in ambulatory surgery patients using white petrolatum vs bacitracin ointment: a randomized controlled trial.
JAMA 1996; 276 (12): 972-7
30. Thomson KF, Wilkinson SM, Powell S, et al. The prevalence
of corticosteroid allergy in two U.K. centres: prescribing implications. Br J Dermatol 1999; 141 (5): 863-6
31. DeShazo RD, Kemp SF. Allergic reactions to drugs and biologic
agents. JAMA 1997; 278 (22): 1895-906
32. Swanbeck G, Dahlberg E. Cutaneous drug reactions: an attempt
to quantitative estimation. Arch Dermatol Res 1992; 284 (4):
215-8
33. Hjorth N, Roed-Petersen J. Occupational protein contact dermatitis in food handlers. Contact Dermatitis 1976; 2: 28-42
34. Hoare C, Li Wan Po A, Williams H. Systemic review of treatments for atopic eczema. Health Technol Assess 2000; 37:
1-191
35. Garcia-Doval I, LeCleach L, Bocquet H, et al. Toxic epidermal
necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? [see
comments]. Arch Dermatol 2000; 136 (3): 323-7
36. Litt J. Drug eruption reference manual. 1st ed. New York (NY):
Parthenon, 1998
37. Photosensitivity reactions to drugs. Med Lett 1995; 37: 35
Adis International Limited. All rights reserved.
835
38. Pellicano R, Lomuto M, Ciavarella G, et al. Fixed drug eruptions with feprazone are linked to HLA-B22. J Am Acad
Dermatol 1997; 36: 782-4
39. Ordoqui E, DeBarrio M, Rodriguez VM, et al. Cross-sensitivity
among oxicams in piroxicam-caused fixed drug eruption: two
case reports. Allergy 1995: 50: 741-4
40. Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome: in vitro assessment of risk. J Clin Invest 1988; 82:
1826-32
41. Shear N, Spielberg SP. An in vitro lymphotoxicity assay for
studying adverse reactions to sulphonamides. Br J Dermatol
1985; 113 Suppl. 28: 112-3
42. Sachs B, Erdmann S, Al-Masaoudi T, et al. In vitro drug allergy
detection system incorporating human liver microsomes in
chlorasepate-induced skin rash: drug-specific proliferation
associated with interleukin-5 secretion. Br J Dermatol 2001
Feb; 144 (2): 136-20
43. Yawalker N, Hari Y, Frutig K, et al. T Cells Isolated from positive epicutaneous test reactions to amoxicillin and ceftiaxone
are drug specific and cytotoxic. J Investig Dermatol 2000;
115: 647-52
Correspondence and offprints: Dr Susan Nedorost, University Hospital Dermatology Associates, 960 Clague Rd,
Westlake, OH 44145, USA.
Drugs Aging 2001; 18 (11)
Anda mungkin juga menyukai
- Beat Eczema: Susan ClarkDokumen24 halamanBeat Eczema: Susan ClarkHenry Nicholas Lee100% (2)
- A Diagnostic Approach To PruritusDokumen8 halamanA Diagnostic Approach To PruritusSpica AdharaBelum ada peringkat
- 14 Common Symptoms of ParasitesDokumen12 halaman14 Common Symptoms of ParasitesMaila Joy Pring FuentesBelum ada peringkat
- Exfoliative DermatitisDokumen7 halamanExfoliative DermatitisRidyah Ning TyasBelum ada peringkat
- Rosacea: Clinical PracticeDokumen11 halamanRosacea: Clinical PracticeLusy Dwitama LeryBelum ada peringkat
- Dermatology For The Small Animal PractitionerDokumen145 halamanDermatology For The Small Animal Practitionerсветлана бугаева100% (5)
- Lidl Bakery Bake Off Nutritional Information NI - July 2020Dokumen3 halamanLidl Bakery Bake Off Nutritional Information NI - July 2020Marta santosBelum ada peringkat
- Cardiac BiomarkersDokumen10 halamanCardiac BiomarkersfelipetheBelum ada peringkat
- Atopic Dermatitis Practical HCP Guide 2nd Ed 2016Dokumen12 halamanAtopic Dermatitis Practical HCP Guide 2nd Ed 2016Tia AfelitaBelum ada peringkat
- Contact DermatitisDokumen70 halamanContact DermatitisThariq Mubaraq DrcBelum ada peringkat
- Eczema Remedies | How to Relieve Eczema With Diet & Natural RemediesDari EverandEczema Remedies | How to Relieve Eczema With Diet & Natural RemediesPenilaian: 4 dari 5 bintang4/5 (1)
- Dermatitis: K. Puviyarasi Dept of MSN MNCDokumen26 halamanDermatitis: K. Puviyarasi Dept of MSN MNCPuviyarasi100% (2)
- Burn ManagementDokumen7 halamanBurn ManagementnrhmhealthBelum ada peringkat
- Everything You Need to Know About PsoriasisDokumen40 halamanEverything You Need to Know About PsoriasisAmrit Preet KaurBelum ada peringkat
- Psoriasis A Case StudyDokumen14 halamanPsoriasis A Case StudyYayin PestañoBelum ada peringkat
- Atopic DermatitisDokumen10 halamanAtopic DermatitistazzycaBelum ada peringkat
- Atopic DermatitisDokumen6 halamanAtopic DermatitisJhuli Elizabeth CBelum ada peringkat
- Hand EczemaDokumen18 halamanHand EczemaNella AztyBelum ada peringkat
- Case Report Dermatitis ContactDokumen10 halamanCase Report Dermatitis ContactMuhammad Aktora Tarigan100% (1)
- Contact Dermatitis: Allergic and IrritantDokumen9 halamanContact Dermatitis: Allergic and IrritantatikaBelum ada peringkat
- Contact Dermatitis: Key PointsDokumen8 halamanContact Dermatitis: Key Pointssaimon reyBelum ada peringkat
- Atopic Dermatitis: Review Open AccessDokumen7 halamanAtopic Dermatitis: Review Open AccessaraaritonangBelum ada peringkat
- EczemaDokumen39 halamanEczemakamardin zakuaniBelum ada peringkat
- BAHAN Chapter 24Dokumen33 halamanBAHAN Chapter 24S FznsBelum ada peringkat
- Identifying The Cause of Contact DermatitisDokumen6 halamanIdentifying The Cause of Contact DermatitiswadejackBelum ada peringkat
- 9930 PDFDokumen9 halaman9930 PDFAnonymous 2BC7omLaWCBelum ada peringkat
- Ebook PDF Current Medical Diagnosis and Treatment Study Guide 2nd PDFDokumen41 halamanEbook PDF Current Medical Diagnosis and Treatment Study Guide 2nd PDFcecil.slocum194100% (38)
- Jurnal Reading DADokumen18 halamanJurnal Reading DAWinendy Deo HaryantoBelum ada peringkat
- Bercak MerahDokumen16 halamanBercak MerahFary SatriadiBelum ada peringkat
- BSN Nursing Practicum Focuses on Integumentary SystemDokumen10 halamanBSN Nursing Practicum Focuses on Integumentary SystemMichelle Gliselle Guinto MallareBelum ada peringkat
- Irritant Contact Dermatitis: Practice EssentialsDokumen4 halamanIrritant Contact Dermatitis: Practice EssentialsHiedajatBelum ada peringkat
- Atopic® 0.1% Ointment Back To The Typical Life: Saleem AlawnehDokumen46 halamanAtopic® 0.1% Ointment Back To The Typical Life: Saleem AlawnehMuhammed AskarBelum ada peringkat
- Diagnosis of Atopic Dermatitis: Mimics, Overlaps, and ComplicationsDokumen35 halamanDiagnosis of Atopic Dermatitis: Mimics, Overlaps, and ComplicationsneleaBelum ada peringkat
- What is Atopy and AllergyDokumen5 halamanWhat is Atopy and AllergyShubhamBelum ada peringkat
- Dissertation On EczemaDokumen7 halamanDissertation On EczemaDoMyPapersUK100% (1)
- Research Paper On EczemaDokumen7 halamanResearch Paper On Eczemauziianwgf100% (1)
- Atopic Dermatitis: A Review of Diagnosis and TreatmentDokumen11 halamanAtopic Dermatitis: A Review of Diagnosis and TreatmentLeona NgadiahBelum ada peringkat
- Allergic Contact Dermatitis GuideDokumen12 halamanAllergic Contact Dermatitis GuideDaisy HamdaliBelum ada peringkat
- Treatment of Atopic Dermatitis (Eczema) - UpToDateDokumen70 halamanTreatment of Atopic Dermatitis (Eczema) - UpToDateKarla Alejandra BriseñoBelum ada peringkat
- Penatalaksanaan Dermatitis AtopicDokumen13 halamanPenatalaksanaan Dermatitis AtopicDini MayrisdayaniBelum ada peringkat
- Dermatology Module 3Dokumen7 halamanDermatology Module 3joseph joshua ngushualBelum ada peringkat
- Allergic (Kriteria DX Dki)Dokumen37 halamanAllergic (Kriteria DX Dki)SORAYA ALAMUDIBelum ada peringkat
- 5: Allergy and The Skin: Eczema and Chronic Urticaria: Mja Practice Essentials - AllergyDokumen6 halaman5: Allergy and The Skin: Eczema and Chronic Urticaria: Mja Practice Essentials - AllergyDavid PattersonBelum ada peringkat
- Treatment of Atopic Dermatitis (Eczema)Dokumen54 halamanTreatment of Atopic Dermatitis (Eczema)David GarcíaBelum ada peringkat
- Irritant Contact Dermatitis from Cosmetic Soap (39chDokumen10 halamanIrritant Contact Dermatitis from Cosmetic Soap (39chstillmysunsetBelum ada peringkat
- Atopic DermatitisDokumen8 halamanAtopic DermatitiskoesantoBelum ada peringkat
- Dermatitis Kontak Alergi (DKA)Dokumen25 halamanDermatitis Kontak Alergi (DKA)tryhabibullahBelum ada peringkat
- Acutesymptomsof Drughypersensitivity (Urticaria, Angioedema, Anaphylaxis, Anaphylacticshock)Dokumen20 halamanAcutesymptomsof Drughypersensitivity (Urticaria, Angioedema, Anaphylaxis, Anaphylacticshock)Daniela VarBelum ada peringkat
- Psoriasis: Posted: 02 Aug 2010 11:18 PM PDTDokumen5 halamanPsoriasis: Posted: 02 Aug 2010 11:18 PM PDTScamb TrekBelum ada peringkat
- Contact Dermatitis BJD Guidelines May 2009Dokumen9 halamanContact Dermatitis BJD Guidelines May 2009Cynthia OktariszaBelum ada peringkat
- Cap. 10. SKIN DISEASES PDFDokumen14 halamanCap. 10. SKIN DISEASES PDFoana policarpovBelum ada peringkat
- Ckobm0ujw001dr42bpo9as0yi 1 1 5 Allergic Contact Dermatitis Nassau 2020 ReviewDokumen16 halamanCkobm0ujw001dr42bpo9as0yi 1 1 5 Allergic Contact Dermatitis Nassau 2020 Reviewarief dpBelum ada peringkat
- Dermatitis & Urticaria: Department of Dermato Venereology Faculty of Medicine Gadjah Mada UniversityDokumen34 halamanDermatitis & Urticaria: Department of Dermato Venereology Faculty of Medicine Gadjah Mada UniversityadystiBelum ada peringkat
- Atopic DermitatisDokumen10 halamanAtopic Dermitatis909 Devang GawasBelum ada peringkat
- Cover Page Mhap 2032Dokumen12 halamanCover Page Mhap 2032Ilham RafisBelum ada peringkat
- Thesis Statement On EczemaDokumen7 halamanThesis Statement On Eczemafjgqdmne100% (2)
- Allergic Contact DermatitisDokumen11 halamanAllergic Contact DermatitisSf AkhadiyatiBelum ada peringkat
- Assignment of Zoology Skin AllergiesDokumen15 halamanAssignment of Zoology Skin Allergieswaji203Belum ada peringkat
- Approach To The Patient With Facial Erythema PDFDokumen38 halamanApproach To The Patient With Facial Erythema PDFFilipa FigueiredoBelum ada peringkat
- Atopic Dermatitis Demystified: Doctor's Secret GuideDari EverandAtopic Dermatitis Demystified: Doctor's Secret GuideBelum ada peringkat
- Complete Medical Guide for Disease Volume VII; Atopic DermatitisDari EverandComplete Medical Guide for Disease Volume VII; Atopic DermatitisBelum ada peringkat
- Brief suicide prevention interventions for at-risk patientsDokumen2 halamanBrief suicide prevention interventions for at-risk patientsfelipetheBelum ada peringkat
- 1 Octeto de DefronzoDokumen23 halaman1 Octeto de DefronzoLesli Rodriguez50% (2)
- Recompensa e ObesidadeDokumen9 halamanRecompensa e ObesidadefelipetheBelum ada peringkat
- Troponina e TiroideDokumen6 halamanTroponina e TiroidefelipetheBelum ada peringkat
- Serie de Casos e RevisaoDokumen4 halamanSerie de Casos e RevisaofelipetheBelum ada peringkat
- Igreja Centrada - 2 - A R Endenção e A CidadeDokumen8 halamanIgreja Centrada - 2 - A R Endenção e A CidadefelipetheBelum ada peringkat
- (2017.01) Blood Pressure Reduction in DMDokumen10 halaman(2017.01) Blood Pressure Reduction in DMfelipetheBelum ada peringkat
- Hipofisite Igg4 - Critical ReviewDokumen10 halamanHipofisite Igg4 - Critical ReviewfelipetheBelum ada peringkat
- BNP e Tiroide 2017Dokumen7 halamanBNP e Tiroide 2017felipetheBelum ada peringkat
- Systemic Effects of Hemodialysis Access: Anil K. AgarwalDokumen7 halamanSystemic Effects of Hemodialysis Access: Anil K. AgarwalfelipetheBelum ada peringkat
- Complicacoes Cateter1Dokumen10 halamanComplicacoes Cateter1felipetheBelum ada peringkat
- Nej Mo A 1506699Dokumen11 halamanNej Mo A 1506699felipetheBelum ada peringkat
- Igg4-Related Hypophysitis Presenting As A Pituitary Adenoma With Systemic DiseaseDokumen5 halamanIgg4-Related Hypophysitis Presenting As A Pituitary Adenoma With Systemic DiseasefelipetheBelum ada peringkat
- Artigo de ExemploDokumen8 halamanArtigo de ExemplofelipetheBelum ada peringkat
- Artigo HiperandrogenismoDokumen13 halamanArtigo HiperandrogenismofelipetheBelum ada peringkat
- Cardiorenal Syndrome Definition, Prevalence, Diagnosis and PathophysiologyDokumen18 halamanCardiorenal Syndrome Definition, Prevalence, Diagnosis and PathophysiologyfelipetheBelum ada peringkat
- Dizziness and VertigoDokumen17 halamanDizziness and Vertigoagrawa03Belum ada peringkat
- Termografia e TireoideDokumen6 halamanTermografia e TireoidefelipetheBelum ada peringkat
- The Relationship of Thyroid Hormone Status With MyocardialDokumen8 halamanThe Relationship of Thyroid Hormone Status With MyocardialfelipetheBelum ada peringkat
- Estudo ANodyneDokumen4 halamanEstudo ANodynefelipetheBelum ada peringkat
- Primary Care Summary of The British Thoracic Society Guidelines For The Management of Community Acquired Pneumonia in Adults: 2009 UpdateDokumen7 halamanPrimary Care Summary of The British Thoracic Society Guidelines For The Management of Community Acquired Pneumonia in Adults: 2009 UpdateMusyfiqoh TusholehahBelum ada peringkat
- Abstracts COMAPI 2014 IJMSDokumen20 halamanAbstracts COMAPI 2014 IJMSfelipetheBelum ada peringkat
- 10 Health Benefits of SpirulinaDokumen10 halaman10 Health Benefits of Spirulinahkm_gmat4849Belum ada peringkat
- Principles of Toxicology SummaryDokumen19 halamanPrinciples of Toxicology SummaryUrugonda VenumadhavBelum ada peringkat
- Bio SSM Term 2 With Cbse QuestionsDokumen148 halamanBio SSM Term 2 With Cbse QuestionsArcha B SuneeshBelum ada peringkat
- Rhinitis Allergic: Elma Wiliandini G4A020043Dokumen10 halamanRhinitis Allergic: Elma Wiliandini G4A020043elma wiliandiniBelum ada peringkat
- Bio-Oil® Product ManualDokumen60 halamanBio-Oil® Product ManualguitarristaclasicosdnBelum ada peringkat
- Runny Nose in The Child Care SettingDokumen2 halamanRunny Nose in The Child Care Settingisaac abbanBelum ada peringkat
- Nursing Diagnosis Therapeutic Management Outcome EvaluationDokumen6 halamanNursing Diagnosis Therapeutic Management Outcome EvaluationClare AlcoberBelum ada peringkat
- Test Specification GuideDokumen176 halamanTest Specification GuideEdogawa RakhmanBelum ada peringkat
- JF Customer Service Text enDokumen46 halamanJF Customer Service Text enTakumi JapaneseBelum ada peringkat
- Allergies Amoung Tactile Defensive Children: Current Allergy and Clinical Immunology May 2004Dokumen4 halamanAllergies Amoung Tactile Defensive Children: Current Allergy and Clinical Immunology May 2004Jody ThiaBelum ada peringkat
- Occupational Risks in BakeriesDokumen7 halamanOccupational Risks in BakeriesKedir DayuBelum ada peringkat
- UNIT 1 LAB Diagnosing and Treating A Jellyfish Sting Mariam SalhienDokumen7 halamanUNIT 1 LAB Diagnosing and Treating A Jellyfish Sting Mariam SalhienMariam SalhienBelum ada peringkat
- BIOLOGY PDFsDokumen168 halamanBIOLOGY PDFsNirmal ChakravarthyBelum ada peringkat
- MR Skin Prick TestingDokumen9 halamanMR Skin Prick TestingAyuAnatrieraBelum ada peringkat
- Allergic Contact Dermatitis Caused by Titanium Screws and Dental ImplantsDokumen7 halamanAllergic Contact Dermatitis Caused by Titanium Screws and Dental ImplantsAlexandra LauraBelum ada peringkat
- Thesis Goat MilkDokumen5 halamanThesis Goat Milkdonnakuhnsbellevue100% (2)
- Agota Statement On COVID 19 Vaccination of Pregnant and Lactating - 17-8-21Dokumen5 halamanAgota Statement On COVID 19 Vaccination of Pregnant and Lactating - 17-8-21prisco linusiBelum ada peringkat
- Unit Test 6Dokumen2 halamanUnit Test 6Daryna BodnarBelum ada peringkat
- Final Copar DeliverableDokumen3 halamanFinal Copar DeliverableSHERMINA HASANBelum ada peringkat
- Extended Safety Data Sheet for Agidol-1Dokumen24 halamanExtended Safety Data Sheet for Agidol-1Sekawan CosmeticsBelum ada peringkat
- Lecture Outline: See Powerpoint Image Slides For All Figures and Tables Pre-Inserted Into Powerpoint Without NotesDokumen81 halamanLecture Outline: See Powerpoint Image Slides For All Figures and Tables Pre-Inserted Into Powerpoint Without NotesDeeptanshu PrakashBelum ada peringkat
- Allergen ImmunotherapyDokumen3 halamanAllergen ImmunotherapytimvrghsBelum ada peringkat
- Observation On Therapeutic Effects of Cupping Therapy For Allergic RhinitisDokumen2 halamanObservation On Therapeutic Effects of Cupping Therapy For Allergic Rhinitischakir BezzahiBelum ada peringkat
- Treatment Protocols Using Nutramedix Products for Medical ConditionsDokumen17 halamanTreatment Protocols Using Nutramedix Products for Medical Conditionsmarkus schnistBelum ada peringkat
- CHAPTER - 1 - Differential Diagnoses - 2011 - Small Animal Dermatology PDFDokumen21 halamanCHAPTER - 1 - Differential Diagnoses - 2011 - Small Animal Dermatology PDFRanjani RajasekaranBelum ada peringkat
- Sulfite Allergy: What You Need To KnowDokumen12 halamanSulfite Allergy: What You Need To KnowMichael McKee100% (1)
- SOP of Flame PhotometerDokumen5 halamanSOP of Flame Photometerfawaz khalilBelum ada peringkat
- Aria Rhinitis Allergic Pocket GuideDokumen8 halamanAria Rhinitis Allergic Pocket GuideAlfani FajarBelum ada peringkat