A Review of The Status of Foam Applications in Enhaced Oil Recovery
Diunggah oleh
Rosales DidierJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
A Review of The Status of Foam Applications in Enhaced Oil Recovery
Diunggah oleh
Rosales DidierHak Cipta:
Format Tersedia
SPE 164917
A Review of the Status of Foam Applications in Enhanced Oil Recovery
Seyed Amir Farzaneh, SPE and Mehran Sohrabi, SPE
Centre for Enhanced Oil Recovery and CO2 Solutions, Heriot-Watt University, Edinburgh, UK
Copyright 2013, Society of Petroleum Engineers
This paper was prepared for presentation at the EAGE Annual Conference & Exhibition incorporating SPE Europec held in London, United Kingdom, 1013 June 2013.
This paper was selected for presentation by an SPE program committee following review of information contained in an abstract submitted by the author(s). Contents of the paper have not been
reviewed by the Society of Petroleum Engineers and are subject to correction by the author(s). The material does not necessarily reflect any position of the Society of Petroleum Engineers, its
officers, or members. Electronic reproduction, distribution, or storage of any part of this paper without the written consent of the Society of Petroleum Engineers is prohibited. Permission to
reproduce in print is restricted to an abstract of not more than 300 words; illustrations may not be copied. The abstract must contain conspicuous acknowledgment of SPE copyright.
Abstract
Foam has been used for controlling gas mobility during injection processes in order to mitigate the adverse effects of low gas
viscosity, reservoir heterogeneity and gravity override. In addition, foam has also been employed in near-wellbore
production, matrix acidizing stimulation, hydraulic fracturing, gas shut-off and water shut-off. The optimization of these
operations requires a good understanding of the physical characteristics of foam and its behavior under reservoir conditions.
Despite numerous experimental and theoretical studies on foam and some field applications reported in the literature, there is
no comprehensive literature survey detailing the knowledge and experience gained through the application of foam. The aim
of this paper is to present a critical review of the literature available on the use of foam injection for enhanced oil recovery.
An extensive appraisal of the current status of foam application in EOR methods is a useful way of presenting advantages,
shortcomings and limitations of the process as well as supplementary advances. Special attention has been given to the
review of new research topics, such as the use of foam as a mobility control agent. Advances in more efficient foaming
agents, additives and boosters are discussed. Moreover, in depth analysis of field applications of foam in EOR projects show
that the main problems encountered during field-scale foam applications are related to foam stability, foam compatibility, as
well as adsorption of the injected chemicals onto the rock surface. To address this, we discuss the evolution of the pilot field
application and the reasons for inconsistency between laboratory results and field scale performance.
Introduction
Favourable mobility ratio between oil bank and chemical slug as well as chemical slug and drive is necessary for efficient
mobilization and displacement of residual oil in chemical EOR methods. Polymers can be added to both the slug and drive
fluid to reduce their mobilities and achieve a more favourable mobility ratio for efficient mobility control. The term mobility
control relates to the stability of the interface (displacement front) between the displacing and displaced fluid. Some
reservoirs do not exhibit the suitable traits that favour polymer injection; or their use may be economically unattractive. High
molecular weight polymers can plug rocks with very low permeability, or if a lower molecular weight polymer is used to
avoid plugging then the cost of using it increases until the point of becoming uneconomical. This reinforces the need for
optimization with the field costs in mind. Physically, some polymers can mechanically degrade due to high shear stress in the
injection line or near well bore regions of low permeability reservoirs. Moreover, high salinity and a severe salinity gradient
in injection and formation waters would require a significant amount of polymer for mobility control. As an alternative to
polymers, gas can also be used to provide mobility control in EOR applications. When gas is injected with the aqueous
chemical solution, simultaneous flow of two phases results in the mobility reduction of each phase. The combined mobility of
two displacing phases is less than their individual mobilities, thus resulting in improved mobility control. Water-alternatinggas (WAG) injection and simultaneous water and gas injection (SWAG) are two processes in which mobility control is
achieved due to simultaneous flow of two phases (Sohrabi et al., 2000, Stephenson et al., 1993; Robie, et al., 1995).
The concept of mobility reduction due to dispersed two-phase flow can be utilized in chemical flooding for achieving
mobility control in the displacement process. Similarly to WAG or SWAG processes, simultaneous flow in porous media can
reduce the mobilities of injected gas and chemicals. Since surfactant is present in the chemical slug, simultaneous injection of
gas and the chemical slug may result in the formation of foam. In foam, gas bubbles are surrounded by thin films of fluids
called lamella. The surface tension on individual lamellae, as well as the drag force on it as it slides along the pore walls
causes it to resist movement out of the pore throats. This resistance to movement results in increased gas apparent viscosity
SPE 164917
and thus increased gas saturation. The increase in gas saturation results in reduction of liquid saturation and liquid relative
permeability. Thus, formation of foam reduces the mobility of the chemical slug and hence improves mobility control in the
process. Similarly, mobility control during drive injection can be achieved by co-injecting gas and surfactant containing drive
fluids.
Gas and liquid injection rates define the quality of the foam formed (i.e. ratio of gas rate to total injection rate), which will
also impact its mobility reduction characteristics (Schramm, 1994a; Chang, 1999). The stability of foam in slug and drive
partially determines the magnitude of mobility reduction for gas and surfactant solution. If foam is stable, gas and liquid
mobilities are reduced and displacement efficiency in the process is improved. On the other hand, unstable foam results in a
smaller reduction in gas and liquid mobilities and thus poor displacement efficiency. Foam is destabilized in the presence of
oil (Nikolov, 198; Friedmann and Jensen, 1987), which is most likely at the leading edge of the foam front. If the slug size is
too small, the entire volume of foamed-slug may be destabilized, resulting in higher slug mobility and an unfavourable
mobility ratio between the oil bank and chemical slug. It is believed that foam propagation is more robust in high
permeability rocks, and vice-versa. Low mobility foam in high permeability regions diverts the injected fluids to lower
permeability regions, resulting in the improvement in volumetric sweep efficiency or conformance control. The improved
conformance control is another advantage of using gas over polymer as the mobility control agent. The mobility reduction
characteristic of polymer is not a strong function of permeability, and hence benefits of conformance control are not achieved
when polymer is used. Foam stability and mobility reduction characteristics depend on the properties of rock and fluids and
process-design parameters such as permeability, injection foam quality and the size of the chemical slug.
The objective of this paper is to review the knowledge and experience of mobility control applications of the foam flooding
process. This literature review concentrates on the history and development of foam-based mobility control technologies to
overcome the geologic and process limitations such as poor sweep efficiency, unfavorable injectivity profiles, gravity
override, high ratios of gas to oil produced, early breakthrough, and viscous fingering. The fundamentals of foam production
and foam termination in porous media as well as the short background of foam based EOR methods are explained. The effect
of different factors: pressure, temperature, salinity, adsorption and crude oil on foam characteristics are reviewed. The
chemical means and application of nano-particles on foam flooding process for mobility control are addressed. Published
literature on foam flooding applicability on fractured porous media and heavy oil reservoir are also evaluated. In addition, the
patents related to foam based EOR processes are surveyed. Besides, field and pilot applications of foam-EOR projects and its
relationship with lab-scale studies are presented.
Foam in Porous Media
A study of foam in porous media must account for three flow regimes encountered in field applications. Firstly, surface
facilities and the well itself, where inertial flow may create bulk foam. Secondly, at the near-wellbore region where the flow
rate and pressure gradient are high. Thirdly, in the formation away from the injection well, where flow rate and pressure
gradient are much lower (Rossen, 1995). Each flow regime results in entirely different flow behaviours and therefore the
foam generation mechanisms. It is commonly accepted that lamella are created by following three mechanisms inside a
realistic porous media:
1. Leave behind is the creation of stabilized liquid films or lenses in pore throats as gas invades adjacent pore bodies through
other throats. Although sometimes cited as a source of weak or ineffective foam the leave behind mechanism can create large
number of lamella. Nevertheless if it is only a lamella creation mechanism, the gas will always have at least one continuous
pathway for flow (Chen et al., 2004).
2. Lamella division denotes the event when two or more lamella is created from a single one. Each time a mobilized lamella
passes through a pore body, with more than one pore throat unoccupied with liquid or another lamella, the lamella must either
break or span several open throats.
3. Snap-off is a third mechanism for lamella generation: lamellas are created in gas-filled pore throats if the local capillary
pressure falls to about half the capillary entry pressure of the throat. While it depends on the geometry of the throat and
wettability of porous medium, the value of one-half is a reasonable representative value for three dimensional pore
geometries (Chen et al., 2004).
Foam Termination Mechanisms in Porous Media
In the absence of oil, foam lamella in porous media rupture by two mechanisms: both mechanisms result in the formation of
one big bubble instead of two smaller bubbles that initially occupied the pore body; however the first one happens through a
fast physical process while the latter takes place through a slow diffusion process.
1. Capillary suction coalescence: moving lamella coalesce when they are rapidly stretched across large pore bodies. For a
given gas flow rate and capillary pressure, pore-throat/pore body combinations with large aspect ratios, serve as termination
SPE 164917
sites. As the gas velocity and/or porous medium capillary pressure increases, an increasing number of throat/body
configurations become termination sites (Manlowe and Radke, 1990).
2. Gas diffusion coalescence: trapped or static bubbles can break by a second mechanism. Whenever two bubbles with
different curvatures are in contact, gas diffuses from the more highly curved bubbles (smaller bubbles) to the less curved
bubbles (bigger bubbles) through the intervening lamella. Eventually, the smaller bubbles disappear along with the common
lamella (Manlowe and Radke, 1990).
Background of Foam for EOR
Foam stability and mobility reduction characteristics depend on the properties of rock and fluids and process-design
parameters such as formation permeability, injection foam quality and the size of the chemical slug. The effects of these
parameters on the performance of the foam flooding process needs to be ascertained in order to determine its optimal
potential for EOR. Most foam owes its existence to the presence of surfactants, that is, materials which are surface active.
They are concentrated at the interface of a fluid and act to reduce the surface tension between interfaces. More importantly
for preventing foam termination, they stabilize the thin fluid film against rupture. In aqueous foams, surfactant molecules are
amphiphilic; their two parts are hydrophobic and hydrophilic so that they can stay on the water surface. In porous media,
foam exists as gas bubbles whose shapes conform to the solid matrix. Hirasaki (1989) defined foam in porous media as a
dispersion of a gas in a liquid such that the liquid phase is continuous and at least some part of the gas phase is made
discontinuous by thin liquid films called lamellae.
Effects of Process Variables Foam Mobility Control
Permeability
Permeability is the most important rock parameter that affects foam propagation in porous media. Injection foam quality and
chemical slug size are the main process-design parameters that influence both foam stability and rheological behaviour.
Experimental evidence indicates that foam reduces gas mobility more in high permeability media than in low permeability
media. For instance, Djabbarah et. al. (1990) examined CO2 displacement efficiency with foaming agents in parallel one
dimensional porous medium with different permeabilities. They found that foam increased the resistance to flow in high
permeability layers and diverted the injected CO2 to less permeable regions. Bertin et al. (1998) constructed a heterogeneous
porous medium with permeable sand surrounding a sandstone core with a permeability contrast of about 70:1. In the absence
of foam, very little gas flowed into the low permeability sandstone core. In the presence of foam however, a significant
portion of injected gas was diverted. Nguyen et al. (2004) conducted a study on foam flow in heterogeneous cores using highresolution computed tomography (CT) to investigate pore scale phenomena. They observed that during the transient
displacement of liquid in the core, the foam front traverses more steadily and uniformly in high permeability layers. Tsau and
Heller (1996) also found that the effective viscosity of foam increased with permeability, and termed this behaviour of foam
as selective mobility reduction. This would help to achieve better conformance control in foam flooding process. This is
lost in polymer applications because its mobility is not a strong function of permeability.
Injection Rate
To an extent, the gas and liquid injection rates determine the in-situ quality of foam during the process. Foam quality is an
important parameter that determines foam propagation and mobility characteristics. Foam propagation will diminish if the
quality is either too high (dry foam) or too low (wet foam). In actual field applications, the amount of available gas may
determine the foam quality that can be realistically achieved during the process. If the gas is not available in large quantities,
only lower foam quality applications are possible. A number of studies on foam quality have shown that foam exhibits
mobility reduction properties where the foam quality is rated between 45 and 95% (Blauer, 1974; Schramm, 1994; Chang,
1998). Above a foam quality of 95%, foam becomes too dry to be stable; below 45% the foam loses its rheological
consistency and reflects the flow behaviour of the constituting liquid phase. Studies have also shown that within a certain
range of foam qualities, the degree of gas dispersion into an aqueous phase increases with the increase in foam quality. The
range of foam quality within which this increase occurs depends on rock, fluid and process parameters.
Pressure
In general, higher pressure favors foam stability. Gases such as CO2 become denser at higher pressure, which enhances the
intermolecular associations between the gas and the hydrophobic tails of the surfactant molecules. In a micromodel study, it
was found that sweep efficiencies associated with CO2 foams flowing at a pressure just below the MMP were just as high as
the efficiencies measured at pressures well above the minimum miscibility pressure (MMP). Therefore, it was concluded that
the high sweep efficiencies can be accomplished using the least amount of CO2 if the foam flood is conducted at the pressure
around MMP rather than much higher pressures (Chang et al., 1994).
Temperature
Because reservoir temperature is a factor that cannot be altered during a foam flood, there are relatively few core flooding
studies in which temperature is varied systematically. Nonetheless, it is apparent that foam flooding at temperatures above
SPE 164917
80C may require more careful design than low temperature floods. A benefit of high temperature formations is that the
adsorption of surfactant in the formation will be lower (Ziegler and Handy, 1981). The primary obstacles for the application
of foams in deep, hot formations include the decrease of surfactant solubility in brine that typically occurs with increasing
temperature, the thermal degradation of the surfactant that is enhanced with increasing temperature (Handy et al., 1982), the
slight increase in the interfacial tension between the CO2 and the brine (Liu et al., 2005a), and diminished foam stability
(Wang, 1984) especially at temperatures above 60C that must be compensated for by higher concentrations of surfactant
(Liu et al., 2005b).
Brine Salinity
In general for a given surfactant, increased salinity may tend to destabilize foam or depending on the surfactant, have little
effect. A decrease in foam stability may be attributable to the increased salinity breaking the foam by decreasing the
electrostatic double layer forces, or by diminishing the surfactant solubility in brine (Alkan et al., 1991). On the other hand,
some anionic surfactants that have excellent foaming properties, such as Chaser CD 1045 that are not significantly influenced
by the salinity of the brine for aqueous solutions containing at least 0.025wt% surfactant (Liu et al., 2005b).
The effect of salinity on surfactants may be more pronounced when the surfactant is dissolved in the CO2 rather than the
brine. Such systems are based on low concentrations of non-ionic surfactants, and the presence of dissolved solids in the
brine can diminish the solubility of the surfactant in the brine and drive it toward the CO2 (Torino et al., 2010). The lower
solubility of non-ionic surfactants in CO2 is also evidenced by the decrease in cloud point temperature (the temperature at
which a 1 wt. % solution of surfactant in an aqueous phase exhibits two-phase liquid-liquid behavior) when brine solutions
are compared to water solutions (Adkins et al., 2010).
Oil
The important parameter to be considered regarding to the capability of foams for mobility control is the effect of oil. It
would be important for foam to reduce the mobility of displacing fluid even when contacting oil in the reservoir. Screening
tests were used to assess the effect of oil on foams, including adding refined hydrocarbons or crude oil to low pressure (air
foam and nitrogen foam)(Dellinger et al., 1994), or high pressure CO2-in-brine foams (Mannhardt et al., 2000).
Also, different investigators suggest that oil becomes detrimental to foam at oil saturations above 5% to 20% (Schramm,
1994b) by various core flooding experiments. Among a number of mechanisms of foam/oil interaction suggested in the
literature, (Schramm, 1994; Schramm and Novosad, 1990; Manlowe and Radke, 1990; Bergeron et al, 1993; Lobo and
Wasan, 1993) three main models have emerged in attempts to predict foam stability to oil: spreading and entering
coefficients, lamella number, and pseudo-emulsion film models. Measurements of the spreading and entering coefficients of
bulk liquid, have been employed with some success (Kristiansen and Holt, 1992; Lau and OBrien, 1988) but these
measurements do not show the linear trend and correlation with foam stability in presence of oil (Schramm and Novosad,
1990; Manlowe and Radke, 1990; Bergeron et al, 1993; Lobo and Wasan, 1993; Hanssen and Dalland, 1990; Raterman,
1989). The lamella number employed to quantify the observation of destabilization of foam by imbibing the emulsified oil
into the foam lamellae (Schramm and Novosad, 1990; Schramm and Novosad, 1992; Schramm and Turta, 1993).
Pseudoemulsion film models state that a foam can only be stable in the presence of oil if the oil is wetted by the aqueous
phase, i.e., if oil and gas phases remain separated by a film of aqueous phase, the pseudoemulsion film, (Manlowe and
Radke, 1990; Bergeron et al., 1993; Lobo and Wasan, 1993; Nikolov, 1986). Although different models have been
successfully applied to different situations, translating the fundamental mechanisms of foam/oil interaction into generally
applicable rules for field application remains difficult.
Perhaps the most important assessments of the effect of oil on CO2 foams are related to oil recovery tests intended to emulate
the CO2 EOR process. Numerous investigators have conducted CO2 injection into core tests that have been waterflooded then
oilflooded to residual water saturation to represent fields that are ready for a secondary recovery process, or a core that has
been waterflooded, then oilflooded to residual water saturation, and then waterflooded to emulate a formation ready for
tertiary recovery. An example of the former case, in which cores initially contained high oil and residual water saturation,
was recently presented (Yin et al., 2009). This study demonstrated that CO2 foams were capable of reducing the residual oil
saturation beyond that achieved by CO2 floods when the foam flood occurred after the CO2 flood. However, CO2 injected
after a CO2 foam flood was not able to yield additional oil.
Chemicals (Foam Agent)
Surfactant
The surfactant has an important role in generation and stability of the foam in porous media. It affects the interfacial forces
between the gas and liquid which in turn affects value of capillary pressure. The appropriate surfactant should have the
capability of generating sufficient and stable foam at the reservoir conditions as well as should have low adsorption and
decomposition losses. Last but not least it should increase the sweep efficiency and the oil recovery all in respect of reservoir
engineering point of view. Additionally, it should be commercially available and inexpensive (Casteel and Djabbarah, 1988).
SPE 164917
Chou (1991) showed that the foam is readily formed during a displacing of the liquid phase by the gas phase whenever the
porous medium is pre-saturated with a surfactant solution. The reduction of surfactant concentration below the Critical
Micelle Concentration (CMC) caused a shift of the transition zone of the flow regimes to lower values of gas fractional flow
and capillary pressure (Alvarez et al., 1999). As it mentioned in the foam termination mechanisms, foam coalescence forces
are inversely proportional to surfactant concentration, thus the foam weakens and the displacement efficiency decreases as
the surfactant concentration decreases (Apaydin and Kovscek, 2000). Friedmann and Jensen (1986) showed that size of foam
bubbles inside porous media slightly decreases with increasing of the surfactant concentration. Li et al. (2006) found that
higher velocity is required to create foam when the surfactant concentration is reduced.
The surfactant should be soluble in brine in order to have the surfactant stabilize CO2 in brine emulsions. Most of the
promising CO2 foaming surfactants are indeed very water soluble, with examples including anionics and nonionics with a
relatively high number of hydrophilic ethylene oxide groups (Borchardt et al., 1988). Most surfactants become less soluble in
brines as the temperature increases and the concentration of total dissolved ions increases; therefore, it is imperative to assess
CO2 foams being considered for field tests at reservoir temperature and pressure using reservoir fluids and formation core
samples (Kuehne et al., 1992).
Many commercial surfactants have been identified in lab tests as viable candidates for CO2 foam generation (Enick and
Olsen, 2012). Different types of water soluble anionic -olefins sulfonates (AOS) have frequently been cited as an excellent
foam agent for the different foam based EOR processes. Chaser CD 1045 has been described by many investigators as a very
good or excellent foaming surfactant (Heller, 1994; Chang and Grigg, 1999; Yaghoobi and Heller, 1994; Khalil and Asghari,
2006; Yaghoobi et al., 1998; Bai et al., 2005; Bai et al., 2010; Tsau and Heller, 1992) along with Alipal CD 128 (Casteel and
Djabbara, 1988; Alkan et al., 1991; Holm and Garrison, 1988; Dellinger et al., 1984). The enormous majority of pilot tests
were conducted with Chaser CD 1045 and/or Alipal CD 128 (Enick and Olsen, 2012). Other examples of surfactant which
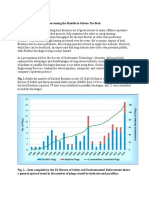
were used in either lab scale or pilot/field scale studies shows in Table 1 which is adapted from work of (Enick and Olsen,
2012) plus some other works from other researcher on nitrogen foam which are cited in the table.
Liu et al., (2005) reported the extensive experimental study on the effect of different parameters on interfacial tension (IFT)
measurement and foam stability with Chaser CD 1045. They showed that the CD1045 foam was stable under all tested
temperatures at surfactant concentrations of 0.1 wt% and above, and decreased with increase of temperature at surfactant
concentrations of 0.05 wt% and below at 1500 psig. Similar tends on the temperature effect on foam stability were observed
at high pressure (2000 psig). However, foam stability increases with CD 1045 concentration at 75C. Foam is stable under all
tested pressures at surfactant concentrations of 0.025 wt% and above, and foam stability decreases with increasing pressure at
the surfactant CD 1045 concentration of 0.005 wt% at 25C. Similar behaviours were observed at high CD 1045
concentrations at 75C. Also, the study showed that, interfacial tension (IFT) does not directly correlate to foam stability and
foam stability cannot be predicted solely by the value of IFT. Asghari et al., (2005) proposed a new foam system which
composed of the mixture of CD1045 and different foam stabilizers. The results showed that the developed stable foam can
significantly reduce the permeability to flow of brine in wormholes. The most stable foam provided with a combination of
CD 1045 and Ultrez 10.
Tsau et al. (1999) used mixed surfactant to improve mobility control in CO2 flooding. The foam durability was measured via
high pressure foam column to assess the stability of CO2 foam. Also, core flooding tests were performed core flooding
experiments in cores with two different permeability sections. Their results showed that mixture of anionic and non-ionic
surfactants exhibited either comparable foam stability than the foam generated using the individual mentioned surfactants.
They observed that a mixture of non-ionic and anionic surfactant showed better foaming stability, mobility reduction and less
adsorption that that generated by an anionic surfactant alone. But, the mixture of anionic AOS and anionic ethoxylated
alcohol sulphate generated more stable foam than non-ionic and anionic surfactant.
The idea of dissolving a surfactant into CO2 during foam mobility control processes for the purpose of generating CO2 in
brine mobility control foams was suggested by Bernard and Holm in their 1967 patent. In recent years, several commercially
available, non-ionic surfactants have been identified that are capable of dissolving in CO2 in dilute concentration at typical
MMP conditions and, upon mixing with brine, stabilizing CO2 in water or CO2 in brine emulsions or foams. Some of these
surfactants include water-soluble branched alkylphenol ethoxylates, linear alkylphenol ethoxylates, and branched alkyl
ethoxylates. Xing et al., (2010) measured the stability of these emulsions, as measured by the rate of their collapse, the results
showed that it influenced by the architecture of the surfactant. The details of these types of surfactants are given elsewhere
(Le et al.,). But, they cannot found direct relationship between slight changes in the alkyl tail and significant differences in
the foam stability. Le et al., (2008) also demonstrated a novel foam flooding method which involves dissolving surfactant in
the CO2. The experimental results showed that this method drastically lowers the injection costs, reduces the loss of
surfactant onto the rock surface due to adsorption, and improves in-situ foam generation to significantly increase oil
recovery. Two different novel methods, continuous CO2 dissolved surfactant injection and water alternating gas with CO2
SPE 164917
dissolved surfactant injection, had been studied. Their results on carbonates core floods demonstrated that CO2 dissolved
surfactants greatly reduce the mobility of the injected gas compared to conventional injection strategies.
Other Foam Agent
Guo et al., (2011) reported results of laboratory study of a novel alkaline surfactant foam process (ASF). They designed the
alkaline surfactant system to provide good foam stability and lowering ability of IFT as well as well defined optimum
salinity. They reported the oil recovery of ASF system to water flooded core was twice as AS flood oil recovery to water
flooded core. The mechanisms were responsible in the higher oil recovery were formation of emulsified oil and mobility
control of generated foam respectively.
Emulsion screening test, core floods and flow visualization experiments using an alpha olefin Sulfonate (AOS), Na2CO3 and
heavy crude oil has shown in work of Lau (2010). They reported that steam foam offers great advantages over regular steam
foam by combining the benefits of thermal and chemical EOR processes. Na2CO3 increased the surfactant propagation
through the core.
Kang et al., (2010) carried out the novel experimental design of ultra low interfacial tension foam agent with combination of
surfactant, HPAM for improving foam stability, and non alkali agent for reducing IFT. The novel non alkali agent has no
reaction with divalent cations. The foam air flow method used for measuring the foamability of proposed formula. The results
of nitrogen foam flooding in the heterogeneous heavy oil saturated cores showed that the oil recovery improvement by factor
of 23% of initial oil in place over water flood recovery.
Syahputra et al., (2000) reported the experimental results of using Lignosulfonate and mixture of surfactant in CO2 foam
flooding. The properties of foam (foam stability) were evaluated using high pressure full sight-glass with ability of
determination of both interfacial tension and foam stability. Also, they evaluated the mobility reduction factor and the oil
recovery efficiency of the custom designed foamer by means of core flooding experiments in heterogeneous composite core.
They informed that the Lignosulfonate was compatible with most of the Chaser CD1040, Chaser CD1045 and Chaser
CD1050 in generating high pressure CO2 foam. Their results also showed that, inexpensive Lignosulfonate was weak foam
former by itself, but, it became a good foaming agent in form of mixture with above surfactant and it improved the oil
production form core flood significantly with lower concentration of surfactant.
Adsorption
The adsorption of surfactant into the rock surface is one of the most unwanted problems in foam based EOR processes (other
than the effect of oil) which is destabilize the foam. Adsorption measurements are commonly performed with reservoir rock
and high surfactant concentration than its required amounts to create desirable stable foam. The cationic surfactants do not
employ in surfactant based methods in sandstone rocks (due to its negative charge) in order to reduce adsorption losses. As a
result anionic, nonionic, and amphoteric surfactants have been more commonly assessed for surfactant based EOR methods
(particularly CO2 foam flooding) of sandstone porous media. However, in carbonate reservoirs with positive charge surface;
anionic and Amphoteric surfactants are not acceptable and cationic surfactants can give lower adsorption. Adsorption of the
surfactant on the reservoir rock reduces the surfactant concentration in the injected fluid. The adsorption is a function of the
surfactant formulation, crude oil and brine compositions, rock mineralogy, and conditions of pressure and temperature of
reservoir (Casteel and Djabbarah, 1988). Novosad et al., (1986) showed that the surfactant adsorption decreases with
increasing the temperature, and increases with the presence of clays in the unconsolidated sandstone core between 50C to
150C. Grigg and Mikhalin (2007) showed that the adsorption is a function of state of the fluid movement alongside the other
mentioned parameters, and the adsorption density on the rock is best described as a function of the available surfactant
volume in the system rather than by surfactant concentration.
Liu et al. (2006) observed that the presence of gas with the surfactant solution in the carbonate rock does not affect the
surfactant adsorption. Bai and Grigg (2005) found that the Lignosulfonate reduced of main surfactant on to the limestone
rock. They also showed that adsorption increased with increasing NaCl and CaCl2. Kuhlman et al., (2000) demonstrated that
the reducing the length of the ethoxylate chain in alcohol ethoxy sulfonates and blinding unethoxylated and ethoxylated
sulfonates to optimized desirables properties, surfactant adsorption on sandstone core was reduced and foam stability
increased. They also illustrated that the surfactant concentration below the CMC have lower adsorption but it also have the
minimal mobility control (due to low surfactant concentration). As it mentioned before, they suggested the ideal surfactant
for CO2 foam in a harsh conditions (high temperature and high salinity), sandstone reservoir could be a mixture of sulfunated
narrow range ethoxylated alcohols with an unethoxylated sulfonate.
SPE 164917
Nitrogen Foam
In this part of the paper, some examples of nitrogen foam floods are presented to identify the essential differences between
CO2 foam and nitrogen foam as well as clarification of the status of research as well as field examples in nitrogen foam flood
process. He et al., (2010) briefly introduced profile modification of nitrogen foam flood to control the fingering and improved
oil recovery in heterogeneous multi layer sandstone reservoir pilot. Static foam test employed to measure the impact of
temperature, salinity and oil saturation on nitrogen foam. The mixture of an unknown surfactant and foam stabilizer was used
to create nitrogen foam suitable for temperature of 50 C and salinity of less than 10000 ppm. Also, pilot test showed the
increasing the reservoir pressures respond quickly.
Li et al., (2010) presented the results of a systematic experimental investigation of foam injection as a substitute for polymer
drive in ASP flooding process. They show that the apparent viscosity of surfactant alternating gas (SAG) is a function of
pressure gradient, permeability and gas fraction. The results also demonstrated that was the dispersed by the foam as oil in
water emulsion. Liu et al., (2008) investigated the reservoir suitability of nitrogen foam such as permeability, heterogeneity;
formation rhythm and oil viscosity for the Shengli oilfield reservoir conditions. They also showed that the effect of operating
parameters including gas liquid injection ratio, injection rate and foam injection time. The experimental results demonstrated
that nitrogen foam has excellent blocking ability and nitrogen foam was more effective in heterogeneous core than in
homogeneous ones. The oil viscosity has adverse effect on the efficiency of nitrogen foam flooding process. Their results
also showed that operational conditions has an important role in the effectiveness of the nitrogen foam flooding.
As it also mentioned in other foam agent section, Guo et al., (2011) reported results of a novel alkaline surfactant foam
process. Lau (2010) showed the successfully application of Na2CO3 in case of steam foam. Kang et al., (2010) carried out the
novel experimental work to design an ultra low interfacial tension and more stable foam agent without alkali and with no
reaction with divalent cations. These studies clearly demonstrated the chemical compatibility of different chemicals with
nitrogen foam, which is more tolerated than CO2 foam. In nitrogen foam different chemicals and additives can be easily
applied to improve foam stability as well as reduction of surfactant adsorption onto the rock surfaces. Also, nitrogen can
produce more stable foam than CO2 (Farzaneh and Sohrabi, 2010). On the other hand, the favorable interaction between CO2
and crude oil (e.g., swelling and viscosity reduction) as well as its higher tolerance to oil are amongst advantages of CO2
foam over nitrogen foam. This has resulted in more research in the area of CO2 foam compared to nitrogen foam.
Nevertheless, research is still ongoing for finding new chemicals or additives which will be compatible with CO2 and be able
to improve stability of CO2 foam.
Foam in Fractured Porous Media
Haugen et al., (2010) reported laboratory experiments using foam to reduce fracture transmissibility and improve the matrix
sweep efficiency in highly fractured, low permeable, and oil wet limestone core and physical model. They used two different
AOS and one internal olefin sulfonate (IOS) and found the optimum surfactant type and concentration by means of bottle
tests. They tried both in situ foam generation by co-injection of surfactant and nitrogen into the fracture and pre-formed foam.
Both water and gas injection yielded low oil recovery with no significant pressure increase observed across the fractures. In
situ foam generation was not observed in the fracture with the smooth wall surfaces and gas mobility was not decreased and
no additional recovery reported. But they reported high oil recovery observed during injection of pre-formed foam for high
injected pore volumes. Other observations were the sensitivity of foam to oil, wettability of the system and matrix/fracture
permeability ratio (most oil recovery obtained at lowest matrix/fracture permeability ratio).
Zuta et al., (2010) carried out a sensitivity analysis to investigate the effect of different injections schemes on oil recovery
efficiency during tertiary pre-formed CO2 foam flooding in both horizontally and vertically fractured core. Their results
showed that the tertiary CO2 foam can improve oil recovery efficiency compared to CO2 injection alone and WAG injection
in non-fractured homogeneous models. Also, CO2 foam flooding was highly effective in fractured model and foam blocked
the fractures. Fjelde et al., (2011) experimentally investigated the co injection of CO2 and different branched ethoxylated
sulphonates in chalk. They showed the loss of surfactant were a function of oil saturation and surfactant type.
Foam Flooding Application in Heavy Oil Reservoirs
Chen et al., (2010) carried out simulation study on improvement of mobility control in steam assisted gravity drainage
(SAGD) by foam assisted SAGD. The results showed that strong steam foam was generated and accumulated in the interwell
region resulted in control steam breakthrough in FA-SAGD. Also, the presence of steam foam in the upper portion of the
steam chamber reduced gas mobility, which resulted in a reduction in the oil production rate. Still, FA-SAGD provided better
overall performance than SAGD and energy efficient.
SPE 164917
Pei et al., (2010) presented the results of laboratory study of polymer enhanced foam flooding with low gas/liquid ratio for
heavy oil in Shengli oilfield. The chemical formula of ASP optimized for good foam forming ability and emulsifying
capacity and can lower IFT of heavy oil and brine. Some core flooding experiments carried out to observe the performance of
foam flood versus ASP flood. Tertiary oil recovery of foam flooding increased with gas/liquid ratio and slug size. Foam
flooding with low gas/liquid ratio can control the earlier breakthrough and has excellent displacement performance. Also, the
ASP flood had lower tertiary oil recovery compared to flooding.
Emadi et al., (2011) documented the results of a series of visualization experiments carried out in high-pressure transparent
micromodels to systematically investigate the performance of subcritical CO2 and CO2-foam injection in heavy oil. CO2 foam
can improve sweep efficiency through displacement of CO2-diluted oil by foam. Their results showed that in addition to
increasing sweep efficiency, there are also microscopic mechanisms taking place during the displacement of oil by CO2-foam
that can significantly impact the performance of foam injection in heavy oil reservoirs. Furthermore, injection a slug of
surfactant solution prior to foam flood was not enough on its own to improve heavy oil recovery; however, it significantly
sped up the process of forming of the strong foam. Direct displacement of heavy oil by foam at the early times of injection
was observed to be much more effective than the double drainage displacement process during CO2 flood. At the later times,
beyond the CO2 foam breakthrough, the upswept oil was displaced by co-current film flow and oil emulsification. Moreover,
a new counter-current film flow mechanisms was recognized which improved the displacement of residual trapped oil inside
dead end pore at slower rate in comparison with interconnected pores. Their study suggested that CO2 injection in heavy oil
reservoirs can be used for combining EOR and CO2 storage by capturing CO2 from nearby industrial sources.
In previous works of Emadi et al., (2010) reported a series of micro-scale visualisation experiments using CO2 for immiscible
displacement and recovery of an extra-heavy crude oil. The results of those experiments showed that, despite a very large
viscosity difference between CO2 and the heavy oil, injection of CO2 for an extended period of time effectively produced the
heavy crude oil due to CO2 dissolution and subsequent viscosity reduction. Also, Emadi et al., (2012) reported a new series of
visualisation experiments carried out in high-pressure micromodels to study the performance of subcritical CO2 and N2 foam
injection in the same crude oil. The results demonstrated that if strong CO2-foam forms in the porous medium, it can
dramatically improve the recovery of extra-heavy oil and accelerate the process of oil recovery by CO2 injection. But the
results of using N2-foam instead of CO2 foam showed that despite forming stronger foam in the micromodel, the oil
displacement process is much less efficient during N2-foam injection compared to the case of CO2-foam.
Nanoparticle Based Foams
The tendency of nanoparticles to migrate to liquid-liquid interfaces has been exploited in the chemical engineering industry
as a means of separating a mixture of extremely fine solids. In general, small, hydrophobic particles can be drawn to the
interface or coat the surface of small droplets of the hydrocarbon liquid, which may accumulate at the interface or settle to the
bottom of the aqueous phase depending on the density of the particle-coated droplet (Enick and Olsen, 2012).
Espinosa et al., (2010) reported very stable supercritical (SC) CO2 in water foam with silica nano particles foam with
simultaneously injection of water and aqueous dispersion of water and nano particles. The foam stability and the normalized
mixture viscosity of nano foam were measured for a range of nanoparticle concentration, water salinity, gas fraction, and
overall flow rate and temperature. The results showed that the successful application of nano particle in foam stability for
mobility control at relatively high temperature (95 C) and 0.05 wt%. The mobility reduction factor of nano foam was
eighteen times higher than that achieved by co-injection of water and SC CO2.
Emulsion remains stable for month with surface coated silica nanoparticles, also, stable foam generated by co injecting SC
CO2 and silica nanoparticle through glass bead and real rock (Zhang et al., 2011). They showed that the produced foam had
10 to 100 times higher viscosity that initial conditions (without nanoparticles). The threshold value of the critical shear rate
and silica nanoparticle concentration was identified by this study. They found that with proper coating retention was below
10% even in low permeability (around 10 mD) core plug.
Yu et al. (2012) performed experimental work on SC CO2 foam by co-injecting CO2 and silica nanoparticles in glass bead
column which was monitor in line with sapphire tube. The results showed that the foam generated at 1500 psi and 25 C at
shear rate higher than 1419 s-1. The foam stability increased as concentration of silica nanoparticle increased. The apparent
viscosity and the mobility reduction factor were 1.5 to 2.5 and nine times higher than CO2-water co injection respectively.
Although there have promising preliminary laboratory scale studies, this technology is still suffers from requirement of high
shear rate for generating foam, high amount of retention of nanoparticles in the porous media and weak tolerance to the high
salinity conditions.
SPE 164917
Patent Survey
Design of low cost chemical slug for enhanced oil recovery has been concerned during last decades of researches. Stable
foam formed by mixing micellar and air/nitrogen. The micellar contained hydrocarbon, surfactant and water. Roszelle (1971)
discussed foam could generate in-situ or on the surface as well.
Surfactant screening was a subject of some patents for finding the potential of different surfactants in desired chemical
processes. Dilgren (1986) categorized some surfactants to improving the oil recovery by steam injection through a process of
forming foam into the reservoir. He gave further details about an improved procedure for determining which surfactant
material is most effective for a particular reservoir. Borchardt (1989) explained an improved process for recovering oil by
injecting a mixture of steam and steam foam surfactant into an oil formation in both steam drive and steam soak operations.
He mentioned that this improvement was provided by using a rapidly foaming steam foam surfactant, either alone or in
combination with a second steam foam surfactant added to an aqueous solution of electrolyte, and optionally also a noncondensable gas in a multi-state surfactant injection process.
Schramm (1991) explained injecting surfactant-based foam having oil imbibing and transporting properties resulted in
enhancing the production of the oil. The effectiveness of enhanced oil recovery methods has been addressed by introducing a
safe form of carbon disulfide comprising one or more salts of tri- and tetrathiocarbonic acid. This was explained in detail in
Kissels patent (1991).
A stable monophasic liquid composition was explained in detail by Keys (1999) which possesses the ability to stabilize
increased amounts of surfactant while retaining the monophasic state. In his patent, Keys clearly stated that another aspect
of this invention is the method of increasing the amount of cationic, anionic, amphoteric, or non-ionic surfactant or a mixture
of two or more of them that can be solubilized in water especially surfactants which exhibit low solubility in water.
Foam Field Application for Improved Mobility Control
Foam can be applied to solve a thief zone or an override problem encountered in an EOR project. The definition of the
problem includes the identification of the offending wells. Also, categorizing the kind of foam to be applied near well bore
blocking/diverting foams or in-depth mobility control foam - is important. Three categories of EOR foams were used in the
field: pre-formed foam, co-injection foam and surfactant alternating gas injection (SAG) foam. Laboratory tests on long
distance foam propagation showed that the main difference between pre-formed foam and other categories of foam is the
behavior of foam in the first sections of the porous medium. The pre-formed foam develops higher resistance factors; it may
even totally block the porous medium. SAG foam has an overall smaller resistance factor than those of other two foams, and
it cannot completely block the porous medium. In this section, a review of mobility control foams in field tests is provided
based on prior reviews (Smith, 1988; Turta and Singhal, 1998; Hanssen et al., 1994).
Steam Foam Field Projects
19 field projects have been produced oil by low pressure foam into high permeability, shallow formations, to solve an
override problem which majorities of them were in the Midway Sunset and Kern River fields in California (Patzec and
Koinis, 1990; Kovscek and Radke, 1994; Friedmann et al., 1994; Ploeg and Duerksen, 1985; Djabbarah et al., 1990). The
most commonly in used surfactants were Chaser without any pre flush slug. The reports of these fields results showed a very
slow foam propagation (it had been confirmed by Kovscek and Radke, 1994). The most successful application was when the
steam quality was in the range 45%-80% and the additives (surfactant and non-condensable gas) were injected in an
intermittent mode, in conjunction with a continuous steam injection. In the most successful steam-foam drive projects short
injection cycles (7 days) were employed.
Miscible Gas Foam Field Projects
The application of foam in gas miscible floods involves a high pressure application in low permeability formations, and it is
associated with high values of mobility reduction factor (foam quality was around 80%). Out of 12 projects, six were located
in the Texas and CD-128 and CD-1045 at the concentration of 0.2% to 0.5% was used as surfactants respectively (Hoefner et
al., 1994). Also, the pre-flush of surfactant was popular before the injection of CO2 foam flood. The most serious problem
was the excessive injectivity reduction. For this reason, the SAG injection has been favoured over co-injection. For the SAG
implementations, both in CO2 and hydrocarbon miscible flooding, the smaller the duration of the injection cycle, the better
the results. For CO2 miscible flooding, the most successful foam test was conducted in a small pattern containing very
heterogeneous sand. The surfactant had a good selective mobility reduction and residual mobility reduction factor, and very
fast SAG was implemented. For the offending well, the GOR decreased by a factor of 9, while oil production increased by 15
times (Martin et al., 1992; Tsau and Heller, 1994; Stevens and Martin, 1994; Harpole and Gerard, 1994). There were only
three reported cases of foam application in hydrocarbon miscible floods. More knowledge is required on foam interaction
with the recovery process, before the technique can be reliably applied in the field (Liu and Besserer, 1988).
10
SPE 164917
Different Injection Scenarios in Foam Field Projects
Three factors should be considered for employing a foam injection scheme. These factors are: reservoir pressure,
permeability and surfactant injection extent. For low pressure and high permeability, the co-injection foam is effective at
normal surfactant concentrations, and it can be considered for long term injection. For high pressure and low permeability,
SAG at medium or even low surfactant concentrations can be considered.
Blaker et al. (1999), Aarra and Skauge (2000), Skauge et al. (2002), and Blaker et al. (2002) presented details about the
worlds largest application of foam in the oil industry: Foam assisted WAG (FAWAG) project in Snorre Field. The in depth
mobility control foams were employed successfully and profitably to recover substantial amounts of oil at Snorre Field in the
North Sea. Two foam pilot projects have been conducted in this field. One project was to reduce the GOR at a production
well and the second project was to control the gas mobility in depth of the reservoir by FAWAG. Both of the projects were
successful to improve the oil recovery. For the FAWAG project, the field application started after two years of planning and
many years of active research (Blaker et al., 1999). The important lesson from this project is the good understanding for
behavior of foam at the target reservoir conditions led to good planning and successful field application.
Relationship between Laboratory experiments and Fields
Precise laboratory experiments should be designed and performed, depending on the circumstances, category of foam and the
injection mode chosen. After choosing the best foam and injection scheme, an investigation of the pressure on foam behavior
should be undertaken, with the intent to reduce the surfactant concentration. Dalland and Hanssen (2002) supposed that,
before work on foaming-agent selection is started the process of which it is going to be a part of should be carefully defined.
The choice of critical parameters to be included in each stage of the selection is dependent on the type of process. The
experimental mode used should reflect the field injection procedure and the desired behaviour of the foam. The presence of
oil is critical for all production-well treatments and for some injection-well treatments. The length of the core is important
when gas-blocking mode is applied. When all this is defined, the foaming agent selection work can start. The selection
procedure should consist of three stages, and a fourth if a successful foamer is selected. In the stages the following should be
included. First stage is pre-screening which consist of brine compatibility, bulk temperature stability, bulk crude oil
compatibility, environmental acceptability and price and availability. Second stage is screening which consist of reservoir
permeability, length, stock tank oil and pressure drop in realistic range. The third stage is qualification that categorized
reservoir rock, reservoir pressure and live oil. The last stage is optimization which including concentration
variation/optimization, adsorption and treatment design parameters.
The 1990 mobility control trial at Joffre Viking (Stephenson et al., 1993) was unsuccessful because the foam propagated only
a few feet from the injector. Also, the 1984 Rock Creek trial (Heller et al., 1985) was somewhat inconclusive since reservoir
fluids and the in situ generated foam did not flow past the observation well as anticipated, although pressure measurements
indicated that a 60% increase in the apparent viscosity of CO2 was attained in regions where the foam formed.
From review of forty two foam applications, Turta and Singhal (1997) found that the most serious problem is the excessive
reduction of injectivity. Kuehne et al., (1990) presented a study for a trial of applying of nitrogen foam injection to reduce the
gas channeling at a dual injector/producer. The trial was unsuccessful, the wrong estimation of injectivity, resulted in
fracturing the formation and opening new gas channels as well as incorrect assessment on required foam for injection.
Conclusions
Many parameters affecting the characteristics of foam have been investigated over many years of research on this
subject. These parameters include rock permeability, injection rate, pressure, temperature, brine salinity and crude oil.
The adsorption of surfactant into the rock surface is one of the most unwanted problems in foam based EOR processes
which is destabilize the foam.
The surfactant has an important role in generation and stability of the foam in porous media. AOS surfactants have been
successful in generating stable foam. CO2 soluble surfactants have also been considered for forming stable foam.
Better Chemical compatibility and availability of nitrogen foam over CO2 foam, led researchers to employ new chemical
and/or mixture of chemicals for improving foam stability and reduction of surfactant adsorption. However, due to
favorable interaction between CO2 and reservoir fluids as well as better tolerance of CO2 foam to oil, CO2 foam has
attracted more attention over nitrogen foam. Nevertheless, finding new chemicals and additives compatible with CO2 and
capable of improving CO2 foam stability is still an active area of research.
Nanoparticle stabilized foams may have potentials for generating mobility control foams and as an alternative to the use
of surfactants in high temperature reservoirs. Although there have been some promising preliminary laboratory scale
studies, this technology is still suffers from requirement of high shear rate for generating foam, high amount of retention
of nanoparticles in the porous media and weak tolerance to the high salinity conditions.
SPE 164917
11
It is difficult to achieve the long term foam stability during a foam field application due to unstable nature of foam and
its destabilizing against the oil. Therefore, the potential of foam mobility control processes may have been
overestimated, particularly in lab scale studies.
Several literature reviews of field applications of foam injection highlighted the excessive reduction of injectivity as
the most serious problem. Wrong estimation of required foam was another reason for unsuccessful field trials.
References
Aarra, M.G., and Skuge, A., 2000. Status of Foam Applications in North Sea Reservoirs. 21st Annual International Energy Agency
Workshop and Symposium, Edinburgh, Scotland, UK.
Adkins, S.S., Chen, X., Chan, I., Torino, E., Nguyen, Q., and Sanders, A., 2010. Morphology and Stability of CO2-in-Water Foams with
Nonionic Hydrocarbon Surfactants, Langmuir, Vol 26, No 8, 5335-5348.
Alkan, H., Goktekin, A., and Satman, A., 1991. A Laboratory Study of CO2-foam Process for Bati Raman Field, Turkey. SPE Middle East
Oil Show, Kingdom of Bahrain.
Alvarez, J.M., Rivas, H.J. and Rossen, W.R., 1999. Unified Model for Steady-State Foam Behavior at High and Low Foam Qualities.
Annual Technical Conference and Exhibition of Society of Petroleum Engineers, Houston, Texas, USA.
Asghari, K., and Khalil, F., 2005. Effect of Operational Parameters on CO2-Foam Process, Petroleum Science and Technology, Vol 23,
189-198.
Apaydin, O.G., and Kovscek, A.R., 2000. Transient Foam Flow in Homogeneous Porous Media: Surfactant Concentration and Capillary
End Effects. SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Bae, J.H., and Petrick, C.B., 1977. Adsorption/Retention of Petroleum Sulfonates in Berea Cores, SPE Journal, 353-357.
Bai, B., Grigg, R.B., Liu, Y., and Zeng, Z., 2005. Adsorption Kinetics of Surfactant Used in CO2-Foam Flooding onto Berea Sandstone.
Annual Technical Conference and Exhibition, Dallas, Texas, USA.
Bai, B., Grigg, R.B., Svec, Y., and Wu, Y., 2010. Adsorption of a Foam Agent on porous Sandstone and its Effect on Foam Stability,
Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol 353, 189-196.
Bergeron, V., Fagan, M.E., and Radke, C.J., 1993. Generalized Entering Coefficients: A Criterion for Foam Stability against Oil in Porous
Media, Langmuir, Vol 9, No 7, 1704.
Bernard, G., and Holm, L., 1967. Method for Recovering Oil from Subterranean Formations, U.S. Patent 3,342,256, issued September 19.
Bertin, H.J.; Apaydin, O.G., Castanier, L.M., and Kovscek, A.R., 1998. Foam Flow in Heterogeneous Porous Media: Effect of Crossflow.
SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Bertin, H.J., Apaydin, O.G., Castanier, L.M., and Kovscek, A.R., 1999. Foam Flow in Heterogeneous Porous Media: Effect of Cross Flow,
SPE Journal, Vol 4, No 2, 75-82.
Blaker, T., Aarra, M.G., Skauge, A., Rasmussen, L., Celius, H.K., Martinsen, H.A., and Vassenden, F., 2002. Foam for Gas Mobility
Control in the Snorre Field: The FAWAG Project, Journal of Reservoir Evaluation & Engineering of Society of Petroleum
Engineers, Vol 5, No 4, 317-323.
Blaker, T., Celius, H.K., Lie, T., Martinsen, H.A., Rasmussen, L., and Vassenden, F., 1999. Foam for Gas Mobility Control in the Snorre
Field: The FAWAG Project. Annual Technical Conference and Exhibition of Society of Petroleum Engineers, Houston, Texas,
USA.
Blauer, R.E., and Kohlhaas, C.A., 1974. Formation Fracturing with Foam. Fall Meeting of the Society of Petroleum Engineers of AIME,
Houston, Texas, USA.
Borchardt, J.K., 1989. US4852653.
Borchardt, J.K, 1988. Structure-Property Relationships for Mobility-Control Surfactants, Ch. 9 of Surfactant-Based Mobility ControlProgress in Miscible Flood Enhanced Oil Recovery, edited by Duane Smith, ACS Symposium Series 373, ACS, Washington DC,
181-204.
Borchardt, J.K., Bright, D.B., Dickson, M.K., and Wellington, S.L., 1985. Surfactants for CO2 Foam Flooding. 60th SPE Annual Technical
Conference and Exhibition, Las Vegas, Nevada, USA.
Casteel, J.F., and Djabbarah, N.F., 1988. Sweep Improvement in CO2 Flooding by Use of Foaming Agents, Journal of Reservoir
Engineering of Society of Petroleum Engineers, Vol 3, No 4, 1186-1192.
Chang, Shih-Hsien, and Grigg, Reid B., 1998. Effects of Foam Quality and flow Rate on CO2-Foam Behavior at Reservoir Conditions.
SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Chang, S.H., and Grigg, R.B., 1999. Effects of Foam Quality and Flow Rate on CO2-Foam Behavior at Reservoir Temperature and
Pressure, Reservoir Evaluation and Engineering Journal, Vol 2, No 3, 248-254.
Chang, Shih-Hsien, Martin, F.D., and Grigg, R.B., 1994. Effect of Pressure on CO2 Foam Displacements: A Micromodel Visualization
Study. SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Chen, H.,L., Ke, M.,J., Chuang, T.,K., Flumerfelt R.,W., 1990. Experimental Studies of Capillary Pressure Effects of Foams in Porous
Media. SPE California Regional Meeting, Ventura, California, USA.
Chen, Q., Gerritsen, M.G., and Kovscek, A.R., 2010. Improving Steam-Assisted Gravity Drainage Using Mobility Control Foams: Foam
Assisted-SAGD (FA-SAGD). SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Chen, M., Yortsos, Y.C., and Rossen, W.R., 2004. A Pore-Network Study of the Mechanisms of Foam Generation. SPE Annual Technical
Conference and Exhibition, Houston, Texas, USA.
Chou, S.I., 1991. Conditions for Generating Foam in Porous Media. 66th SPE Annual Technical Conference and Exhibition, Dallas, Texas,
USA.
Dalland, M., and Hanssen J.E., 2002. Foam Application in North Sea reservoirs, II: Efficient Selection of Products for Field Use. SPE/DOE
10th Symposium on Improved Oil Recovery, Tulsa Oklahoma, USA.
Dellinger, S.E., Patton, J.T., and Holbrook, S.T., 1984. CO2 Mobility Control, SPE Journal, Vol 24, No 2, 191-196.
De Vries, A., Wit, K., 1990. Rheology of Gas/Water Foam in the Quality Range Relevant to Steam Foam, SPE Reservoir Engineering
12
SPE 164917
Journal, Vol 5, No 2.
Di-Julio, S.S., and Emanuel, A.S., 1989. Laboratory Study of Foaming Surfactant for CO2 Mobility Control, SPE Reservoir Engineering,
136-142.
Dilgren, R.E., 1986. US4601336.
Djabbarah, N.F., Weber, S.L, Freeman, D.C., Muscateloo, J.A., and Covington, T.E., 1990. Laboratory Design and Field Demonstration of
Steam Diversion with Foam. The 60th California Regional Meeting, Ventura, California, USA.
Emadi, A., Sohrabi, M., Jamiolahmady, M., Ireland, S., and Robertson, G., 2011. Mechanistic Study of Improved Heavy Oil Recovery by
CO2-Foam Injection, SPE Enhanced Oil Recovery Conference, Kuala lumpur, Malaysia.
Emadi, A., Sohrabi, M., Jamiolahmady, M., Ireland, S. and Robertson, G., 2011. Visual Investigation of Extra Heavy Oil Recovery by
CO2/N2 Foam Injection. EAGE 16th European Symposium on Improved Oil Recovery, Cambridge, UK.
Emadi, A., Sohrabi, M., Jamiolahmady, M., and Ireland, S., 2012. Visualization of Oil Recovery by CO2-Foam Injection; Effect of Oil
Viscosity and Gas Type. SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Enick R.,M., and Olsen, D.,K., 2012. Mobility and Conformance Control for Carbon Dioxide Enhanced Oil Recovery (CO2-EOR) via
Thickeners, Foams, and Gels A Detailed Literature Review of 40 Years of Research. National Energy Technology Laboratory,
Espinosa, D., Caldesas, F., Johnston, K., Bryant, S.L., and Huh, C., 2010. Nanoparticle-Stabilized Supercritical CO2, Foams for Potential
Mobility Control Applications. SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma. USA.
Farzaneh, S.A., and Sohrabi, M., 2010. Report on Enhanced Heavy Oil Recovery. Technical report, Heriot-Watt University, Edinburgh,
Scotland, UK (unpublished).
Fjelde, I., Zuta, J., and Berenblyum R., 2011. Retention of CO2-Foaming Agent in CO2-Foam Flooding of Heterogeneous Carbonate
Reservoirs. Brasil Offshore, Maca, Brazil.
Friedmann, F., and Jensen, J.A., 1986. Some Parameters Influencing the Formation and Propagation of Foams in Porous Media. California
Regional Meeting of Society of Petroleum Engineers. Oakland, California, USA.
Friedmann, F.F., Smith, M.E., Guice, W.R., Gump, J.M., and Nelson, D.G., 1994. Steam-Foam Mechanistic Field Trial in the MidwaySunset Field, SPE Reservoir Engineering.
Grigg, R.B., and Mikhalin, A.A., 2007. Effects of Flow Conditions and Surfactant Availability on Adsorption. International Symposium on
Oilfield Chemistry of Society of Petroleum Engineers, Houston, Texas, USA.
Guo, H., Faber, R., Buijse, M., and Zitha, P.L.J., 2011. A Novel Alkaline-Surfactant-Foam EOR Process. SPE Enhanced Oil Recovery
Conference, Kuala Lumpur, Malaysia.
Handy, L.L., Amaefule, J.O., Ziegler, V.M., and Ershaghi, I., 1982. Thermal Stability of Surfactants for Reservoir Application. SPE
Journal, Vol 22, No 5, 722-730.
Hanssen, J.E., and Dalland, M., 1990. Foams for Effective Gas Blockage in the Presence of Crude Oil, SPE/DOE Symposium on Enhanced
Oil Recovery, Tulsa, Oklahoma, USA.
Hanssen, J.E., Holt, T., and Surguchev, L.M., 1994. Foam Processes: An Assessment of Their Potential in North Sea Reservoirs Based on a
Critical Evaluation of Current Field Experience. SPE/DOE 9th Symposium on Improved Oil Recovery, Tulsa, Oklahoma, USA.
Harpole, K.J., and Gerard, M.G., 1994. CO2-Foam Field Verification Pilot Test at EVGSAU: Phase lllC Reservoir Characterization and
Response to Foam Injection. SPE/DOE 9th Symposium on Improved Oil Recovery, Tulsa, Oklahoma, USA.
Haugen, A., Ferno, M.A., Graue, A., and Bertin, H.J., 2010. Experimental Study of Foam Flow in Fractured Oil-Wet Limestone for
Enhanced Oil Recovery. SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
He, L., Peng, Y., Wei, L., Li, S., and Yan Z., 2010. Application of Nitrogen Foam for Profile Modification in a Heterogeneous Multi-layer
Sandstone Oilfield. SPE Asia Pacific Oil & Gas Conference and Exhibition, Brisbane, Queensland, Australia.
Heller, J.P., Boone, D.A.; Watts, R.J., 1985. Field Test of CO2 Mobility Control at Rock Creek. 60th SPE Annual Technical Conference and
Exhibition, Las Vegas, Nevada, USA.
Heller, J.P, CO2 Foams in EOR, Chapter 5 in Foams: Fundamentals and Applications in the Petroleum Industry, American Chemical
Society, L.L. Schramm (ed.), Washington DC, 1994.
Hirasaki, G.J., 1989. The Steam-Foam Process--Review of Steam-Foam Process Mechanisms. Supplement to paper 19505.
Hoefner, M.L., Evans, E.M., Buckles, J.J., and Jones, T.A., 1994. CO2 Foam: Field Trials. SPE/DOE 9th Symposium on Improved Oil
Recovery, Tulsa, Oklahoma, USA.
Holm, L.W., and Garrison, W.H., 1988. CO2 Diversion with Foam in an Immiscible CO2 Field Project, SPE Reservoir Engineering, 112118.
Hudgins, D.A., Chung, T.,H., 1990. Long-Distance Propagation of Foams. SPE/DOE Enhanced Oil Recovery Symposium, Tulsa,
Oklahoma, USA.
Kang, W., Liu, S., Meng, L., Cao, D., and Fan H., 2010. A Novel Ultra-low Interfacial Tension Foam Flooding Agent to Enhance Heavy
Oil Recovery. SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Keys, R.O., 1999. US5977189.
Kissel, C.L., 1991. US5076358.
Khalil, F., and Asghari, K., 2006. Application of CO2-Foam as a Means of Reducing Carbon Dioxide Mobility, JCPT, Vol 45, No 5, 37-42.
Kovscek, A.R., and Radke, C.J., 1994. Fundamentals of Foam Transport in Porous Media, in Foams: Fundamentals and Applications in
the Petroleum Industry, Schramm, L.L., editor. American Chemical Society, Washington, D.C., USA.
kristiansen, T.S., and Holt, T., 1992. Properties of Flowing Foam in Porous Media Containing Oil. SPE/DOE Symposium on Enhanced Oil
Recovery, Tulsa, Oklahoma, USA.
Kuehne, D.L., Ehman, D.I., EmanueL, A.S., and Magnanl, Ch.F., 1990. Design and Evaluation of a Nitrogen-Foam Field Trial, Journal of
Petroleum Technology, Vol 42, No 4, 504-512.
Kuehne, D.L., Frazier, R.H., Cantor, J., Horn, Jr., W., 1992. Evaluation of Surfactants for CO2 Mobility Control in Dolomite Reservoirs.
SPE/DOE Eighth Symposium on Enhanced Oil Recovery, Tulsa, Oklahoma, USA.
Kuhlman, M.I., Lau, H.C., and Falls, A.H., 2000. Surfactant Criteria for Successful Carbon Dioxide Foam in Sandstone Reservoirs, SPE
Reservoir Evaluation and Engineering, Vol 3, No 1, 35-41.
SPE 164917
13
Kuhlman, M.I., 1988. Visualizing the Effect of Light Oil on CO2 Foams, SPE/DOE Enhanced Oil Recovery Symposium, Tulsa, Oklahoma,
USA.
Kuhlman, M.I., 1990. Visualizing the Effect of Light Oil on CO2 Foams, JPT, 902-908.
Lau, H.C., and Obrien, S.M., 1988. Effects of Spreading and Non spreading Oils on Foam Propagation through Porous Media, SPERE,
893.
Lee, H., and Heller, J.P, 1988. Carbon Dioxide-Foam Mobility Measurements at High Pressure. Ch. 19 of Surfactant-Based Mobility
Control-Progress in Miscible Flood Enhanced Oil Recovery, edited by Duane Smith, ACS Symposium Series 373, ACS,
Washington DC, 375-386.
Lee, H.O., and Heller, J.P., 1990. Laboratory Measurements of CO2-Foam Mobility, SPE Reservoir Engineering, 193-197.
Lee, H.O., Heller, J.P., and Hoefer, A.M.W., 1991. Change in Apparent Viscosity of CO2-Foam with Rock Permeability, SPE Reservoir
Engineering, 421-428.
li, B., Hirasaki, G.J., and Miller, C.A., 2006. Upscaling of Foam Mobility Control to Three Dimensions. SPE Symposium on Improved Oil
Recovery, Tulsa, Oklahoma, USA.
Li, R.F., Yan, W., Liu, S., Hirasaki, G.J., and Miller, A., 2010. Foam Mobility Control for Surfactant Enhanced Oil Recovery, SPE
Journal, Vol 15, No 4, 928-942.
Li, R.,F., Hirasaki, G.,J., Miller, C.,A., and Masalmeh S., K., 2012. Wettability Alteration and Foam Mobility Control in a Layered, 2D
Heterogeneous Sandpack, SPE Journal, Vol 17, No 4.
Liu, P.C., and Besserer, G.J., 1988. Application of Foam Injection in Triassic Pool, Canada: Laboratory and Field Tests Results. SPE 63rd
Annual Technical Conference, Houston, Texas, USA.
Liu, R., Liu, H., Li, X., and Fan, Z., 2008. The Reservoir Suitability Studies of Nitrogen Foam Flooding in Shengli Oilfield. SPE Asia
Pacific Oil and Gas Conference and Exhibition, Perth, Australia.
Liu, Y., Grigg, R.B., and Bai, B., 2005. Salinity, pH , and Surfactant Concentration Effects on CO2-Foam. SPE International Symposium
on Oilfield Chemistry, Woodlands, Texas, USA.
Liu, Y., Grigg, R.B., and Svec, R.K., 2005. CO2 Foam Behavior: Influence of Temperature, Pressure, and Concentration of Surfactant. SPE
Production Operations Symposium, Oklahoma City, Oklahoma, USA.
Liu, Y., Grigg, R.B., and Svec, R.K., 2006. Foam Mobility and Adsorption in Carbonate Core. SPE/DOE Improved Oil Recovery
Symposium, Tulsa, Oklahoma, USA.
Liu, X.B., Wang, F.S., Zhang, Z.Y., Wang, X., Zhou, W.F., Han, Z.L., Wang, H.J., Zu, D.F., LI, G., and Fu, H.R., 2010 Acid-Resistant
Foamer Used to Control Gas Breakthrough for CO2 Drive Reservoi. SPE Asia Pacific Oil and Gas Conference and Exhibition,
Brisbane, Queensland, Australia.
Lobo, L., and Wasan, D.T., Mechanisms of Aqueous Foam Stability in the Presence of Emulsified Non-Aqueous-Phase Liquids: Structure
and Stability of the Pseudoemulsion Film, LangmuirI, Vol 9, No 7, 1668.
Manlowe, D.J., and Radke, C.J., 1990. A Pore-Level Investigation of Foam/Oil Interactions in Porous Media. SPE Reservoir Engineering
Journal,Vol 5, No 4.
Mannhardt, K., Novosad, J.J., and Schramm, L.L., 2000. Comparative Evaluation of Foam Stability to Oil, SPE Reservoir Evaluation and
Engineering, Vol 3, No1, 23-34.
Martin, F.D., Heller, J.P., Weiss, W.W., Tsau, J.S., Zornes, D.R., Sugg, L.A., and Stevens, J.E., 1992. CO2-Foam Field Verification Pilot
Test at EVGSAU Injection Project Phase l:Project Planning and Initial Results. SPE/DOE Eighth Symposium on Enhanced Oil
Recovery, Tulsa, Oklahoma, USA.
Mohammadi, S.,S., Tenzer, J.,R., Steam-Foam Pilot Project at Dome-Tumbador, Midway Sunset Field: Part 2. SPE/DOE Enhanced Oil
Recovery Symposium, Tulsa, Oklahoma, USA.
Nguyen, Q.P., 2004. Dynamics of Foam in Porous Media. PhD Dissertation, Delft University, Netherlands.
Nikolov, A.D., Wasan, D.T., Huang, D.W., and Edwards, D.A., 1986. The Effect of Oil on Foam Stability: Mechanisms and Implications
for Oil Displacement by Foam in Porous Media. SPE Annual Technical Conference and Exhibition. New Orleans, Louisiana,
USA.
Novosad, J., Maini, B.B., and Huang, A., 1986. Retention of foam-forming surfactants at elevated temperature, Journal of Canadian
Petroleum Technology, 42-46.
Patzec, T.W., and Koinis, M.T., 1990. Kern River Steam-Foam Pilots. JPT.
Pei, H., Zhang, G., Ge, J., Wang, J., Ding, B., and Liu, X., 2010. Investigation of Polymer-Enhanced Foam Flooding with Low Gas/Liquid
Ratio for Improving Heavy Oil Recovery. Canadian Unconventional Resources and International Petroleum Conference,
Calgary, Alberta, Canada.
Ploeg, J.F., and Duerksen, J.H., 1985. Two successful Steam/Foam Field Tests, Sections 15A and 26C, Midway-Sunset Field. California
Regional Meeting, Bakersfield, California, USA.
Prieditis, J., and Paulett, G.S., 1992. CO2-Foam Mobility Tests at Reservoir Conditions in San Andres Cores. SPE/DOE Eighth Symposium
on Enhanced Oil Recovery, Tulsa, Oklahoma, USA.
Raterman, K.T. 1989. An Investigation of Oil Destabilization of Nitrogen Foams in Porous Media, SPE Annual Technical Conference and
Exhibition, San Antonio, Texas, USA.
Robie, JR, D.R., Roedell, J.W., Wackowski, R.K., 1995. Field Trial of Simultaneous Injection of CO2 and Water, Rangely Weber Sand
Unit, Colorado. SPE Production Operations Symposium. Oklahoma City, Oklahoma, USA.
Rossen, W.R., Zhou, Z.H., and Mamun, C.K., 1995. Modeling Foam Mobility in Porous Media. SPE Advanced Technology Series Journal,
Vol 3, No 1.
Roszelle, W.O., 1971. US3599715.
Schramm, L.L., Ayasse, C., Mannhardt, K., and Novosad, J.J., 1991. US5060727.
Schramm, L.L., and Novosad, J.J., 1990. Microvisualization of Foam Interactions With a Crude Oil, Colloids Surf., Vol 46, No 21.
Schramm, L.L., and Novosad, J.J., 1992. The Destabilization of Foams for Improved Oil Recovery by Crude Oils: Effect of the Nature of
the Oil, J. Pet. Sci. Eng., Vol 7, No 77.
14
SPE 164917
Schramm. L.L., 1994. Foam: Fundamentals and Applications in the Petroleum Industry. Advances in Chemistry, Series 242.
Schramm, L.L., 1994. Foam Sensitivity to Crude Oil in Porous Media in Foams, Fundamentals and Applications in the Petroleum Industry,
Schramm L.L., ed., Advances in Chemistry, Series 242, American Chemical Society, Washington, DC, 165-197.
Schramm, L.L., Turta, A.T., and Novosad, J.J., 1993. Microvisual and Coreflood Studies of Foam Interactions with a Light Crude Oil,
SPERE, 201.
Skauge, A., Aarra, M.G. and Surguchev, L.M. et al. 2002. Foam- Assisted WAG: Experience from the Snorre Field. SPE/DOE Improved
Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Smith, D., 1988. Injectivity and Surfactant-Based Mobility Control: Field Tests, Ch. 22 of Surfactant-Based Mobility Control-Progress in
Miscible Flood Enhanced Oil Recovery, edited by Duane Smith, ACS Symposium Series 373, ACS, Washington DC, 429-438.
Sohrabi, M., Henderson, G.D. and Tehrani, D.H., et al. 2000. Visualisation of Oil Recovery by Water Alternating Gas (WAG) Injection
Using High Pressure Micromodels - Water-Wet System. SPE Annual Technical Conference and Exhibition. Dallas, Texas, USA.
Stephenson, Derril J., Graham, Andrew G., Luhning, Richard, W., 1993. Mobility Control Experience in the Joffre Viking Miscible CO2
Flood. SPE Reservoir Engineering Journal, Vol 8, No 3.
Stevens, J.E., and Martin, F.D., 1994. CO2-Foam Field Verification Pilot Test at EVGSAU: Phase lllB-Project Operations and Performance
Review. SPE/DOE 9th Symposium on Improved Oil Recovery, Tulsa, Oklahoma, USA.
Syahputra, A.E., Tsau, J.S., Grigg, R.B., 2000. Laboratory Evaluation of Using Lignosulfonate and Surfactant Mixture in CO2 Flooding.
SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Torino, E., Reverchon, E., and Johnston, K.P., 2010. Carbon Dioxide/Water, Water/Carbon Dioxide Emulsions and Double Emulsions
Stabilized With a Nonionic Biocompatible Surfactant, J. Colloid Interface Science, Vol. 348, 469-478.
Tsau J.S., and Heller, J.P., 1994. CO2-Foam Field Verification Pilot Test at EVGSAU: Phase lllA-Surfactant Performance Characterization
and Quality Assurance. SPE/DOE 9th Symposium on Improved Oil Recovery, Tulsa, Oklahoma, USA.
Tsau, J.S., and Heller, J.P., 1992. Evaluation of Surfactants for CO2-Foam Mobility Control. Permian Basin Oil and Gas Recovery
Conference, Midland, Texas, USA.
Tsau, J.S., and Heller, J. P., 1996. How Can Selective Mobility Reduction of CO2-Foam Assist in Reservoir Floods. The Permian Basin Oil
and Gas Recovery Conference, Midland, Texas, USA.
Tsau, J.S., Syahputra, A.E., Yaghoobi, H., et al. 1999. Use of Sacrificial Agents in CO2 Foam Flooding Application. SPE Annual
Technical Conference and Exhibition, Houston, Texas, USA.
Turta, A.T., and Singhal, A.K., 1998. Field Foam Applications in Enhanced Oil Recovery Projects: Screening and Design Aspects. Paper
Presented at the International Oil and Gas Conference and Exhibition in China of Society of Petroleum Engineers, Beijing,
China.
Wang, G.C., 1984. A Laboratory Study of CO2 Foam Properties and Displacement Mechanism. SPE Enhanced Oil Recovery Symposium,
Tulsa, Oklahoma, USA.
Wasan, D., Nikolav, A., Huang, D., and Edwards, D., 1988. Foam Stability: Effects of Oil and Film Stratification, Ch. 7 of SurfactantBased Mobility Control Progress in Miscible Flood Enhanced Oil Recovery, edited by Duane Smith, ACS Symposium Series
373, ACS, Washington DC, 136-162.
Xing, D., Wei, B., Trickett, K., Mohamed, A., Eastoe, J., Soong, Y., and Enick R., 2010. CO2-Soluble Surfactants for Improved Mobility
Control. SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA.
Yaghoobi, H., and Heller, J.P., 1994. Laboratory Investigation of Parameters Affecting CO2-Foam Mobility in Sandstone at Reservoir
Conditions. SPE Eastern Regional Meeting, Charleston, West Virginia, USA.
Yaghoobi, H., Tsau, J.S., and Grigg, R.B., 1998. Effect of Foam on CO2 Breakthrough: Is This Favorable to Oil Recovery?. SPE Permian
Basin Oil and Gas Recovery Conference, Midland, Texas, USA.
Yin, G., Grigg, R.B., and Svec, Y., 2009. Oil Recovery and Surfactant Adsorption during CO2-Foam Flooding. Offshore Technology
Conference, Houston, Texas, USA.
Yu, J., An, C., Nig Lio, D., and Lee, R., 2012. Foam Mobility Control for Nanoparticle-Stabilized CO2 Foam. SPE Improved Oil Recovery
Symposium, Tulsa, Oklahoma, USA.
Yu, H., Yang, B., Xu, G., Wang, J., Ren, S.R., Lin, W., Xiao, L., Gao, H., 2008. Air Foam Injection for IOR: from Laboratory to Field
Implementation in Zhongyuan Oilfield China. SPE/DOE Symposium on Improved Oil Recovery, Tulsa, Oklahoma, USA.
Ziegler, Victor M., Handy, and Lyman L., 1981. Effect of Temperature on Surfactant Adsorption in Porous Media, SPE Journal, Vol 21,
No 2, 218-228.
Zhang, T., Espinoda D.,A., Yoon, K.,Y., and Rahmani, A.,R., et al., 2012. Engineered Nanoparticles as Harsh-Condition Emulsion and
Foam. Offshore Technology Conference. Houston, Texas, USA.
Zuta, J., Fjelde, I., and Berenblyum, R. 2010a. Experimental and Simulation of CO2-Foam Flooding in Fractured Chalk Rock at Reservoir
Conditions: Effect of Mode of Injection on Oil Recovery. SPE EOR Conference, Oil & Gas West Asia, Muscat, Oman.
Zuta, J., and Fjelde, I., 2010b. Transport of a CO2-foaming agent during CO2-foam Processes in Fractured Chalk Rock, SPE Reservoir
Evaluation & Engineering, Vol 13, No 4, 710-719.
SPE 164917
15
Table 1: A summary of different surfactant studies adapted from Enick and Olsen (2012)
Surfactant Name(s)
Authors
Surfactant Type
Fluid
Chaser CD 1045
Tsau and Heller, 1992; Heller
1994; Yaghoobi and Heller,
1994; Yaghoobi et al., 1998;
Chang and Grigg, 1999; Bai et
al., 2005; Bai et al., 2010
Dellinger et al., 1984; Casteel
and Djabbara, 1988; Holm and
Garrison, 1988; Hudgins and
Chung, 1990; Alkan et al., 1991
Yaghoobi and Heller, 1994
Amphyterics
CO2-Foam
Anionic
CO2-Foam
Anionic
CO2-Foam
Non-ionic
CO2-Foam
Anionic
CO2-Foam
Anionic
CO2-Foam
Alipal CD 128
Chaser CD 1040
Chaser CD 1050
NES-25, Avanel S30
Shell Enordet X2001
Tsau and Heller, 1992;
Yaghoobi and Heller, 1994
Tsau and Heller, 1992
Lee and Heller, 1990; Lee,
Heller and Hoefer, 1991;
Yaghoobi and Heller, 1994;
Kuhlman et al., 2000
Lee and Heller, 1988; Lee and
Heller, 1990
Casteel and Djabbara, 1988
Anionic
CO2-Foam
Anionic
CO2-Foam
Alkan et al., 1991
Anionic/Non-Ionic
CO2-Foam
TRS 18 and TRS 40 blend
Bae and Petrick, 1977
Anionic
Alkaline-Surfactant
AOS C14-C16 Shell AOS
Bertin and Apaydin, 1999
Anionic
CO2-Foam
AOS
Mohammadi and Tenzer, 1990
Anionic
N2-Foam
CRSO 85/66
Di Julio and Emanuel, 1989
Anionic
CO2-Foam
DOWFAX
Kuhlman et al., 2000
Anionic
CO2-Foam
Triton X 200 octylphenol
ethoxylate, Neodol 25-9, NEGS
25-12
Witcolate 1247H and 1276,
Witconate 3203 and AOS12,
Stepanflo 10, Pluronic F-68
EO-PO copolymer, Dowfax
8390, Dow XSS-84321.05 and
XSS 84321.12, Ethoquad C/12
40 surfactants
Kuhlman 1990
Anionic
CO2-Foam
Prieditis and Paulett, 1992; Lee
and Heller, 1990
Anionic
CO2-Foam
Borchardt, 1985
Anionic
CO2-Foam
5 unknown surfactants
Djabbarah et al., 1990
N/A
N2-Foam
AS-40
Chen et al., 1990
N/A
N2-Foam
Siponate OS 10
De Vries and Wit, 1990
N/A
N2-Foam
AOS 1618, Siponate DS-10,
Neodene 1618, Siponate A 168
ZY 01
Patzek and Kolnis, 1990
N/A
N2-Foam
Yu et al., 2008
N/A
Air-Foam
NEODOL 67-7PO, IOS 15-18,
AOS 16-18, NI, NIB, IB
Li et al., 2012
Anionic
Air-Foam
Sherex Varion CAS
Plurafoam NO-2N, Witcolate
1247-H
S-50, C-10, N-40, O-Sap
Anda mungkin juga menyukai
- SPE 183676 Production Optimization of High Temperature Liquid Hold Up Gas WellDokumen12 halamanSPE 183676 Production Optimization of High Temperature Liquid Hold Up Gas WellEdgar GonzalezBelum ada peringkat
- Confined Fluid Phase Behavior and CO2 Sequestration in Shale ReservoirsDari EverandConfined Fluid Phase Behavior and CO2 Sequestration in Shale ReservoirsBelum ada peringkat
- Advanced Water Injection for Low Permeability Reservoirs: Theory and PracticeDari EverandAdvanced Water Injection for Low Permeability Reservoirs: Theory and PracticePenilaian: 4 dari 5 bintang4/5 (2)
- Fluid Chemistry, Drilling and CompletionDari EverandFluid Chemistry, Drilling and CompletionQiwei WangBelum ada peringkat
- Journal of Petroleum Science and EngineeringDokumen10 halamanJournal of Petroleum Science and EngineeringMohammed Al-shargabiBelum ada peringkat
- 06b Fluid Loss and Diverting AgentsDokumen19 halaman06b Fluid Loss and Diverting AgentsErick Carballo CabreraBelum ada peringkat
- Mechanism of An Asphaltene Inhibitor PDFDokumen50 halamanMechanism of An Asphaltene Inhibitor PDFTEXOPED Parsian KishBelum ada peringkat
- Spe 59537Dokumen14 halamanSpe 59537cmkohBelum ada peringkat
- Phosphonate Scale Inhibitor Adsorption/Desorption and The Potential For Formation Damage in Reconditioned Field CoreDokumen14 halamanPhosphonate Scale Inhibitor Adsorption/Desorption and The Potential For Formation Damage in Reconditioned Field CoreLaura Natalia SalcedoBelum ada peringkat
- Polymer Systems For Water Shutoff and Profile Modification: A Review Over The Last DecadeDokumen15 halamanPolymer Systems For Water Shutoff and Profile Modification: A Review Over The Last DecadeLeopold Roj DomBelum ada peringkat
- PDFDokumen11 halamanPDFizzyguyBelum ada peringkat
- SPE-174699-MS Dalia/Camelia Polymer Injection in Deep Offshore Field Angola Learnings and in Situ Polymer Sampling ResultsDokumen18 halamanSPE-174699-MS Dalia/Camelia Polymer Injection in Deep Offshore Field Angola Learnings and in Situ Polymer Sampling ResultslimbergBelum ada peringkat
- Preventing and Removing Wax Deposition in PipelinesDokumen2 halamanPreventing and Removing Wax Deposition in PipelinesDhea SamanthaBelum ada peringkat
- Positive Reactions in Carbonate Reservoir Stimulation: Ealian Al-Anzi Majdi Al-MutawaDokumen18 halamanPositive Reactions in Carbonate Reservoir Stimulation: Ealian Al-Anzi Majdi Al-MutawaBolsec14Belum ada peringkat
- Applications of Petroleum Geochemistry To Exploration and ReDokumen32 halamanApplications of Petroleum Geochemistry To Exploration and ReAndrea Soledad Olmos MoralesBelum ada peringkat
- Literature Review of Implemented Polymer Field Projects - JPSE 2014Dokumen15 halamanLiterature Review of Implemented Polymer Field Projects - JPSE 2014Wilmer ArcosBelum ada peringkat
- Euncl PCC 002 PDFDokumen19 halamanEuncl PCC 002 PDFAnvesh DonthulaBelum ada peringkat
- MEG Scaling in Oil Gas EnvironmentDokumen209 halamanMEG Scaling in Oil Gas EnvironmentAdrian YongBelum ada peringkat
- Wax Deposition IntroductionDokumen27 halamanWax Deposition IntroductionHoa NguyenBelum ada peringkat
- Surfactant Flooding 2020MSPET103Dokumen49 halamanSurfactant Flooding 2020MSPET103Sunny BbaBelum ada peringkat
- SPE30714 Fevang WhitsonDokumen16 halamanSPE30714 Fevang WhitsonMohamed El KikiBelum ada peringkat
- 25 One Year Experience With The Injection of Nitrate To Control Souring in Bonga Deepwater Development Offshore NigeriaDokumen9 halaman25 One Year Experience With The Injection of Nitrate To Control Souring in Bonga Deepwater Development Offshore NigeriaCatalinaManjarresBelum ada peringkat
- 2013 AutumnDokumen68 halaman2013 AutumnbahmangBelum ada peringkat
- SPE-169715-MS Chemical EOR For Heavy Oil The Canadian Experience - Heavy OilDokumen31 halamanSPE-169715-MS Chemical EOR For Heavy Oil The Canadian Experience - Heavy OilGilbert OmittaBelum ada peringkat
- Validation of ScaleSoftPitzer - Calcite SI, Density PDFDokumen20 halamanValidation of ScaleSoftPitzer - Calcite SI, Density PDFAmita GuptaBelum ada peringkat
- 2019 Recent Development and Remaining Challenges of Iron Sulfide Scale Mitigation in Sour-Gas WellsDokumen8 halaman2019 Recent Development and Remaining Challenges of Iron Sulfide Scale Mitigation in Sour-Gas WellsBeatriz Joo LeeBelum ada peringkat
- 56 - Remedial Cleanup, Sand Control and Other Stimulation TreatmensDokumen9 halaman56 - Remedial Cleanup, Sand Control and Other Stimulation Treatmensrizal tri susiloBelum ada peringkat
- Active Heated Pipe Technologies For Field Development Optimisation (OTC 26578-MS)Dokumen21 halamanActive Heated Pipe Technologies For Field Development Optimisation (OTC 26578-MS)ArsénioBelum ada peringkat
- SPE 126719 Matrix Acid Systems For Formations With High Clay ContentDokumen15 halamanSPE 126719 Matrix Acid Systems For Formations With High Clay ContentJose Miguel GonzalezBelum ada peringkat
- SPE-155651-MS Wettability For Low SalinityDokumen15 halamanSPE-155651-MS Wettability For Low Salinitykmilo04Belum ada peringkat
- Formation DamageDokumen26 halamanFormation DamagerajneeshgogoiBelum ada peringkat
- Modelling LSWFDokumen13 halamanModelling LSWFChun YanBelum ada peringkat
- Table 1. Synthetic Polymer Acid Gelling AgentsDokumen4 halamanTable 1. Synthetic Polymer Acid Gelling AgentsLeo Rojas DomBelum ada peringkat
- A Review of Polymer Nanohybrids For Oil RecoveryDokumen59 halamanA Review of Polymer Nanohybrids For Oil RecoverySayaf Salman100% (1)
- 4 Iron ControlDokumen26 halaman4 Iron ControlGilangFarhanaBelum ada peringkat
- SPE 160703 Simultaneous Well Stimulation and Scale Squeeze Treatments in Sandstone and Carbonate ReservoirsDokumen21 halamanSPE 160703 Simultaneous Well Stimulation and Scale Squeeze Treatments in Sandstone and Carbonate ReservoirsJose Miguel GonzalezBelum ada peringkat
- Spe 17140 Pa PDFDokumen5 halamanSpe 17140 Pa PDFJose RamirezBelum ada peringkat
- Production Chemistry Risks in Oil & GasDokumen3 halamanProduction Chemistry Risks in Oil & Gasyrdna nawaiteosBelum ada peringkat
- Rotating Vane Rheometry-AreviewDokumen14 halamanRotating Vane Rheometry-AreviewgoldennanukBelum ada peringkat
- Carbonates Acidizing PPTDokumen61 halamanCarbonates Acidizing PPTBolsec14Belum ada peringkat
- Volume II - Well Bore TreatmentsDokumen235 halamanVolume II - Well Bore TreatmentsADARSH KUMARBelum ada peringkat
- EOR of VNDokumen26 halamanEOR of VNVann Torng BmcBelum ada peringkat
- Evaluation of Scale Inhibitors PerformanceDokumen14 halamanEvaluation of Scale Inhibitors PerformanceLaura Natalia SalcedoBelum ada peringkat
- Matrix Acidizing of Carbonate Formations: A Case Study: AbstractDokumen8 halamanMatrix Acidizing of Carbonate Formations: A Case Study: AbstractAdriyan SyahBelum ada peringkat
- New and Novel Fracture Stimulation Technologies For The Revitalization of Existing Gas Storage Wells: Interim Project ResultsDokumen87 halamanNew and Novel Fracture Stimulation Technologies For The Revitalization of Existing Gas Storage Wells: Interim Project ResultsPinkesh ShahBelum ada peringkat
- Ranking Oil Viscosity in Heavy Oil ReservoirsDokumen12 halamanRanking Oil Viscosity in Heavy Oil ReservoirsJORGE SALINAS SALCEDOBelum ada peringkat
- Improved Production With Mineralogy-Based Acid DesignsDokumen11 halamanImproved Production With Mineralogy-Based Acid Designsmohamadi42Belum ada peringkat
- Otc19160 Subsea Cottonwood WaxDokumen13 halamanOtc19160 Subsea Cottonwood Wax1mmahoneyBelum ada peringkat
- Chemical Stimulation Techniques For Geothermal WellsDokumen11 halamanChemical Stimulation Techniques For Geothermal WellsMustafa AkyolBelum ada peringkat
- Asphaltene Deposit Removal Long-Lasting Treatment With A CO-SOLVENTDokumen8 halamanAsphaltene Deposit Removal Long-Lasting Treatment With A CO-SOLVENTRamanamurthy PalliBelum ada peringkat
- Spe 184869 MSDokumen16 halamanSpe 184869 MSSS100% (1)
- 2015 09 SPE-175610-MS Experimental Study of Wettability Alteration During Nanofluid Enhanced Oil Recovery Process and Its Effects On Oil RecoveryDokumen11 halaman2015 09 SPE-175610-MS Experimental Study of Wettability Alteration During Nanofluid Enhanced Oil Recovery Process and Its Effects On Oil RecoveryPetra StevenBelum ada peringkat
- Surfactant FloodingDokumen14 halamanSurfactant FloodingSagar DadhichBelum ada peringkat
- SRB & TCB Tests EvaluationDokumen25 halamanSRB & TCB Tests EvaluationEmad BehdadBelum ada peringkat
- 2010 Effect of PH and Scale Inhibitor Concentration On Phosphonate-Carbonate InteractionDokumen18 halaman2010 Effect of PH and Scale Inhibitor Concentration On Phosphonate-Carbonate Interactionandrea cunhaBelum ada peringkat
- Spe 56772Dokumen7 halamanSpe 56772curumnBelum ada peringkat
- Mercury Contamination RisksDokumen78 halamanMercury Contamination RisksguruhnurizalBelum ada peringkat
- Riopipeline2019 1014 Rio 2019 Paper v4Dokumen10 halamanRiopipeline2019 1014 Rio 2019 Paper v4Marcelo Varejão CasarinBelum ada peringkat
- Recent Advances in Viscoelastic Surfactants For Improved Production From Hydrocarbon Reservoirs PDFDokumen35 halamanRecent Advances in Viscoelastic Surfactants For Improved Production From Hydrocarbon Reservoirs PDFLê CôngBelum ada peringkat
- Petrel ShaleDokumen4 halamanPetrel ShaleAnderson Portilla BenavidesBelum ada peringkat
- Fracturing Engineering Manual - 4243456 - 02Dokumen903 halamanFracturing Engineering Manual - 4243456 - 02Pablo Antezana100% (2)
- Formation Testing and Sampling - 16-20 - Sep - 2019Dokumen70 halamanFormation Testing and Sampling - 16-20 - Sep - 2019Vasy Stancu100% (1)
- PVT Analysis For Oil EnglishDokumen4 halamanPVT Analysis For Oil EnglishPaolo RTBelum ada peringkat
- Eremor Core Lab PVT ReportDokumen45 halamanEremor Core Lab PVT ReportMartha Patricia Medina CasasBelum ada peringkat
- Well TestingDokumen8 halamanWell TestingstoianzBelum ada peringkat
- Calsep Nova Brochure OnlineDokumen4 halamanCalsep Nova Brochure OnlineedgarelerBelum ada peringkat
- Selection and Application Methods For Adsorption and Precipitation Scale Inhibitors For Squeeze Treatments in North Sea OilfieldsDokumen20 halamanSelection and Application Methods For Adsorption and Precipitation Scale Inhibitors For Squeeze Treatments in North Sea OilfieldsGuilherme MonteiroBelum ada peringkat
- W8 Series: Seal Support ReservoirDokumen2 halamanW8 Series: Seal Support ReservoirDumitrescuBelum ada peringkat
- 2011 Horizontal Underbalanced Drilling Technology Successfully Applied in Field AA - Libya PDFDokumen11 halaman2011 Horizontal Underbalanced Drilling Technology Successfully Applied in Field AA - Libya PDFcarlorgsBelum ada peringkat
- Chapter 7 Production UTMDokumen56 halamanChapter 7 Production UTMNurzanM.JefryBelum ada peringkat
- Paper Scale OnepetroDokumen15 halamanPaper Scale OnepetroRani JuliariniBelum ada peringkat
- Building A New Regulatory Framework For E&P in MexicoDokumen24 halamanBuilding A New Regulatory Framework For E&P in Mexicoguille5407Belum ada peringkat
- Archie (1952)Dokumen21 halamanArchie (1952)William S. FreitasBelum ada peringkat
- PVT Properties of Crude Oils: Understanding Density, Viscosity & Phase BehaviorDokumen121 halamanPVT Properties of Crude Oils: Understanding Density, Viscosity & Phase BehaviorRavi Shankar PatelBelum ada peringkat
- Preventing and Removing Wax Deposition Inside Vertical Wells: A ReviewDokumen17 halamanPreventing and Removing Wax Deposition Inside Vertical Wells: A ReviewmehranBelum ada peringkat
- EquationsDokumen9 halamanEquationstsar mitchelBelum ada peringkat
- Zeta WareDokumen4 halamanZeta WarerizuBelum ada peringkat
- Exploring Oil and Gas ReservoirsDokumen5 halamanExploring Oil and Gas ReservoirsSunil GoriahBelum ada peringkat
- Drilling For Non Technical PeopleDokumen87 halamanDrilling For Non Technical Peopleislima100% (1)
- Deoiling Manual: RestrictedDokumen137 halamanDeoiling Manual: RestrictedYoga Pratama100% (1)
- Temizel2020 Heavy OilDokumen29 halamanTemizel2020 Heavy OilCarlos Alberto Torrico BorjaBelum ada peringkat
- Flow Assurance For Subsea Tie BacksDokumen18 halamanFlow Assurance For Subsea Tie BacksDaniel InemughaBelum ada peringkat
- Case Studies of Expanding Cement To Prevent Microannular FormationDokumen8 halamanCase Studies of Expanding Cement To Prevent Microannular FormationEmad JamshidiBelum ada peringkat
- Spe 58987 Propped Fracturing in Gas Carbonate Formations MexicoDokumen12 halamanSpe 58987 Propped Fracturing in Gas Carbonate Formations MexicoJose Gregorio FariñasBelum ada peringkat
- What Is Pad DrillingDokumen11 halamanWhat Is Pad Drillingfredo405Belum ada peringkat
- Coren Technical Report 2019Dokumen3 halamanCoren Technical Report 2019Thankel IzuBelum ada peringkat
- Li Thesis 2012 PDFDokumen253 halamanLi Thesis 2012 PDFbu7amudBelum ada peringkat
- (Bret-Rouzaut, Nadine, Favennec, Jean-Pierre) Oil PDFDokumen291 halaman(Bret-Rouzaut, Nadine, Favennec, Jean-Pierre) Oil PDFanellbmcBelum ada peringkat