Repr
Diunggah oleh
mada2220 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
52 tayangan9 halamanadresa catre fac mv timisoara
Judul Asli
repr
Hak Cipta
© Attribution Non-Commercial (BY-NC)
Format Tersedia
RTF, PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen Iniadresa catre fac mv timisoara
Hak Cipta:
Attribution Non-Commercial (BY-NC)
Format Tersedia
Unduh sebagai RTF, PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
52 tayangan9 halamanRepr
Diunggah oleh
mada222adresa catre fac mv timisoara
Hak Cipta:
Attribution Non-Commercial (BY-NC)
Format Tersedia
Unduh sebagai RTF, PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 9
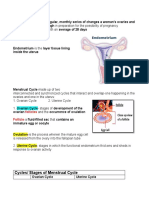
The ovaries, like the testicles, exert a double function,
exocrine and endocrine, consisting of the production of
gametes, the oocytes, as well as sex hormones, estrogens
and progesterone. Whereas in the testicles the two functions
are assured permanently from puberty onwards by two
different structures, however, in the ovary they are exerted
cyclically, between puberty and the menopause, and result
from the evolution of a same morphological unit, the ovarian
follicle, situated within the cortical stroma.
Histology of ovarian organelles
The gametogenic follicles
These follicles correspond to different stages of the evolution
of primordial follicles up to the rupture of the mature follicle
(ovulation). Each one contains an oocyte and is the site of
oogenesis and of the production of steroid hormones.
The primordial follicle.
Around the 7th month of embryonic development, the
ovarian cortex contains a definitive stock of several million
primordial follicles which progressively diminishes up to the
menopause. Each follicle, within the cortical stroma, is made
up of a 1st order oocyte (oocyte 1) surrounded by a layer of
flattened follicular cells, these cells being covered by a basal
membrane (membrane of Slavjanski). Oocyte 1 measures
about 30 µm in diameter.
The primary follicle.
It is characterized by the transformation of the flattened
follicular cells into cubic cells.
The secondary follicle.
This follicle is called secondary once the multiplication of the
follicular cells constitutes a second layer around the oocyte.
The diameter of the follicle progressively increases up to
about 180 µm. The follicular cells reach about 5000 in
number and together constitute the granulosa. Oocyte 1
begins its growth and its diameter increases from 40 up to
60 µm. At the last stage of its development, the secondary
follicle appears surrounded by irregularly spaced islets of
differentiated epithelioid cells from stromal fibroblasts and in
relation with the capillaries. Together the epithelioid cells
constitute the internal theca (theca interna) of the follicle.
The secondary follicle, provided with its theca interna is
called a preantral follicle.
The tertiary follicle.
Also called cavitary follicle or antral follicle, it is
characterized by the presence of a cavity (antrum) in the
granulosa and a theca externa, a fibrous layer around the
theca interna. It increases considerably in volume because of
the rapid multiplication of the follicular cells which will reach
about 50 million in number. At the end of its development,
the follicle (roughly 2 cm in diameter) will become a
preovulatory or mature follicle.
In the clumps of the granulosa there appear small drops of
liquid whose confluence forms the antrum which contains
the follicular fluid produced by the follicular cells. Around the
oocyte, the granulosa projects into the follicular cavity—the
cumulus oophorus. The theca interna, separated from the
granulosa by the membrane of Slavjanski, is made up of
numerous clusters of epithelioid cells. Electron microscopy
reveals that these cells have the characteristics of
steroidogenic cells, identical to those observed in Leydig
cells. The theca externa is made up of a thick layer of
collagen fibres, traversed by numerous blood capillaries; it
contains myofibroblasts differentiated from fibroblasts of the
stroma.
Up to the preovulatory stage of follicular evolution, the
oocyte harboured in the cumulus is an oocyte 1 blocked at
the end of the prophase (diakinesis stage). Cytoplasmic
growth continues and the oocyte attains around 120 µm in
diameter.
The preovulatory period and ovulation.
At the end of its growth, the mature follicle reacts to a
discharge of gonadotropic hormones by great
transformations which end in follicular rupture (ovulation).
The cumulus cells secrete large quantities of hyaluronic acid
which accumulates in the intercellular space and provokes
the dissociation of the cumulus followed by its rupture: the
oocyte surrounded by certain quantity of follicular cells is
released into the follicular fluid. The apical region, the
ovarian stroma, is the site of a vasoconstriction which results
in an ischemia followed by necrosis, within a few hours, of
the stroma and the follicular wall. The gonadotropic
discharge will give rise to a release of histamine and
bradykinin, leading to an edema of the theca. At the same
time, the secretion of a plasminogen activator will also
activate collagenases which will dissociate the theca
externa, this action being reinforced by the release of
prostaglandins. Lastly, the cells of the ovarian epithelium in
the apical region, would appear to be subject to autolysis,
leading to the release of lysosomal hydrolases and thus to
the dissociation of the apex (a mechanism which could be
deficient in the luteinized unruptured follicle [LUF]
syndrome).
The oocyte completes its cytoplasmic and nuclear
maturation in the cytoplasm, the cortical granules migrate to
the periphery and attach to the plasma membrane. Meiosis
resumes but is again blocked in 2nd division metaphase
(metaphase II). Ovulation commences with the rupture of the
necrosed tissues of the apex (stigma). The viscous follicular
fluid begins to flow. The decrease in pressure of the follicular
liquid induces a series of rhythmic contractions of the
myofibroblasts of the theca externa and of all the cortical
stroma which lead to the expulsion of the follicular fluid and
oocyte II surrounded by cumulus cells.
The corpus luteum
After expulsion of the oocyte, the follicle presents a pleated
aspect. It’s then called a dehiscent follicle. The membrane of
Slavjanski disappears completely and the blood capillaries of
the theca rapidly invade the granulosa thereby provoking
the transformation of these cells (luteinization) by the
constitution of the corpus luteum.
The blood vessels completely traverse the granulosa and
open up in the follicular cavity, thereby causing a
circumscribed and rapidly coagulated hemorrhage (central
coagulum). The granulosa cells are transformed into large
luteal cells, approximately 40 µm in diameter, whose
ultrastructure is the same as that of steroidogenic cells. The
cells of the theca interna (hardly modified) constitute the
small luteal or paraluteinic cells, situated at the periphery of
the corpus luteum and forming strings that penetrate more
or less deeply into the layer of the large cells.
Follicular atresia and luteolysis
Between the 7th month of fetal life and the menopause,
most of the gametogenic follicles undergo an involution
(involutive or atretic follicles). Only 300-400 follicles will
reach the preovulatory stage. All the involutive follicles
which preserve for a certain time their theca interna are
called thecogenic follicles. The theca cells of these follicles
as a whole constitute the interstitial gland of the ovary.
Involution of the corpus luteum, or luteolysis, occurs most
often in the form of a fibrous or fibro-hyalin degeneration
with cell lysis and marked collagen fibre synthesis, which
ends in the formation of a voluminous organelle called "
corpus albicans ". The process is relatively slow and spread
over several weeks.
Dynamics of follicular growth
In the human being, the stock of primordial follicles, called "
reserve follicles ", is about 1 million at birth, and at the
beginning of puberty a few hundred thousands. As already
emphasized, practically all the follicles (over 99%) will be
affected by the phenomenon of atresia, but at variable
stages in the course of development. The interregulation of
these two physiological phenomena—growth and atresia—is
governed by complex mechanisms, which are now beginning
to be elucidated in the human female, through the works of
Gougeon in particular.
It has been established that an average of 85 days—i.e.
corresponding to 3 ovarian cycles—separate the moment
when a follicle becomes preovulatory (stage 8 of Gougeon’s
classification) and the moment when it has differentiated its
theca interna (i.e. is at stage 1 or " preantral "). This means
that a preovulatory follicle enters the preantral stage 85
days earlier, in mid-cycle, at the time of the preovulatory
discharge of the gonadotropic hormones follicle-stimulating
hormone (FSH) and luteinizing hormone (LH).
As it is also recognised that entry into the preantral stage
occurs randomly at any moment during the cycle, it may be
deduced that all the follicles that differentiate their theca at
a time that does not correspond to the preovulatory period
will evolve more or less rapidly to atresia. A hypothesis that
has been advanced is that the concentration of plasma FSH
at the time of theca differentiation conditions the future
quality of the theca, and more generally of the follicle to
which it belongs.
It is nevertheless recognized that, up to a diameter of 2-4
mm (stage 4-5), follicular growth requires only a minimal
concentration (basal) of FSH. Follicles up to 4 mm diameter
may be found in impuberal girls or in women using hormonal
contraception. Further follicular growth requires stimulation
by gonadotropic hormones, and more especially by FSH. We
can thus distinguish three steps:
Follicular recruitment, corresponding to entry into terminal
growth of a group of follicles (stages 5 to 8).
Follicular selection, which will result in the emergence of the
future ovulatory follicle.
Follicular dominance, exerted by the selected follicle and
which will lead to the atretic evolution of the other follicles.
In the human female, recruitment occurs during the first
days of the cycle and affects a maximum of 5 follicles per
ovary, 3-5 mm in diameter (stage 5). It corresponds to an
elevation in the plasma FSH level observed at the beginning
of the cycle. Selection becomes more obvious shortly after: it
concerns the follicle with the highest mitotic index and,
generally, with the largest diameter. This follicle will
continue its growth (stages 6-7) whereas the FSH level
decreases (under the action of a negative feedback due to
the increase in estradiol), and signs of atresia appear in the
other follicles. It is of interest to note that if exogenous FSH
is supplied, pure or associated with LH (human menopausal
gonadotropin [hMG]) these follicles can be " recuperated "
and thereby avoid atresia. It is the principle of stimulatory
treatments of ovarian functions (hMG or pure FSH) which
lead to multiple ovulations.
The dominance of the selected follicle is clearly evident in
the second part of the follicular phase: growth continues
(stages 7-8) while the level of FSH continues to decrease:
such a phenomenon may account for a better uptake of FSH,
but also for an amplified response to FSH, bringing into play
an autocrine mechanism, corresponding to the production of
growth factors, as IGF-I, by the granulosa cells. In fact, for
these large follicles, evolution to continued growth or atresia
is directly linked to the aromatization potentialities of the
granulosa cell which will terminate in the transformation into
estrogens of androgens originating from the theca interna.
The dominant follicle possesses, up to the preovulatory
gonadotropic discharge, a high aromatic activity. It might
secrete a protein, called " regulatory ", which could perhaps
inhibit the aromatase activity of the other follicles through a
paracrine mechanism.
Regulation of ovarian functions
Ovarian functions are under the control of cyclic pituitary
gonadotropic hormones, which in turn are subjected to
stimulation by the hypothalamic peptide gonadotropin-
releasing hormone (GnRH). Plasma FSH increases at the
beginning of a cycle, then decreases before a peak which
reaches its summit about 24 hours before ovulation (i.e. D
13) and is thus synchronous with that of LH, constituting the
preovulatory discharge of gonadotrophins.
Estradiol levels rise progressively during the follicular phase:
estradiol is secreted by all the follicles recruited at the
beginning of the cycle, then, as atresia gradually affects the
majority of these follicles, it is secreted by the dominant
follicle. It is accepted that estradiol exerts first a classical
negative feedback on the pituitary gland which then
becomes positive as from a certain level, and then triggers
the gonadotropic discharge in the 24 hours following the
estradiol peak. Progesterone then begins to be secreted by
the mature preovulatory follicle, and can be detected in the
follicular fluid, but it is only once the corpus luteum is
formed that it appears in large concentrations in the blood to
reach a maximum at the 21st day.
The important features may be summarized as follows: when
the follicle reaches a diameter of approximately 5 mm
(stages 5-6), the mitotic indices of the theca and granulosa
cells decrease, whereas their respective secretory functions
occur in a coordinated manner: stimulated by LH (only small
quantities are needed), the theca cells produce increasing
quantities of androgens, which are transformed into
estrogens by the granulosa cells exhibiting increased
aromatization capacities through FSH stimulation. FSH
induces two important syntheses in these cells: the
enzymatic complex responsible for aromatization on the one
hand, LH receptors on the other hand.
There occurs a reciprocal slowing-down in the synthesis of
progesterone and aromatization, and therefore of estradiol
synthesis. Up to the gonadotropic surge, this balance is in
favour of aromatization (inhibited progesterone synthesis). In
contrast, in the 24-48 hours before ovulation, the LH level
rises whereas the number of its receptors increases, and
luteinization of the follicle begins, with slowing down of
aromatization. In clinical practice it is known that
luteinization of a follicle that is still immature will perturb the
ovarian functions and ovulation in particular.
After constitution of the corpus luteum, the granulosa luteal
cells are mainly responsible for progesterone secretion,
whereas the theca luteal cells acquire the possibility to
aromatize the androgens, and thus directly secrete estradiol.
The granulosa cell is subjected to a complex paracrine and
autocrine regulation, whose general purpose is to control
aromatase activity. Among the positive effectors known, IGF-
I is essentially important. Negative effectors are more
numerous: progesterone, inhibin (autocrine control),
epidermal growth factor and 5a-dihydrotestosterone
(paracrine control).
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Physiology of MenstrationDokumen33 halamanPhysiology of MenstrationYiel Ellie Balase Moldin100% (1)
- Biology 30 Menstrual Cycle Worksheet Answer Key PDFDokumen2 halamanBiology 30 Menstrual Cycle Worksheet Answer Key PDFRoger DogerBelum ada peringkat
- Menstrual Cycle-WPS OfficeDokumen6 halamanMenstrual Cycle-WPS OfficeWebster TemboBelum ada peringkat
- Q3 Science 10 Module 2 - NK4ADokumen17 halamanQ3 Science 10 Module 2 - NK4ALyzhafel AlingalanBelum ada peringkat
- CBL Wk6 Foundation Mod.Dokumen3 halamanCBL Wk6 Foundation Mod.Savera NarejoBelum ada peringkat
- Roles of HormonesDokumen13 halamanRoles of Hormoneshafizah_90100% (6)
- Central Precocius PubertyDokumen29 halamanCentral Precocius PubertyNurhidayat DayatBelum ada peringkat
- Cycles/ Stages of Menstrual Cycle: Follicles FollicleDokumen6 halamanCycles/ Stages of Menstrual Cycle: Follicles FollicleAaliyah CarlobosBelum ada peringkat
- Normal Menstrual CycleDokumen20 halamanNormal Menstrual CycleTrevor UratelBelum ada peringkat
- Menstrual CycleDokumen20 halamanMenstrual CycleJomz AbaigarBelum ada peringkat
- Sakala Step 2 CK GYN 2018 Color Handout 2 - PageDokumen12 halamanSakala Step 2 CK GYN 2018 Color Handout 2 - PageJurian F. Kaunang100% (1)
- Graphing Hormones LabDokumen4 halamanGraphing Hormones LabSarahlynn CampbellBelum ada peringkat
- Artigo - Vegetarianism and Menstrual Cycle Disturbances - Susan I BarrDokumen6 halamanArtigo - Vegetarianism and Menstrual Cycle Disturbances - Susan I Barrlayla.oliveiraBelum ada peringkat
- SCIENCE 5 - Q2 - Mod2Dokumen17 halamanSCIENCE 5 - Q2 - Mod23tj internetBelum ada peringkat
- 057 - Endocrinology Physiology) OvulationDokumen4 halaman057 - Endocrinology Physiology) Ovulationhasanatiya41Belum ada peringkat
- Activity 6 Menstrual Cycle Bracelet 1Dokumen3 halamanActivity 6 Menstrual Cycle Bracelet 1Ludy LynBelum ada peringkat
- Follicular Dynamics in Bovine and Ovine 1Dokumen19 halamanFollicular Dynamics in Bovine and Ovine 1israr yousafBelum ada peringkat
- DLP - Menstrual CycleDokumen5 halamanDLP - Menstrual CycleRigel Del CastilloBelum ada peringkat
- The Three Phases of The Menstrual CycleDokumen3 halamanThe Three Phases of The Menstrual CycleMelai MarceloBelum ada peringkat
- Science 10 Q3 - M2Dokumen14 halamanScience 10 Q3 - M2Avha CortesBelum ada peringkat
- UTERVINDokumen1 halamanUTERVINsabiinfotechBelum ada peringkat
- TJS Matungao, Bulakan, Bulacan: Name: Grade and Section: I. Matching TypeDokumen3 halamanTJS Matungao, Bulakan, Bulacan: Name: Grade and Section: I. Matching Typenina lykka calaraBelum ada peringkat
- Mestrual CycleDokumen15 halamanMestrual CycleMary-Ann SanchezBelum ada peringkat
- B11.5 Interpreting The Menstrual CycleDokumen7 halamanB11.5 Interpreting The Menstrual CycleBenjamin WatsonBelum ada peringkat
- MENSTRUAL CYCLEI-Investigatory-project-of-biologyDokumen22 halamanMENSTRUAL CYCLEI-Investigatory-project-of-biologyAkshayBelum ada peringkat
- 0009KK003556F2Dokumen3 halaman0009KK003556F2Sunny SharmaBelum ada peringkat
- Q3W2Dokumen28 halamanQ3W2pjBelum ada peringkat
- Science10 Q3 M1 W2Dokumen6 halamanScience10 Q3 M1 W2Chris John SantosBelum ada peringkat
- Reproductive Hormones and Their FunctionsDokumen6 halamanReproductive Hormones and Their FunctionsOwolabi PetersBelum ada peringkat
- Normal Menstrual CycleDokumen28 halamanNormal Menstrual CycleCristóbal ConchaBelum ada peringkat