Re Aipmt 2015 Paper
Diunggah oleh
Sudhanshu JoshiDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Re Aipmt 2015 Paper
Diunggah oleh
Sudhanshu JoshiHak Cipta:
Format Tersedia
AIPMT - 2015
25-07-2015
Time: 3 Hrs.
l e;: 3 ?kaVs
CODE - (D)
Max. Marks : 720
v f/kd re v ad
: 720
INSTRUCTIONS (funs
'k)
Z
Important Instructions:
egRoiw.kZfunsZ'k %
1.
1.
The Answer Sheet is inside this Test Booklet. When you
are directed to open the Test Booklet, take out the Answer
Sheet and fill in the particulars on Side-1 and Side-2
carefully with blue/black ball point pen only.
2.
The test is of 3 hours duration and Test Booklet contains
180 questions. Each question carries 4 marks. For each
correct response, the candidate will get 4 marks. For
each incorrect response, one mark will be deducted
from the total scores. The maximum marks are 720.
3.
Use Blue/Black Ball Point Pen only for writing particulars
on this page/marking responses.
4.
Rough work is to be done on the space provided for this
purpose in the Test Booklet only.
5.
On completion of the test, the candidate must
havdover the Answer Sheet to the invigilator in the
Room/Hall. The candidates are allowed to take away
this Test Booklet with them.
6.
The CODE for this Booklet is F. Make sure that the CODE
printed on Side-2 of the Answer Sheet is the same as
that on this Booklet. In case of discrepancy, the candidate
should immediately report the matter to the Invigilator
for replacement of both the Test Booklets and the
Answer Sheets.
7.
The Candidates should ensure that the Answer Sheet
is not folded. Do not make any stray marks on the Answer
Sheet. Do not write your roll no. anywhere else except in
the specified space in the Test Booklet/Answer Sheet.
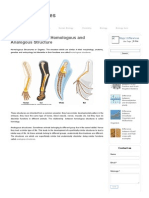
8.
Use of white fluid for correction is NOT permissible on
the Answer Sheet.
mkj i=k bl ijh{kk iqfLrd k d svUnj j[kk gSA t c vkid ks
ijh{kk iqfLrd k [kksy usd ksd gk t k,] rksmkj i=k fud ky
d j i`"B-1 ,oai`"B-2 ij d soy uhy s@ d ky sckWy ikWbaV isu
lsfooj.k HkjsaA
2. ijh{kk d h vof/k 3 ?ka
VsgS,oaijh{kk iq
fLrd k es
a180 iz
'u
gS
A iz
a
R;s
d iz
'u 4 va
d d k gS
A iz
R;s
d lgh mkj d sfy,
ijh{kkFkhZd ks4 va
d fn, t k,a
xs
A iz
R;s
d xyr mkj d sfy,
dq
y ;ks
x es
als,d va
d ?kVk;k t k,xkA vf/kd re va
d 720
gSaA
3. bl i`
"B ij fooj.kva
fd r d jus,a
o mkj i=k ij fu'kku yxkus
d sfy, d osy uhys
@ d kysckyW ikbWVa is
u d k iz
;kxs d js
Aa
jQ d k;Zbl ijh{kk iqfLrd k esafu/kkZfjr LFkku ij gh d jsaA
5. ijh{kk lEiUu gks
usij] ijh{kkFkhZd {k@gkWy NksM uslsiwoZ
mkj i=k d {k fujh{kd d ksv o'; lkSai nsaA ijh{kkFkhZv ius
lkFk iz'u iqfLrd k d ksy st k ld rsgSaA
4.
bl iqfLrd k d k lad sr gSF A ;g lqfuf'pr d j ysafd bl
iqfLrd k d k lad sr] mkj i=k d si`"B-2 in Nislad sr ls
feyrk gSA vxj ;g fHkUu gks rks ijh{kkFkhZ nwl jh ijh{kk
iqfLrd k vkSsj mkj i=k ysus d s fy, fujh{kd d ks rqjUr
voxr d jk,aA
7. ijh{kkFkhZlq
fuf'pr d jsafd bl mkj i=k d kseksM+k u t k,
,oaml ij d ksbZvU; fu'kku u yxk,aA ijh{kkFkhZviuk
vuq ekad iz'u iqfLrd k@mkj i=k es fu/kkZfjr LFkku d s
vfrfjDr vU;=k uk fy[ksaA
6.
8.
mkj i=k ij fd lh izd kj d sla'kks/ku gsrqOgkbV +y wbM d s
iz;ksx d h vuqefr ughagSA
In case of any ambiguity in translation of any question, English version shall be treated as final.
iz'uksad sv uqokn esafd l h v Li"Vrk d h fLFkfr esa] v axzst h l aLd j.k d ksgh v fUre ekuk t k;sxkA
Name of the Candiate (in Capital letters) : ____________________________________________________________
Roll Number : in figures :
in words : _______________________________________________
Name of Examination Centre (in Capital letters) : ________________________________________
Candidate's Signature : ______________________________ Invigilator's Signature : ___________________________________
AIPMT-2015 |
CODE - (D)
BIOLOGY
| DATE: 25-07-2015
PART A : BIOLOGY
1.
Ans
Sol.
In his classic experiments on pea plants, Mendel did not use
(1) Pod length
(2) Seed shape
(3) Flower position
(1)
Pod length did not use by mendel for ihs experiment.
eVj d sikS/ksaij viusvkn'kZiz;ksx esaesaM y usfd l mi;ksx ughafd ;k\
(1) Q y hd hy EckbZ
(2) cht d kvkd kj
(3) iq
"i d hfLFkfr
2.
(4) Seed colour
(4) cht
d k jax
Which one of the following is not applicable to RNA?
(1) 5 phosphoryl and 3 hydroxyl ends
(2) Heterocyclic nitrogenous bases
(3) Chargaffs rule
(4) Complementary base pairing
fuEufy f[kr esalsd kSu lk RNA ij y kxwughagksrk
(1) 5 Q kW
LQ ksfjy vkSj 3 gkbMksfDly fljs
(2) fo"kep h; ukbV
ks
t uhcsl
(3) pkjxkS
Q fu;e
(4) la
iwjd csl ;qXeu
Ans
(3)
3.
Male gametophyte in angiosperms produces:
(1) Single sperm and a vegetative cell
(2) Single sperm and two vegetative cells
Sol.
(3) Three sperms
(4) Two sperms and a vegetative cell
Male gametophyte in angiosperm is 3-celled containing 2 male gametes (sperms) an vegetative cell.
vko`r cht h ikniksaesauj ;qXed D;k cukrsgSa\
(1) ,d 'kq
k.kqvkSj ,d d kf;d d ksf'kd k
(2) ,d 'kq
k.kqvkSj nksd kf;d d ksf'kd k;sa
(3) rhu 'kq
k.kq
(4) nks'kq
k.kqvkSj ,d d kf;d d ksf'kd k
Ans
4.
(4)
Which of the following are not membrane-bound?
(1) Ribosomes
(2) Lysosomes
(3) Mesosomes
Sol.
Ribosome is naked cell organelle.
fuEufy f[kr esalsd kSu f>Yy h esaugh f?kjsjgrs\
(1) jkbcks
l kes
(2) ykblks
l ks
e
Ans
(3) e/;d k;
eht ks
l ks
e
(4) Vacuoles
(4) jl/kkfu;k
(1)
Page || 3
AIPMT-2015 |
5.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
The chitinous exoskeleton of arthropods is formed by the polymerisation of:
(1) D- glucosamine
(2) N- acetyl glucosamine
(3) lipoglycans
(4) keratin sulphate and chondroitin sulphate
vkFkz
ksiksM ksad kd kbfVuhckg~; d ad ky fd ld scgqy d hd j.kcurkgS\
(1) D- Xy w
d ksl sfeu d s
(2) N- ,lhfVy Xy w
d ksl sfeu d s
(3) fyiks
Xykbd s
uks
ad s
(4) d S
jkfVu lYQ sV vkSj d kWUMksbfVu lYQ sV d s
Ans
6.
(2)
Among china rose, mustard, brinjal, potato, guava, cucumber, onion and tulip, how many plants have superior
ovary?
(1) Six
(2) Three
(3) Four
(4) Five
Sol.
China rose, mustard, brinjal , potato, onion and tulip total six plants have superior ovary.
xqM +gy ]ljlksa]cSaxu]vky w]ve: n][khjk]I;kt vkSj V~;wfy i esalsfd ruksaesa /oZorhZv.Mk'k; gSa\
(1) N%
(2) rhu
(3) pkj
(4) ik
p
Ans
7.
(1)
The function of the gap junction is to
(1) facilitate communication between adjoining cells by connecting the cytoplasm for rapid transfer of ions,
small molecules and some large molecules.
(2) separate two cells from each other
(3) stop substance from leaking across a tissue
(4) performing cementing to keep neighbouring cells together.
xsi&t ad 'ku d k d k;ZgS%
(1) iM+
ksl hd ksf'kd kvksad schp laizs"k.kesaenn d jusd sfy ,]d ksf'kd kvksad kst ksM +sj[kusd sfy , rkfd vk;Ul NksVsv.kqvkSj
d qN cM+sv.kqrhozxfr esaLFkkukUrfjr gk ld saA
(2) nksd ks
f'kd kvksad ks,d &nwl jslsi`Fkd d sfy ,A
(3) fd lh inkFkZd ks rd d sikj fud y uslsjks
d usd sfy ,
(4) iM+
ksl hd ks
f'kd kvksad ksijLij t ksM +sj[kusd sfy ,A
Ans
Sol.
8.
(1)
Colostrum has high levels of IgA, which provide passive immunity to foetus.
Which of the following immunoglobulins does constitute the largest percentage in human milk?
(1) Ig M
(2) Ig A
(3) Ig G
(4) Ig D
fuEufy f[kr esalsd kSu ?kVd izfrj{kkXy ksC;wfy u ekuo nqX/k esalclsvf/kd izfr'krrk esaik;k t krk gS\
Ans
(1) Ig M
(2)
(2) Ig A
(3) Ig G
(4) Ig D
Page || 4
AIPMT-2015 |
9.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
In mammalian eye, the fovea is the center of the visual field, where:
(1) the optic nerve leaves the eye.
(2) only rods are present.
(3) more rods than cones are found.
(4) high density of cones occur, but has no rods.
Lru/kkjh izk.kh d sus=k esa[kkr Q ksc;k n`'; y si d k d sUnzgSt gk
(1) pk{kq
i raf=kd k us=k esackgj fud y usesa
(2) d s
oy 'ky kd k,sagksrhgSA
(3) 'ka
d qv ksad hvis{kk'ky kd k,savf/kd gksrhgSa
(4) 'ka
d qv ksad hl?kurkvf/kd gksrhgS]y sfd u 'ky kd k,saughagksrhA
Ans
Sol.
10.
(4)
Fovea is present at centre of macula lutea, whcih is centre of highest resolution has only cones.
Doctors use stethoscope to hear the sounds produced during each cardiac cycle. The second sound is heard
when:
(1) Ventricular walls vibrate due to gushing in of blood from atria
(2) Semilunar valves close down after the blood flows into vesels from ventricles
(3) AV node receives signal from SA node
(4) AV valves open up
izR;sd g`n p d snkSjklu mRi gksusoy h /ofu rajxksad kslquusd sfy , fpfd Rld LVsFkksLd ksi d kmi;ksx d jrsgSaA nlwj /
ofu ml le; lqukbZnsrh gSat c%
(1) vfy a
nksals: f/kj d scy iwoZd fuy ; esavkusd sd kj.k fuy h; fHkfk;kesad ai gksusy xrk gSA
(2) fuy ;ks
alsokfgd kvksaesa: f/kj d scgusd sckn v/kZp anzkd kj d ikV can gkst krsgSaA
(3) AV ioZ
l af/k SA ioZl af/klslad sr izkIr d jrhgSA
(4) AV d ikV [kq
y t krsgSaA
Ans
11.
(2)
Coconut water from a tender coconut is:
(1) Free nuclear endosperm
(2) Innermost layers of the seed coat
(3) Degenerated nucellus
(4) Immature embryo
d Ppsukfj;y d k ukfj;y ikuh D;k gS%
(1) eq
d sUnz
d hHkz
w.kiks
"k
(2) cht pks
y d h lclscanj oky h lrgsa
(3) viHkz
"V cht k.Md k;
(4) vifjiDo Hkw
z.k
Ans
(1)
Page || 5
AIPMT-2015 |
CODE - (D)
BIOLOGY
| DATE: 25-07-2015
12.
The cutting of DNA at specific locations became possible with the discovery of:
(1) Probes
(2) Selectable markers
(3) Ligases
(4) Restriction enzymes
Ans
Sol.
(4)
Restriction endomiclease enzyme cut the DNA at specific site
Mh-,u-,-d k fof'k"V LFkkuksaij d kV nsuk fd ld svfo"d kj lslaHko gqv k\
(1) iz
ks
cl~
(2) ly s
DVscy ekd Zjl~
(3) ykbxs
t
(4) js
fLVD'ku ,sat kbe
13.
Ans
Sol.
Which of the following structures is not found in a prokaryotic cell?
(1) Ribosome
(2) Mesosome
(3) Plasma membrane (4) Nuclear envelope
(4)
Procaryotic celld does not contain distinct nucleus and nuclear membrane.
fuEufy f[kr esalsd kSu lh lajpuk izkd ~d sUnzd h d ksf'kd k esaugh ik;h t krh \
(1) jkbcks
l kes
(2) e/;d k; eht ks
l ks
e (3) Iy kTek d y k
14.
(4) d s
Unzd
vkoj.k
Arrange the following events of meiosis in correct sequence:
(a) Crossing over
(b) Synapsis
(c) Terminalisation of chiasmata
(d) Disappearance of nucleolus
(1) (b), (a), (c), (d)
(2) (a), (b), (c), (d)
(3) (b), (c), (d), (a)
(4) (b), (a), (d), (c)
v/kZl w=khfoHkku d h/kVukvksad hlgh e esaO;ofLFkr d hft ,&
(a) kfla
x vksoj t hu fofue;
(b) fluS
fIll lw
=k;qXeu
(c) d k,Tes
Vk d k var
(d) d s
fnzd kd kvn`'; gksuk
Ans
Sol.
15.
(1) (b), (a), (c), (d)
(2) (a), (b), (c), (d)
(3) (b), (c), (d), (a)
(1)
In prophase I of meiosis I, The correct sequence of events are
b synapsis in zygotene
c corssingover in pachytene in diakensis
d Disappearance of nucleolus in diakinesis
(4) (b), (a), (d), (c)
A column of water within xylem vesels of tall trees does not break under its weight because of:
(1) Tensile strength of water
(2) Lignification of xylem vessels
(3) Positive root pressure
(4) Dissolved sugars in water
,d y acso`{k d ksnk: okfgd kvksaesat y d k LrEHk viusij lsugh VwVrkA bld k d kj.k gS%:
(1) t y d h lrr 'kf
(2) nk: okfgd kvks
ad kfy fXuud j.k
(3) /kukRed ew
y nkc
(4) t y es
a?kqfy r 'kd Zjk
Ans
(1)
Page || 6
AIPMT-2015 |
16.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
The imperfect fungi which are decomposer of litter and help in minearl cycling belong to:
(1) Basidiomycetes
(2) Phycomycetes
(3) Ascomycetes
(4) Deuteromycetes
viwoZd od t ksd jd V d svi?kVd gSvkSj [kfut ksad sp esalgk;rk d jrsgSa]osfd llslEcfU/kr gSa\
(1) cS
flMh;ks
ekbflVht
(2) Q kbd ks
ekbflVht
(3) ,Ld ke
skbflVht
(4) M~
;w
jkuskbflVht
Ans
Sol.
(4)
The members of deuteromycetes do not show sexual reproduction therefore they are called fungi infercti.
17.
The structures that help some bacteria to attach to rocks and / or host tissues are:
(1) Fimbriae
(2) Mesosomes
(3) Holdfast
(4) Rhizoids
cgqlajpuk t ksd qN t hok.kqv ksad kspkuksa;k iks"kh rd la;kst h gksusesalgk;rk d jrh gS]D;k gSa\
(1) >kyj
(2) eht ks
l ks
e
(3) gkY
sMQ kLV
(4) ew
y kHkkl
Ans
18.
(1)
The DNA molecule to which the gene of interest is integrated for cloning is called:
(1) Vector
(2) Template
(3) Carrier
(4) Transformer
ml Mh-,u-,-v.kqd ksD;k d grsgSaft lesaDy ksuu d sfy , : fp oky h t hu d kslekd fy r fd ;k t krk gS\
(1) la
okgd
(2) : ink
(3) okgd
(4) : ikUrjd
Ans
19.
(1)
Pick up the wrong statement:
(1) Protista have photosynthetic and heterotrophic modes of nutrition
(2) Some fungi are edible
(3) Nuclear membrane is present in Monera
(4) Cell wall is absent in Animalia
xy e d Fku d kspqfu,%
(1) iz
ksfVLVkesaiks"k.kd hfof/k;kizd 'kla'y s"k.kh, fo"keHkkst hgksrhgSaA
(2) d q
N d od [kkus;ksX; gksrsgSaA
(3) eks
usjk esad sUnzd d y k mifLFkr gksrh gSA
(4) ,fues
fy ;kes
ad ksf'kd kfHkfkvuqifLFkr gksrhgSA
Ans
(3)
20.
Metagenesis refers to:
(1) Alternation of generation between asexual and sexual phases of an organisms
(2) Occurrence of a drastic change in form during post-embryonic development
(3) Presence of a segmented body and parthenogenetic mode of reproduction
(4) Presence of different morphic forms
esVkt susfll : ikraj.kfd ld klad sr nsukgS&
(1) ,d t ho d ksvy S
afxd vkSj y Sfaxax izkoLFkkvksad sczksu ih<+h,ad krj.k
(2) Hkz
w.ki'oksaifjo/kZu d snkSjku Lo: i esaifjorZu d kik;kt kukA
(3) ,d lca
n 'kjhj vkSj t uu d ksvuf'kpd fo"k d k ik;k t kukA
(4) fofo/kLo: iks
aesaik;kt kukA
Ans
Sol.
(1)
Metagenesis is alternation of generations found in cnidaria phylum eg. obelia.
Page || 7
AIPMT-2015 |
21.
CODE - (D)
BIOLOGY
| DATE: 25-07-2015
Which of the following events is not associated withovulation in human female?
(1) Full development of Graffian follicle
(2) Release of secondary oocyte
(3) LH surge
(4) Decrease in estradiol
fuEufy f[kr ?kVukvksaesalsd kSu lh ?kVuk h esaizkMksl t Zl lacfU/kr ugh gS\
(1) Xkz
kQ hiqVd d kiw.kZfod kl
(2) f}rh;d va
M d d hfoeksp u
(3) LH iz
okg LH lht
(4) bLV
sM ksy esad eh
Ans
Sol.
22.
(4)
At ovulation, LH surge occur due to hypersecretion of estrogen, which gives positive feed back to anterior
pituitary for secretion of LH.
Which of the following joints would allow no movement?
(1) Cartilaginous joint
(2) Synovial joint
(3) Ball and Socket joint (4) Fibrous joint
fuEufy f[kr esalsd kSu lh laf/k fd lh d h vuqefr ugh nsrh gSa\
(1) mikfLFky la
f/k
(2) lk;uks
fo;y la
f/k
(3) d a
nqd [kfYy d klaf/k
Ans
23.
(4) js
'knkj laf/k
(4)
Match the following list of microbes and their importance:
(a)
Sacharomyces cerevisiae
(b)
Monascus purpureus
(c )
Trichodemra polysporum
(d)
(i) Production of immunosuppressive agents
(ii) Ripening of Swiss cheese
(iii) Commerical production of ethanol
Propionibacterium sharmanii (iv) Production of blood cholesterol lowering agents
(a)
(iii)
(b)
(ii)
(c)
(i)
(d)
(iv)
(2)
(3)
(4)
(iv)
(iii)
(iii)
(ii)
(i)
(iv)
(i)
(iv)
(i)
(iii)
(ii)
(ii)
(1)
lw{et hoksad h vkSj mud segRo d h fuEufy f[kr lwp h d k fey ku d hft ,%
Ans
(a)
lS
dS
jkekbl ht l foZ
fl v kbZ
(i)
iz
frj{kh l l a
ned d kjd ks
ad k mRiknu
(b)
eks
us
Ld l iI;w
fj;l
Z
(ii)
fLol pht d ksid kuk
(c )
V
kbd ks
M ekZiks
y hLiks
je
(iii)
bZ
Fks
ukW
y d k O;ol kf;d mRiknu
(d)
iz
ks
fiv kfu cS
DVhfj;e 'kekZ
ukbZ
(iv)
: f/kj es
ad ks
ys
LV
ky d e d jusd k d kjd
(iv)
(2)
(3)
(4)
(4)
(a)
(iii)
(iv)
(iii)
(iii)
(b)
(ii)
(ii)
(i)
(iv)
(c)
(i)
(i)
(iv)
(i)
(d)
(1)
(iii)
(ii)
(ii)
Page || 8
AIPMT-2015 |
24.
CODE - (D)
The UN conference of Parties on climate change in the year 2012 was helf at:
(1) Doha
(2) Lima
(3) Warsaw
(4) Durban
o"kZ2012 esat y ok;qifjorZu ij ny ksd k ;w-,u lEesy u d gkgqv k Fkk\
(1) nks
gk
(2) yhek
(3) okjlk
Ans
25.
BIOLOGY
| DATE: 25-07-2015
(4) McZ
u
(1)
If you suspect major deficiency of antibodies in a person, to which of the following would you look for confirmatory evidences?
(1) Serum albumins
(2) Haemocytes
(3) Serum globulins
(4) Fibrinogin in plasma
;fn vki fd lh O;f esaizfrjf{k;ksad h xaHkhj d eh d k vuqeku y xk jgsgSa]rksvki iqf"V d sfy , fuEufy f[kr fd l lsizek.k
izkIr d jsaxs\
(1) lhje ,YC;w
feu
(2) gheks
l kbV
(3) lhje Xy ks
C;wfy u
(4) Iy kTekes
afQ fczuksft u
Ans
Sol.
26.
(3)
Antibodies are -Globulin.
Chromatophores take part in:
(1) Growth
(2) Movement
(3) Respiration
(4) Photosynthesis
o.kZd h y od kseSVksQ ksj fd l f ;k esaHkkx y srsgSa\
(1) o`
f)
(2) xfr
(3) 'olu
(4) iz
d k'kla
'y s
"k.k
Ans
Sol.
(4)
Chromatophores
photosynthesis
27.
Acid rain is caused by increase in the atmospheric concentration of:
(1) SO3 and CO
contain
pigments
(2) CO2 and CO
and
they
are
(3) O3 and dust
vEy o"kkZokrkoj.k esafd ld hlkanzrk esavf/kd rk d sd kj.kgksrh gS
(1) SO3 vkS
j CO
(2) CO2 vkS
j CO
(3) O3 vkS
j /kwy
Ans
Sol.
28.
found
in
blue
green
algae
(4)
SO2 and NO2
(4)
SO2 vkS
j NO2
for
(4)
The main contribution in acid rain is 60-70 SO2 and 20 30% NO2.
During ecological seccession:
(1) the establishment of a new biotic communityh is very fast in its primary phase.
(2) the numbers and types of animals remain constant.
(3) the changes lead to a community that is in near equilbrium with the environment and is called pioneer
community
(4) the gradual and predictable change in species composition occurs in a given area
ikfjLFkfrd h; vuq e.kd snkSjku
(1) bld hiz
kFkfed izkoLFkkesau;kt hoh; leqnk; rhozxfr lsLFkkfir gksrhgSa
(2) t a
rqv ksad hla[;kvkSj fd LesafLFkj jgrhgSaA
(3) ml leq
nk; esagksusoky sifjorZuksad sd kj.k t ksi;kZIr d slkE; d slehi gksrk gS]iqjksxkeh lenqk; d grsgSa
(4) fd lh Lih'kht d h la
?kVuk esa fed vkSj igy scik;st k ld usoky sifjorZu fd lh ,d {ks=k esa;qXe 'kkfey gS\
Ans
(4)
Page || 9
AIPMT-2015 |
29.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
The oxygen evolved during photosynthesis comes from water molecules. Which one of the following pairs of
elements is involved in this reaction?
(1) Manganese and Potassium
(2) Magnesium and Molybdenum
(3) Magnesium and Chlorine
(4) Manganese and Chlorine
izd k'kla'y s"k.kd snkSjku fu"d kf"kr vkWDlht u t y HkvkrhgSA bl vfHkf ;kesafuEufy f[kr rRoksad kd kSu ;qXe 'kkfey gS\
(1) eS
axuht vkSj ikSVsf'k;e
(2) eS
fXuf'k;e vkSj eksfy CMsue
(3) eS
fXuf'k;e vkSj Dy ksjhu
(4) eS
xuht vkSj Dy ksjhu
Ans
Sol.
(4)
photolysis of water is initiated by Mn++ and Cl ions.
30.
Which of the followin pairs is not correctly matched?
Mode of reproduction
(1)
Rhizome
(2)
Binary fission
(3)
Conidia
(4)
Offset
Example
Banana
Sargassum
Penicillium
Water hyacinth
fuEufy f[kr esalsd kSu lk ;qXe lgh lqesfy r ughagS\
izt uu fof/k
(1)
iz
d Un
(2)
f}[ka
Mu
(3)
d kfsufM;k
(4)
HkLwrkjh
mnkgj.k
ds
yk
lkjxkle
iS
fuflfy;e
t y gk;flUFk
Sol.
Ans
31.
Binary fission is usually found in amoeba, Paramoecium, euglena.
(2)
In the following human pedigree, the filled symbols represent the affected individuals. Identify the type of given
pedigree.
fuEufy f[kr ekuo oa'kkoy h esaHkjsgq, lad sr izHkkfor O;f ;ksad kfu: i.k d jrsgSaA nksx;hoa'kkoy h d sizd kj d k igpkfu,%
(1) X- linked recessive
(1) X- lgyXu
Ans
(2) Autosomal recessive (3) X-linked dominant
viz
Hkkfork (2) vfya
xlw
=khviz
Hkkoh
(3) X-lgyXu iz
Hkkoh
(4) Autosomal dominant
(4) vfya
xlw
=khiz
Hkkoh
(2)
Page || 10
AIPMT-2015 |
32.
CODE - (D)
BIOLOGY
| DATE: 25-07-2015
Which one of the following animals has two separate circulatory pathways?
(1) Lizard
(2) Whale
(3) Shark
(4)
Frog
fuEufy f[kr t arqv ksaesalsfd l ,d esanksvy x ifjlap kjh iFk gksrsgSa\
(1) fNid yh
(2) s
y
(3) 'kkd Z
esa<d
(4)
Ans
Sol.
(2)
Whale is a mammal and has 4 chambered heart with 2 atria & 2 ventricles, oxygenated & deoxygenated blood
flow on separate sides.
33.
Flowers are unisexual in:
(1) Cucumber
(2) China rose
(3) Onion
(4) Pea
fd lesaiq"i ,ad fy axh gksrsgSa\
(1) [khjk
(2) pk;ukjks
t
(3) I;kt
(4) eVj
Ans
34.
Ans
35.
(1)
Which one of the follownig fruits is parthenocarpic?
(1) Apple
(2) Jackfruit
(3) Banana
(4) Brinjal
fuEufy f[kr esalsd kSu lk Q y vfu"ksd Q y gS
(1) ls
c
(2) d Vgy
(4) cS
axu
(3) d s
yk
(3)
A pleiotropic gene:
(1) is a gene evolved during Pliocene.
(2) controls a trait only in combinatio with another gene
(3) controls multiple traits in an individual.
(4) is expressed only in primitive plants
,d cgq
izHkkfor t hu%
(1) vR;Ur uw
ru d ky esafod flr gqv k t hu
(2) vU; t hu lsla
;ksft r gksd j d soy ,d y {k.k d h fu;fU=kr d jrk gSA
(3) ,d O;f"V es
acgqfo/k y {k.kksad ksfu;fU=kr d jr gSA
(4) d s
oy vn~; ikniksaesavfHkO;f gksrk gSA
Ans
36.
(3)
Which of the following is not a function of the skeletal system?
(1) Storage of minerals
(2) Production of body heat
(3) Locomotion
(4) Production of erythrocytes
fuEufy f[kr esalsd kSu&lk d ad ky &ra=k d k;Zugh gSa&
(1) [kfut ks
ad kHkaMkj.k
(2) ns
g& "ek d k mRiknu
(3) la
py u
(4) j k.kq
v ksad kmRiknu
Ans
(2)
Page || 11
AIPMT-2015 |
37.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
A jawless fish, which lays eggs in fresh water and whose ammocoetes larvae after metamorphosis retun to the
ocean is:
(1) myxine
(2) Neomyxine
(3) Petormyzon
(4) Eptatretus
t cM+kghu eNy h]t ksviusvaM svy o.k t y esansrh gSaft lesa,seksl hV y kjosd k;raj.k d sckn okil leqnzt krsgSa]gS
(1) feDlkbu
(2) fu;kf
sElkbu
(3) is
V
ks
ekbt kW
u
(4) ,IVkV
Vl
s
Ans
38.
(3)
Filiform apparatus is characteristic feature of:
(1) Nucellar embryo
(2) Aleurone cell
(3) Synergids
(4) Generative cell
rUrq: i mid j.k fd ld k y k{kf.kd xq.k gS\
(1) cht k.Md kf;d Hkw
.zk
(2) ,Y;w
jks
u d ks
f'kd k
(3) lgkd d ks
f'kd k,
(4) t uu d ks
f'kd k
Ans
39.
(3)
Read the different components from (a) to (d) in the list given below and tell the correct order of the components with reference to their arrangement from outer side to inner side in a woody dicot stem
(a) Secondary cortex
(b) Wood
(c) Secondary phloem
(d) Phellem
The correct order is:
(1) (a), (b), (d), (c)
(2) (d), (a), (c), (b)
(3) (d), (c), (a), (b)
(4) (c), (d), (b), (a)
uhpsnhx;h lwp hesa(a) ls(d) rd fofHk vo;oksavkSj ,d d ks"Bh; f}cht i=khrusesackgj lsHkhrj d h vmud hO;oLFkkd k
lgh e crk;sa%
(a) f}rh;d oYd q
V
(b) d k"B
(c) f}rh;d iks
"kokg
(d) d kx
lgh e gS%
Ans
(1) (a), (b), (d), (c)
(2)
(2) (d), (a), (c), (b)
(3) (d), (c), (a), (b)
(4) (c), (d), (b), (a)
Sol.
The correct sequence from outerside towards inner side in a wood dicot stem is
Phelluum Secondary cortex Secondary phloem Wood
(d)
40.
(a)
(c)
(b)
Which one of the following hormones is not involved in sugar metabolism ?
(1) Aldosterone
(2) Insulin
(3) Glucagon
(4) Cortisone
fuEufy f[kr gkWeksZuksaesalsd kSulk ,d gkWekZsu 'kd Zjk mikip; esa'kkfey ughagksrk gS\
(1) ,s
YMkLsVs
jkW
u
(2) bUlq
fyu
(3) Xy w
d SxkWu
(4) d kW
fVZ
l ks
u
Ans
Sol.
(1)
Al dosterone is secreted by adrenal cortex and is responible for regulation of Na+ & K+ levels in body
Page || 12
AIPMT-2015 |
41.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
Golden rice is a genetically modified crop plant where the incorporated gene is meant for biosynthesis of :
(1) Vitamin C
(2) Omega 3
(3) Vitamin A
(4) Vitamin B
lqugjsxksYMu pkoy ,d vkuqoaf'kd : ikarfjr Q ly h ikni gSA blesafuosf'kr t hu fd ld st Sfod la'y s"k.k d sfy , gS\
(1) foVkfeu C
(2) vks
esxk3
(3) foVkfeu A
(4) foVkfeu B
Ans
42.
(3)
Outbreeding is an important strategy of animal husbandry because it :
(1) is useful in producing purelines of animals.
(2) is useful in overcoming inbreeding depression.
(3) exposes harmful recessive genes that are eliminated by selection.
(4) helps in accumulation of superior genes.
i'kqiky u esacfg%izt uu ,d egRoiw.kZf ;kfof/k gSD;ksafd ;g %
(1) t a
rqv ksad s'kq) oa'k eksad ksmRiUu d jusesami;ksxh gSA
(2) va
r%izt uu d svolkn d ksnwj d jusesami;ksxh gSA
(3) gkfud kjd viz
Hkkoh t huksad ksvuko`r d j nsrkgS]ft Ugsap;u }kjkfu"d kflr fd ;kt k ld rkgSA
(4) cs
grj t huksad s,d =khd j.k esaenn d jrk gSA
Ans
43.
(2)
A gene showing codominance has:
(1) alleles tightly linked on the same chromosome
(2) alleles that are recessive to each other
(3) both alleles independently expressed in the heterozygote
(4) one allele dominant on the other
lg izHkkfork n'kkZusoky h t hu esaD;k gksrk gS\
(1) ;q
Xefod Yih ,d gh xq.klw=k ij d l d j lgy fXur gksrsgSaA
(2) os;q
Xefod Yih t ks,d nwl jsd sfy , vizHkkoh gksrsgSaA
(3) fo"ke ;q
Xet esanksuksa;qXefod Yih Lora=k : i lsvfHkO;Dr gksrsgSA
(4) ,d ;q
Xefod Yihnwl jsij izHkkohgksrkgSA
Ans
44.
(3)
Which one of the following hormones through synthesised elsewhere is stored and released by the master
gland ?
(1) Luteinizing hormone
(2) Prolactin
(3) Melanocyte stimulating hormone
(4) Antidiuretic hormone
fuEufy f[kr gkWeksZuksaesalsd kSulk gkWeksZu]gky kafd d gh vU; LFkku ij la'y sf"kr gksrk gS]y sfd u mld kHkaM kj.k vkSj fueksZp u
izeq[k xzafFk }kjk gksrk gS\
(1) Y;w
Vhukbft ax gkW
eksZ
u
(2) iz
ks
yS
fDVu
(3) es
y kuks
l kbV mn~nhid gkWeksZu
(4) iz
frew
=k.kgkWeks
Zu
Ans
Sol.
45.
(4)
ADH and oxytocin are secreted by hypothalamus & stored in posterior pituitary.
Increase in concentration of the toxicant at successive trophic levels is known as :
(1) Biodeterioration
(2) Biotransformation
(3) Biogeochemical
(4) Biomagnification
vkuq fed iks"kh Lrj ij fo"k d h lkanzrk c<+usd ksD;k d grsgSa\
(1) t S
o vid "kZ
.k
(2) t S
o : ikUrj.k
(3) t S
o Hkwl k;fud p
Ans
(4) t S
o vko/kZu
(4)
Page || 13
AIPMT-2015 |
46.
Ans
47.
CODE - (D)
BIOLOGY
| DATE: 25-07-2015
Industrial melanism is an example of :
(1) Natural selection
(2) Mutation
(3) Neo Lamarckism
(4) Neo Darwinism
vkS| ksfxd vfrd `".krk ,d mnkgj.k gS%
(1) iz
kd `frd oj.kd k
(2) mRifjrZ
u dk
(3) fu;ks
y Sekfd ZTe
(4) fu;ks
MkfoZ
fuTe
dk
dk
(1)
The primary dentition in human differs from permanent dentition in not having one of the following type of teeth
:
(1) Premolars
(2) Molars
(3) Incisors
(4) Canine
ekuo esaizkFkfed narfoU;kl LFkk;hnarfoU;kl lsbl ukrsfHkUu gksrkgSfd izkFkfed narfoU;kl esafuEufyf[kr d kSu lsizd kj
d snkar ughagksrs\
(1) vxz
p oZ.kd
(2) poZ
.kd
(3) d `
ard
(4) jnud
Ans
(1)
Sol.
Dental formula for milk teeth is
48.
The wheat grain has an embryo with one, large, shield-shaped cotyledon known as:
(1) Coleorrhiza
(2) Scutellum
(3) Coleoptile
(4) Epiblast
2102
so premolars are absent.
2102
xsagwd snkusesaHkzw.k esa,d cM+k <ky d svkd kj d k cht i=k gksrk gSA og D;k d gy krk gS\
(1) ew
y kad qj pksy
(2) Ld w
Vsy e
(3) iz
kad qj pksy
(4) vf/kd ks
jd
Ans
49.
(2)
The body cells in cockroach discharge their nitrogenous waste in the haemolymph mainly in the form of :
(1) Potassium urate
(2) Urea
(3) Calcium carbonate (4) Ammonia
fry psd h 'kjhj&d ksf'kd k,viusukbVkst uh vif'k"V d ksgheksfy EQ easiz/kku : i lsbl : i esaMky nsrh gSa%
(1) iks
VSf'k;e ;wjSV
(2) ;w
fj;k
(3) d S
fYl;e d kcksZ
us
V
(4) vekf
su;k
Ans
50.
(1)
Which of the following biomolecules does have phosphodiester bond ?
(1) Monosaccharides in a polysaccharide
(2) Amino acids in a polypeptide
(3) Nucleic acids in a nucleotide
(4) Fatty acids in a diglyceride
fuEufy f[kr t So v.kqv ksaesalsfd l esaQ kWLQ ksM kb,LVj ca/k gksrk gSS\
(1) ,d iks
y hlSd sjkbM esaeks
uksl Sd sjkbM
(2) ,d iks
y hisIVkbM esavehuksvEy
(3) ,d U;w
fDy ;ksVkbM esaU;wDy hd vEy
(4) ,d MkbZ
Xy hlSjkbM esaolkvEy
Ans
51.
Ans
(3)
The term linkage was coined by :
(1) T. Boveri
(2) G. Mendel
(3) W. Sutton
(4) T.H. Morgan
lgy Xurk fy ad st 'kCn fd lusiz;ksx fd ;k Fkk \
(1) Vh-cks
ojh
(2) t h-es
.My
(3) MCY;w
-lVu
(4) Vh-,p-eks
XkZu
(4)
Page || 14
AIPMT-2015 |
52.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
Which one is wrong statement ?
(1) Mucor has biflagellate zoospores
(2) Haploid endosperm is typical feature of gymnosperms
(3) Brown algae have chlorophyll a and c and fucoxanthin
(4) Archegonia are found in Bryophyta, Pteridophyta and Gymnosperms.
fuEufy f[kr esalsd kSulk d Fku xy r gS\
(1) E;w
d j esaf}d 'kkfHkd py cht k.kqgksrsgSaA
(2) vxq
f.kr Hkzw.kiks"kvuko`r chohikniksad kizk: fid y {k.kgSaA
(3) Hkw
js'kSoky ksaesai.kZgfjr a ,oac rFkk;wd kst sUFkhu gksrkgSA
(4) L=kh/kkuh]cz
k;ksQ kbVk]VsfjMksQ kbVkvkS
j vuko`khikniks
aes
aik;ht krhgSA
Ans
53.
(1)
Ectopic pregnancies are referred to as:
(1) Implantation of embryo at site other than uterus.
(2) Implantation of defective embryo in the uterus
(3) Pregnancies terminated due to hormonal imbalance.
(4) Pregnancies with genetic abnormality.
viLFkkfud lxHkZrk,t kuht krhgS%
(1) xHkkZ
'k; d svfrfjDr Hkzw.kd kfd lhvU; LFkku ij varjksZi.kA
(2) nks
"k;qDr Hkzw.kd kxHkkZ'k; esavarjksZi.kA
(3) lxHkZ
rk,t ksgkWekZsu d svlarqy u gksuslsvar gkst krh gSA
(4) lxHkZ
rk,ft uesavkuqoa
f'kd fo"kerk,gksA
Ans
54.
(1)
Most animals that live in deep oceanic waters are :
(1) secondary consumers
(2) tertiary consumers
(3) detritivores
(4) primary consumers
T;knkrj t Urqt ksxgjsleqnzh; ikuh esajgrsgSa]osgksrsgSa%
(1) ek/;fed miHkks
Drk
(2) r`
rh;d miHkksDrk
(3) vijnHkks
th
(4) iz
kFkfed miHkks
Drk
Ans
55.
Ans
Sol.
56.
(3)
Which of the following diseases is caused by a protozoan ?
(1) Influenza
(2) Babesiosis
(3) Blastomycosis
(4) Syphilis
fuEufy f[kr esalsd kSulk jksx izksVkst ksv k d sd kj.k gksrkgS?
(1) ba
yw,at k
(2) cS
cfslvkfsll
(3) CykLVks
ekbd ks
fll
(4) flQ fyl
(2)
Babesiosis is caused by sporozoan protozoon-babesia. In this disease haemoglobinuric fever occur.
In which of the following interactions both partners are adversely affected ?
(1) Predation
(2) Parasitism
(3) Mutualism
(4) Competition
fuEufy f[kr esalsfd l ikjLifjd f ;k esanksuksalaxh izfrd wy : i esaizHkkfor gksrsgSa\
(1) ijHk{k.k
(2) ijt hfork
(3) lgki
sd kfjrk
(4) Li/kkZ
Ans
(4)
Page || 15
AIPMT-2015 |
57.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
Identify the correct order of organisation of genetic material from largest to smallest :
(1) Genome, chromosome, nucleotide, gene
(2) Genome, chromosome, gene, nucleotide
(3) Chromosome, genome, nucleotide, gene
(4) Chromosome, gene, genome, nucleotide
lclscM+slsizkjaHk d jd slclsNksVsd s e esat hoksad svkuqokaf'kd inkFkZd slgh e d ksigpkfu,A
(1) t huks
e]xq.klw=k]U;wfDy ;ks
VkbM]t hu
(2) t huks
e, xq.klw=k, t hu, U;wfDy;ksVkbM
(3) xq
.klw=k, t hukse, U;wfDy ;ksVkbM , t hu
(4) xq
.klw=k, t hu, t hukse, U;wfDy;ksVkbM
Ans
58.
(2)
A colour blind man marries a woman with normal sight who has no history of colour blindness in her family.
What is the probability of their grandson being colour blind ?
(1) 1
(2) Nil
(3) 0.25
(4) 0.5
,d o.kk/kO;fDr lkekU; n`
f"V okyh,d ,s
l hefgyklsfookg d jrkgS]ft ld sifjokj d kd ksbZHkhlnL; o.kk/kughagSA bl
naifr d siksrksad so.kk/k gksusd h D;k laHkkouk gS\
(1) 1
(2) 'kw
U;
(3) 0.25
(4) 0.5
Ans
(3)
Sol.
The daughters of this couple will normal eye sight & carrier if one of the carrier daughter marries with normaleyed
man.
only 25% crand son will colourblind.
Page || 16
AIPMT-2015 |
59.
Ans
60.
CODE - (D)
BIOLOGY
| DATE: 25-07-2015
Photosynthesis the light-independent reactions take place at :
(1) Photosystem-I
(2) Photosystem-II
(3) Stromal matrix
(4) Thylakoid lumen
izd k'kla'y s"k.kesaizd k'k&Lora=kvfHkf ;k;sad gkgksrhgSa\
(1) iz
d k'kra
=k-I
(2) iz
d k'kra
=kII
(3) ihfBd k; vk/kk=kh
(4) Fkkbys
d kbWM
vod kf'kd k
(3)
In which of the following both pairs have correct combination ?
(1)
(2)
(3)
(4)
Gaseous nutrient cycle
Carbon and sulphur
Sedimentary nutrient cycle
Nitrogen and Phosphorus
Gaseous nutrient cycle
Nitrogen and sulphur
Sedimentary nutrient cycle
Carbon and Phosphorus
Gaseous nutrient cycle
Sulphur and Phosphorus
Sedimentary nutrient cycle
Carbon and Nitrogen
Gaseous nutrient cycle
Carbon and Nitrogen
Sedimentary nutrient cycle
Sulphur and Phosphorus
fuEufy f[kr esalsfd lesanksuksa;qXeksaesalgh la;kst u gS\
(1)
(2)
(3)
(4)
xS
l h; iks
"k.k p
v ol knh iks
"k.k p
d kcZ
u v kS
j l YQ j
ukbV
ks
t u v kS
j Q kLQ ks
jl
xS
l h; iks
"k.k p
v ol knh iks
"k.k p
xS
l h; iks
"k.k p
ukbV
ks
t u v kS
j l YQ j
d kcZ
u v kS
j Q kLQ ks
jl
l YQ j v kS
j Q kLQ ks
jl
v ol knh iks
"k.k p
xS
l h; iks
"k.k p
d kcZ
u v kS
j ukbV
ks
tu
d kcZ
u v kS
j ukbV
ks
tu
v ol knh iks
"k.k p
l YQ j v kS
j Q kLQ ks
jl
Ans
(4)
61.
The introduction of t-DNA into plants involves :
(1) Altering the pH of the soil, then heat shocking the plants
(2) Exposing the plants to cold for a brief period
(3) Allowing the plant roots to stand in water
(4) Infection of the plant by Agrobacterium tumefaciens
ikniksaesaVh&Mh,u, (t-DNA) d sizos'k lsD;k gksrk gSA
(1) e`
nk d spH esacny ko vkrk gSvkSj ikni esarki iz?kkr gksrk gSA
(2) ikniks
ad ksFkksM +svYid ky d sfy , 'khr esamn~Hkkflr d juk iM+rkgSA
(3) ikni ew
y ksad kst y esa[kM+sjgusnsrk gSA
(4) ikni es
a,xzkscSDVhfj;e V~;wfeQ sf'k,Ul }kjkla e.kgksrkgSA
Ans
(4)
Page || 17
AIPMT-2015 |
62.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
The wings of a bird and the wings of an insect are :
(1) analogous structures and represent convergent evolution
(2) phylogenetic structures and represent divergent evolution
(3) homologous structures and represent convergent evolution
(4) homologous structures and represent divergent evolution
i{kh d sia[k vkSj d hV d sia[k %
(1) vuq
: i lajpuk,gSavkSj lal `r fod kl d ksn'kkZrhgSaA
(2) oa
'kkoy hlajpuk,gSavkSj vilkjhfod kl d ksn'kkZrhgSaA
(3) let krh; la
jpuk,gSavkSj lal `r fod kl d ksn'kkZrhgSA
(4) let krh; la
jpuk,gSavkSj vilkjhfod kl d ksn'kkZrhgaSA
Ans
63.
(1)
Root pressure develops due to :
(1) Low osmotic potential in soil
(3) Increase in transpiration
ewy nkc fd ld h ot g lsfod flr gksrk gS\
(1) e`
nkesafuEu ijklj.khfoHko d sd kj.k
(3) ok"iks
Rlt Zu esac<+ko d sd kj.k
Ans
64.
(2) Passive absorption
(4) Active absorption
(2) fuf" ;
vo'kks"k.kd sd kj.k
(4) lf ; vo'kks
"k.kd sd kj.k
(4)
Human urine is usually acidic because :
(1) excreted plasma proteins are acidic
(2) potassium and sodium exchange generates acidity
(3) hydrogen ions are actively secreted into the filtrate.
(4) the sodium transporter exchanges one hydrogen ion for each sodium ion, in peritubular capillaries.
ekuo ew=k vkerkSj lsvEy h; gksrk gSD;ksafd %
(1) mRlft Z
r Iy kTekizksVhusavEy h; gksrhgSA
(2) iks
Vsf'k;e vkSj lksfM;e fofue; esavEy rk iSnk gkst krh gSA
(3) gkbM
kst u vk;u lf ; : i esafuL;an lsL=kfor gkst krsgSa
(4) ifjufy d kd kj d ks
f'kd kvksaesa]lksfM;e Vkal iksVZj izR;sd lksfM;e vk;u d kfofue; ,d gkbMkst u vk;u lsd j nsrkgSA
Ans
65.
(3)
A protoplast is a cell :
(1) without nucleus
(3) without cell wall
t honzO;d ,d d ksf'kd k gS:
(1) d s
Unzd jfgr
(3) d ks
f'kd kfHkfkjfgr
Ans
(2) undergoing division
(4) without plasma membrane
(2) foHkkft r
gksrhgqbZ
(4) iz
nzO; f>Yy hjfgr
(3)
Page || 18
AIPMT-2015 |
66.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
The species confined to a particular region and not found elsewhere is termed as :
(1) Alien
(2) Endemic
(3) Rare
(4) Keystone
,d fof'k"V {ks=k esalhfer jgusoky h t kfr d kst ksvU;=k ughaik;h t krh gSmlsD;k d gk t krk g\
(1) fons
'kh
(2) fo'ks
"k{ks
=kh
(3) nq
yZ
Hk
(4) d hLVks
u
Ans
67.
(2)
Select the wrong statements :
(1) W.M. Stanley showed that viruses could be crystallized
(2) The term 'contagium vivum fluidum' was coined by M.W. Bejerinek
(3) Mosaic disease in tobacco and AIDS in human being are caused by viruses
(4) The viroids were dicovered by D.J. Ivanowski
xy r d Fku d kspqfu, :
(1) MCY;w
.,e. LVS
Uy susn'kkZ;k d h fo"kk.kqf LVy h r gksld rsgSA
(2) 'contagium vivum fluidum' in ,e.MCy ;w
. fct s
fjususfn;kFkkA
(3) rEckd qes
afd ehZj jskx vkSj euq"; esa,-vkbZ-Mh-,l-fo"kk.kqv ksad s}kjk gksrk gSA
(4) fo"kk.kq
Hk]Mh-t s-bokuksoLd h}kjk[kkst sx;sFksA
Ans
(4)
68.
Axile placentation is present in
(1) Lemon
(2) Pea
LraHkh; cht k.M U;kl fd lesagksrkgS\
(1) uha
cw
(2) eVj
Ans
69.
(3) Argemaone
(4) Dianthus
(3) vkt hZ
eks
u
(4) Mkb,UFkl
(1)
A childless couple can be assisted to have a child through a technique called GIFT. The full form of this
technique is :
(1) Gamete intra fallopian transfer
(2) Gamete internal fertillization and transfer
( 3 )
Germ cell internal fallopian transfer
(4) Gemete inseminated fallopian transfer ,d fu%la
rku na
ifk
d ksGIFT uked rd uhd d st fj, cPpk izkIr d jusesaenn d h t k ld rh gSA bl rd uhd d k iwjk uke gSA
(1) va
r%Q Sy ksih ufy d k esa;qXed
d k LFkkukarj.k
(2) ;q
Xed d kvkarfjd fu"ksp u vkSj LFkkukarj.k
(3) vka
rfjd Q Sy ksihufy d kest uu d ksf'kd kd kLFkkukarj.k
(4) oh;Z
l sfpr Q Sy ksihufy d k esa;qXed d k LFkkukarj.k
Ans
70.
(1)
Destruction of the anterior horn cell of the spinal cord would result in loss of :
(1) voluntary motor impulses
(2) commissural impulses
(3) integrating impulses
(4) sensory impulses
eS: jTt qd h vxzgksuZd h d ksf'kd k,;fn u"V gkst k,rksbld sifj.kke Lo: i fd ld k y ksi gksxk\
(1) ,s
fPNd izs
jd izfrorZ
(2) la
/kk;hiz
frorZ
(3) lekos
'khbaVhxzsfVaxizfrorZ
(4) la
os
nhizfrorZ
Ans
Sol.
(1)
In poliomyelitis, anterior horn cells of spinal cord are destroyed which result in loss of motor activities of limbs.
Page || 19
AIPMT-2015 |
71.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
During biological nitrogen fixation, inactivation of nitrogenase by oxygen poisoning is prevented by :
(1) Xanthophyll
(2) Carotene
(3) Cytochrome
(4) Leghemoglobin
ukbVkst u fLFkfjd j.kd snkSjku vkDlht u d sfo"kSy sizHkko d ksukbVkst u d kfuf" ;d j.kfd l }kjkjksd kt krkgS\
(1) t U
SFkfsQ y
(2) d S
jks
fVu
(3) lkbVk
s ke
(4) y s
XgheksXy kschu
Ans
72.
(4)
An association of individuals of different species living in the same habital and having functional interactions is:
(1) Biotic community
(2) Ecosystem
(3) Population
(4) Ecological niche
,d ghi;kZokl esajg jghfofHkUu Lizh'kht ksd hO;f"V;ksad kikjEifjd lac/k vkSj f ;kRed f ;kd jkukgS:
(1) t hoh; leq
n;k
(2) ikfjra
=k
(3) lef"V
(4) ikfjfLFkfrd fud s
r
Ans
(1)
73.
Name the pulmonary disease in which alveolar surface area involved in gas exchange is drastically reduced
due to damage in the alveolar walls.
(1) Emphysema
(2) Pneumonia
(3) Asthma
(4) Pleurisy
ml Q qQ ql hjksx d k uke crkb, ft lesad wfid h; fHkfk;ksad s{k; gkst kusd sd kj.kxSl &fofue; esa'kkfey d wfid h; lrgh
{ks=k cgqr vf/kd d e gkst krk gSA
(1) okrLQ hfr
(2) U;w
eks
fu;k
(3) vLFkek
(4) Iyw
fjlh
Ans
74.
(1)
Balbiani rings are sites of :
(1) Nucleotide synthesis
(3) RNA and protein synthesis
(2) Polysaccharide synthesis
(4) Lipid synthesis
cfYc;kuh oy ; LFky gS:
(1) U;fDy vks
VkbM la'y s
"k.kd s
(3) RNA vkS
j izksVhu la'y s
"k.kd s
(2) iks
y hlSd SjkbM
la'ys"k.kd s
(4) fy fiM la
'y s"k.kd s
Ans
(3)
75.
Mathe the columns and identify the correct option.
Column-I
Column-II
(a) Thylakoids
(i) Disc-shaped sacs in Golgi apparatus
(b) Cristae
(ii) Condensed structure of DNA
(c) Cisternae
(iii) Flat membranous sacs in stroma
(d) Chromatin
(iv) Infoldings in mitochondria
d kWy eksad schp fey ku d hft , vkSj lgh fod Yi pqfu,
d kWy e-I
(a) Fkk;ykW
d kW
bM
(b) f LVh
(c) flLVuhZ
(d) ks
eS
fVu
Ans
(1)
(2)
(3)
(4)
(1)
(iii)
(iii)
(iii)
(iv)
(iv)
(i)
(iv)
(iii)
(i)
(iv)
(ii)
(i)
d kWy e-II
(i) xkW
Yt hmid j.kd sfMLd uqekd ks"k
(ii) DNA d hla
?kfur lajpuk
(iii) LV
ksek esapiVsf>Yy he; d ks"k
(iv) ekbVks
d kW
fUM
;kes
avaroZ
yu
(ii)
(ii)
(i)
(ii)
Page || 20
AIPMT-2015 |
76.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
Cellular organelles with membranes are :
(1) chormosomes, ribosomes and endoplasmic reticulum
(2) endoplasmic reticulum, ribosomes and nuclei
(3) lysosomes, Golgi apparatus and mitochondria
(4) nuclei, ribosomes and mitochondria
f>Yy h;qDr d ksf'kd h; vaxd gS:
(1) xq
.klw=k, jkbcksl ks
e vkSj ,a
M ks
Iy kfLed jsfVd q
ye
(2) ,a
MksIykfLed js
fVd q
y e, jkbcks
l ks
e vkSj d sUnzd
(3) y k;lks
l ks
e, xkWYt hmid j.kvkS
j ekbVksd kW
fUM
;k
(4) d s
Unzd , jkbcks
l ks
e vkS
j ekbVks
d kW
fUM;k
Ans
77.
(3)
Auxin can be bioassayed by :
(1) Hydroponics
(3) Lettuce hypocotyl elongation
vkDt hu d k fd ld s}kjk t So fd ;k t k ld rk gS\
(1) t y loa
o/kZu ls
(3) y s
V~;wl cht i=kk/kkj d sy Ecu ls
Ans
78.
(2) Potometer
(4) Avena coleoptile curvature
(2) iks
Vks
ehVj ls
(4) ,ohuk iz
kd qj pksy
d so .k ls
(4)
Which of the following layers in an antral follicle is acelluar ?
(1) Theca interna
(2) Stroma
(3) Zona pellucida
(4) Granulosa
,UVeh ?kqVd esfufEufy f[kr esalsd kSulh vd ksf'kd h; gksrh gS\
(1) Fkhd kbZ
Vjuk(va
rj izkojd )
(2) LV
ksek(ihfBd k)
(3) t ks
ukis
Y;q
flMk(ijn'khZva
Mkoj.k)
(4) xS
zuq
y ks
l k(d f.kd h;)
Ans
Sol.
79.
(3)
Zona pellucida is secreted by secondary oocyte around it self
Satelliete DNA in important because it :
(1) shows high degree of polymorphism in population and also the same degree of polymorphism in an individual, which is heritable form parents to children.
(2) does not code for proteins and is same in all members of the population
(3) codes for enzymes needed for DNA replication
(4) codes for proteins needed in cell cycle.
vuq"kaxh DNA egoiw.kZgksrk gSD;ksafd ;g :
(1) lef"V es
amPp d ks
fV d hcgq
: irkvkS
j lkFkgh,d O;fDr es
amruhghd ks
fV d hcgq
: irkiz
nf'kZr d jrkgSft ld hoa
'kkxfr
t ud ksalscPpksard gksld rh gSA
(2) iz
ksVhuksad sfy , d ksM u ughad jrk vkSj lef"V d slHkh lnL;ksaesa,sl k gksrk gSA
(3) mu ,a
t kbekasd sfy , d ksM u d jrk gSft ud k DNA d sizfr rh;u d sfy , t : jr gksrh gSA
(4) mu iz
ksVhuksad sfy , d ksM u d jrk gSft ud h d ksf'kd k p d sfy , t : jr gksrh gSA
Ans
(1)
Page || 21
AIPMT-2015 |
80.
Cell wall is absent in :
(1) Funaria
(2) Mycoplasma
fd lesad ksf'kd kfHkfkd kvHkko gksrkgS\
(1) ;q
us
fj;k
(2) ekb ks
IykTek
Ans
81.
CODE - (D)
| DATE: 25-07-2015
(3) Nostoc
(4) Aspergillus
(3) uks
LVkd
(4) ,Lift yl
BIOLOGY
(2)
In angiosperms, microsporogenesis and megasporogenesis :
(1) form gametes without further divisions
(2) Involve meiosis
(3) occur in ovule
(4) occur in anther
vko`r cht hikniksaesay ?kqcht k.kqt uu vkSj xq: cht k.kqt uu:
(1) fcuk vxzfoHkkt u d s;q
Xed cukrsgSA
(2) v}Zlw
=kfoHkkt u }kjkgksrsgSA
(3) cht k.M es
agksrkgSA
(4) ijkxd ks
"kesagksrhgSA
Ans
Sol.
82.
(2)
Each microspore mother cell of anther undergoes meiosis to form microspore tetrad while megspore moter
cell of ovule undergoes meiosis to form megaspore tetrad.
Roots play insignificant role in absorption of water in :
(1) Pistia
(2) Pea
(3) Wheat
(4) Sunflower
fd lesat M+st y 'kks"k.k esaux.; d k;Zd jrh gS\
(1) fifLr;k
(2) eVj
(3) xs
gw
(4) lw
;Z
eq
[kh
Ans
Sol.
(1)
Pistia is hydrophyte where absorption of water by root is insignificant
83.
Which of the following are most suitable indicators of SO2 pollution in the environment?
(1) Conifers
(2) Algae
(3) Fungi
fufEufy f[kr esalsd kSu ,d i;kZoj.k esaSO2 iznw"k.k d k lcls;ksX; lad srd gS?
(1) 'kd
aq
/kkjh
(2) 'kS
oky
(3) d od
Ans
84.
(4) Lichens
(4) ykbd s
u
(4)
Grafted kidney may be rejected in a patient due to :
(1) Cell-midiated immune response
(2) Passive immune responce
(3) Innate immune responce
(4) Humoral immune responce
fd lhjksxksesaizR;kjksfir o`Dd fd Muhd ksvLohd kj fd l d kj.kfd ;kt kld rkgS\
(1) d ks
f'kd kekf/;r izfrj{kkvuqf ;k
(2) fuf" ; iz
frj{kkvuq
f ;k
(3) lgt iz
frj{kkvuq
f ;k
(4) f=knks
'kt g~;w
eks
jy izfrj{kkvuq
f ;k
Ans
85.
(1)
Body having meshwork of cell, internal cavities lined with food filtering flagellated cells and indirect development are the characteristics of phylum.
'kjhj esa
d ksf'kd kvksad kt y gks
uk][kkn~; inkFkZd kfuL;a
nu d jusokyhd 'kkfHkd ke; d ksf'kd kvksalsvLrfjr vkarfjd xqgkvksad kik;k
t kuk]rFkkvizR;{k ifjo/kZu d kgksukfd l Q kby e d hfof'k"Vrk,gS\
(1) ikW
fjQ s
jk
(2) eks
y Ld k
(3) iz
kVsks
t ks
vk
(4) lks
ys
UVjs
Vk
(1) Porifera
(2) Mollusca
(3) Protozoa
(4) Coelenterate
Page || 22
AIPMT-2015 |
Ans
86.
CODE - (D)
| DATE: 25-07-2015
BIOLOGY
(1)
In which group of organisms the cell walls form two thin overlapping shells which fit together?
(1) Euglenoids
(2) Dinoflagellates
(3) Slime moulds
(4) Chrysopytes
t hoksaesfd l lewg esad ksf'kd k fHfk nksiry h vfrO;kih d opksad h cuh gksrh gSt ks,d lkFk vklaft r gksrh gS\
(1) ;w
XyhukabM
(2) Mk;us
y Sft ysV
(3) voia
d d od
(4) kblks
Q kbV
Ans
Sol.
87.
(4)
Diatoms (chrysophytes) body is look like soap box and fit together.
Choose the wrong statements:
(1) Neurospora is used in the study of biochemical genetics
(2) Morels and truffles are poisonoues mushrooms
(3) Yeast is unicellular and useful in fermentation
(4) Penicillium is multicellular and produces antibiotics
xy r d Fku d kspqfu,%
(1) U;w
jksLiksl d kst Sojlk;u vuqokaf'kd hd sv/;;u esami;ksx fd ;kt krkgSA
(2) ekW
jsy vkSj VwQ sy fo"kSy sN=kd gSA
(3) ;hLV ,d d ks
f'kd h; gSvkSj fd .ou esami;ksxh gSA
(4) iS
fulhfy ;e cgqd ksf'kd h; gSvkSj iz
frt Sfod mRikfnr d jrkgSA
Ans
Sol.
88.
Ans
89.
(2)
Morel and truffles are edible and members of Ascomycetes in fungi
In human females, meiosis-II in not complete until?
(1) fertilization
(2) uterine implantation (3) birth
(4) puberty
ekuo eknkvksaesa]v/kZl w=khfoHkkt u-II fd ld siw.kZgkst kusij ghgksrkgS\
(1) fu"ks
pu
(2) xHkkZ
'k; esavr%LFkkiu (3) t Eu
(4) ;ko
Sukja
Hk
(1)
Eutrophication of water bodies leading to killing of fishes is mainly due to non-availability of :
(1) light
(2) essential minerals
(3) oxygen
(4) food
t y h; fud k;ksad k ;wVksfQ d s'ku ft ld sd kj.k eNfy ;kejusy xrh gS]fd ld h miy C/krk u gksusd sd kj.k gksrk gS\
(1) iz
d k'k
(2) vko';d [kfut
(3) vkW
fDlt u
(4) Hkks
tu
Ans
90.
(3)
The enzyme that is not present in succus Entericus is :
(1) nucleases
(2) nucleosidase
(3) lipase
cg ,at kbe t ksld l ,aVsfjd l (vka=k jl ) esaekSt wn ughagksrk\
(1) U;w
fDy ,st
(2) U;w
fDyvkfslMs
t
(3) ykbis
t
Ans
(4) maltase
(4) ekYVs
t
(1)
Page || 23
AIPMT-2015 |
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
PART B : CHEMISTRY
91.
Reaction of phenol with chloroform in presence of dilute sodium hydroxide finally introduces which one of the
following functional group ?
fQ ukW
y d h f ;k Dyks
jks
Q kW
e d slkFk ruqNaOH es
ad jokusij fuEufyf[kr es
alsva
rr%d kS
u&lkf ;kRed lew
g yxrkgS\
(1) CH2Cl
(2) COOH
(3) CHCl2
(4) CHO
Ans.
(4)
Sol.
dil.NaOH
Ph OH + CHCl3
92.
If the equilibrium constant for N 2(g) + O 2 (g)
2NO(g) is K, the equilibrium constant for
>
It is Reimer - Tiemann reaction
1
1
N2(g) + O2(g)
NO(g) will be :
2
2
1
1
;fn N2(g) + O2(g)
d K gS]rc N2(g) + O2(g)
2NO(g) d k lkE;koLFkk fLFkjka
NO(g) d k lkE;koLFkk
2
fLFkjkad gksxk:
(1) K 12
(2)
Ans.
(1)
Sol.
[NO]2
K=
[N2 ][O2 ]
1
K
2
(3) K
(4) K2
NO
K' =
1/ 2
[N2 ]
[O2 ]1/ 2
K' K
Page || 24
AIPMT-2015 |
93.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample ?
(XI)
20.0 g eS
Xuhf'k;e
d kcksZusV d suewusd ksxeZd jusij vi?kfVr gksd j d kcZu MkbZvkWDlkbM ,ao 8.0 g eSXuhf'k;e vkWDlkbM
nsrk gSA uewusesaeSXuhf'k;e d h 'kq) rk d k izfr'kr D;k gksxk \
(1) 75
(2) 96
(3) 60
(4) 84
(At. Wt.: Mg = 24)
Ans.
(4)
Sol.
MgCO3 MgO + CO2
84 g
40g
8g MgO will be from
% Purity =
94.
84
g
5
84 100
84%
5
20
The number of water molecules is maximum in :
(1) 18 molecules of water
(2) 1.8 gram of water
(3) 18 gram of water
(4) 18 moles of water
t y v.kqv ksad h vf/kd re la[;k gS%
(1) ikuhd s18 v.kq
v ksaesa
(3) 18 xz
ke ikuh esa
(2) 1.8 xz
ke
Ans.
(4)
Sol.
1 mole contains 6.021023 molecule
ikuh esa
(4) 18 eks
y ikuh esa
18 mole will contain 186.021023
Page || 25
AIPMT-2015 |
95.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
The formation of the oxide ion O2 (g), from oxygen atom requires first an exothermic and then an endothermic
step as shown below :
O(g) + e O(g) ;
= 141 kJ mol1
O (g) + e O2(g) ;
= +780 kJ mol1
Thus process of formation of O2 in gas phase is unfavourable even though O2 is isoelectronic with neon. It is
due to the fact that
(1) Electron repulsion outweighs the stability gained by achieving noble gas configuration
(2) O ion has comparatively smaller size than oxygen atom
(3) Oxygen is more electronegative
(4) Addition of electron in oxygen results in larger size of the ion.
vkW
DlkbM vk;u O2 (g), d k vkW
Dlht u ijek.kqlscuusd sfy;sigys "ek{ks
ih ,oackn es
a "ek'kks
"kh in uhpsfn;sx;sgS
a%
O(g) + e O(g) ;
= 141 kJ mol1
O (g) + e O2(g) ;
= +780 kJ mol1
xSl h; voLFkk esaO2 d k cuuk izfrd qy gS;|fi O2 fuvkWu d k leby sDVkWuh gSA ;g fd l rF; d sd kj.k gS\
(1) uks
cy xSl d sfoU;kl izkfIr d sd kj.kLFkk;hRo ls]by sDVkWu izfrd "kZ.kizHkko'kky hgksrkgSaA
(2) O vk;u d k vkd kj vkW
Dlht u ijek.kqd h rqy uk esaNksVk gksrk gSA
(3) vkW
Dlht u T;knkoS| qr _ .kkRed gSA
(4) vkW
Dlht u esaby sDVkWu d st ksM +lsvk;u d k vkd kj cM+k gksrk gSA
Ans.
(1)
96.
What is the mole fraction of the solute in a 1.00 m aqueous solution ?
1.00 m t y h;
foy ;u esfoy s; d h eksy va'k gS%
(1) 0.177
Ans.
(4)
Sol.
Molality =
97.
(2) 1.770
(3) 0.0354
(4) 0.0177
1000 n
1000 n
n
18
1
N M
N 18 = N 1000
n
18
0.0177
n N 1018
The rate constant of the reaction A B is 0.6 103 mole per second. If the concentration of A is 5 M then
concentration of B after 20 minutes is :
vfHkf ;kA B d sfy , osx fLFkjkad 0.6 103 eksy izfr lSd .M gSA ;fn A d hlkUnzrk5 M gSrks20 fefuV i'pkr B d h
lkUnzrk gS%
(1) 1.08 M
(2) 3.60 M
Ans.
(4)
Sol.
con.
It is zero order reaction 6104 = 20 60
(3) 0.36 M
(4) 0.72 M
conc.of B 0.72M
Page || 26
AIPMT-2015 |
98.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
Decreasing order of stability of O2 ,O2 ,O2 and O2
is :
2
O2 ,O2 ,O2
rFkk O2
d sLFkk;hRo d k ?kVrk gqv k e gS%
2
(1) O2 O2 O 2 O22
(2) O22 O2 O2 O 2
(3) O2 O 2 O 22 O2
(4) O2 O22 O2 O 2
Ans.
(1)
Sol.
B.O.
99.
Which one of the following esters gets hydrolysed most easily under alkaline conditions ?
O2 O2 O 2 O22
2.5
1.5 1.0
fuEufy f[kr esalsd kSu&lk ,LVj {kkjh; n'kk esalclsljy rk lst y vi?kfVr gksrk gS\
(1)
(2)
(3)
(4)
Ans.
(1)
Sol.
In basic medium rate of hydrolysis increases with electron withdrawing group (-M effect predominates)
100.
On heating which of the following releases CO2 most easily ?
fuEu esalsfd ld ksxeZd jusij CO2 lokZf/kd vklkuh lsmRlft Zr gksxh \
(1) K2CO3
(2) Na2CO3
(3) MgCO3
Ans.
(3)
Sol.
BeCO3 to BaCO3 stability increases
101.
Which one of the following pairs of solution is not an acidic buffer ?
(4) CaCO3
fuEufy f[kr foy ;uksad s;qXeksaesalsd kSu&lk vEy h; cQ j ughagS\
(1) HClO4 and ,oaNaClO4
(2) CH3COOH and ,oaCH3COONa
(3) H2CO3 and ,oaNa2CO3
(4) H3PO4 and ,oaNa3PO4
Ans.
(1)
Sol.
Stong acid with its salt can not form duffer solution.
Page || 27
AIPMT-2015 |
102.
CODE - (D)
CHEMISTRY
| DATE: 25-07-2015
The sum of coordination number and oxidation number of the metal M in the complex [M(en)2(C2O4)]Cl (where
en is ethylenediamine) is :
lad qy [M(en)2(C2O4)]Cl (t gken bZfFky huMkb,sehu gS) es
a/kkrqM d hmilgla;ks
t u la[;k,ao vkW
Dlhd j.kla[;kd k;ksx
gS%
(1) 9
(2) 6
Ans.
(1)
Sol.
C.No. = 6
(3) 7
(4) 8
O.No. = +3
103.
Which of the statements given below is incorrect ?
(1) Cl2O7 is an anhydride of perchloric acid
(2) O3 molecule is bent
(3) ONF is isoelectronic with O2N
(4) OF2 is an oxide of fluorine
uhpsfn;sd Fkuksaesalsd kSu&lk xy r gS\
(1) Cl2O7 ijDyks
jhd vEy d k,uMkbMkbZM gSA
(3) ONF leby s
DV
kWuhgSO2N d slkFk
(2) O3 v.kqeq
M +kgqv kgSA
(4) OF2 y ks
jhu
d k vkWDlkbM gSA
Ans.
(4)
Sol.
OF2 is oxygen difluoride.
104.
In the reaction with HCl, an alkene reacts in accordance with the Markovnikov's rule, to give a product 1-chloro1-methylcyclohexane. The possible alkene is :
,d ,Yd hu HCl lsvfHkf ;kd jd sekjd ks
uhd kWQ fu;e d svuql kj mRikn 1-Dy ks
jks-1-esfFky lkbDy ks
gsDls
u nsrkgSA laHkkfor
,Yd hu gS%
(1)
Ans.
Sol.
(A)
(2)
(B)
(3) (A) and vkS
j (B)
(4)
(3)
HCl
HCl
Cl
Cl
Page || 28
AIPMT-2015 |
105.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
2,3-Dimethyl-2-butene can be prepared by heating which of the following compounds with a strong acid ?
fuEufy f[kr esalsfd l ;kSfxd d ksizcy vEy d slkFk xeZd jusij 2,3-MkbZesfFky -2-C;wVhu d kscuk;k t k ld rk gS\
Ans.
(1)
(2) (CH3)3C CH = CH2
(3) (CH3)2C = CH CH2 CH3
(4) (CH3)2CH CH2 CH = CH2
(2)
Sol.
106.
The following reaction
is known by the name :
(1) Friedel-Craft's reaction
(2) Perkin's reaction
(3) Acetylation reaction
(4) Schotten-Baumen reaction
fuEu vfHkf ;kThe following reaction
fd l uke lst kuh t krh gS\
(1) hMs
y & kV vfHkf ;k
(3) ,s
l hVkby s
'ku ,s
fly uvfHkf ;k
(2) ifd u
Z vfHkf ;k
(4) 'kkW
Vu&ckeu
vfHkf ;k
Ans.
(4)
Sol.
Name reaction
107.
In the extraction of copper from its sulphide ore, the metal finally obtained by the reduction of cuprous oxide
with :
(1) iron (II) sulphide
(2) carbon monoxide
(3) copper (I) sulphide
(4) sulphur dioxide
lYQ kbM v;Ld ks
aes
alsd kW
ij d sfu"d "kZ
.kes
a/kkrqd ksizkIr d jusd sfy;svarr%D;w
iz
l vkW
DlkbM d kvip;u fd ld slkFkgksrk
gS\
(1) vkbju (II) lYQ kbM
(2) d kcZ
u eksuksDlkbM
(3) d kW
ij (I) lYQ kbM
(4) lYQ j MkbZ
v kW
DlkbM
Ans.
(3)
Page || 29
AIPMT-2015 |
108.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
If Avogadro number NA, is changed from 6.022 1023 mol1 to 6.022 1020 mol1 this would change :
(1) the definition of mass in units of grams
(2*) the mass of one mole of carbon
(3) the ration of chemical species to each other in a balanced equation.
(4) the ratio of elements to each other in a compound
;fn vkokxknzksla[;k NA, 6.022 1023 mol1 lsifjofrZr gksd j 6.022 1020 mol1 gksrk gSrksbllsifjorZu gksxk :
(1) nz
O;eku
d h ifjHkk"kk g ;wfuV esaA
eksy d kcZu d k nzO;ekuA
(3) la
rqfy r lehd j.kesaijLij lek;fud Lih'kht d kvuqikrA
(4) ;kS
fxd esaijLij rRoksad kvuqikrA
(2) ,d
109.
The variation of the boiling point of the hydrogen halides is in the order HF > HI > HBr > HCl.
What explains the higher boiling point of hydrogen fluoride ?
(1) The electronegativity of fluorine is much higher than for other elements in the group.
(2) There is strong hydrogen bonding between HF molecules
(3) The bond energy of HF molecules is greater than in other hydrogen halides.
(4) The effect of nuclear shielding is much reduced in fluorine which polarises the HF molecule.
gkbMkst u gSy kbMksad sDoFkukad esaifjorZu d k e fuEu gSHF > HI > HBr > HCl
gkbMkst u y ksjkbM d smPpre DoFkukad d h O;k[;k D;k gS\
(1) y ks
jhu d h oS| qr _ .kkRed rk lewg d snwl jsrRoksalscgqr vf/kd gksrh gSA
(2) HF v.kq
v ksaesagkbMkst u vkca/kvf/kd gksrkgSA
(3) HF v.kqd hvkca
/k t kZnwl jsgkbMkst u gSy kbMksalsvf/kd gSA
(4) y ks
jhu esaukfHkd h; ifjj{k.k izHkko cgqr d e gksrk gSt ksfd HF v.kqd ks/kqzfor d jrk gSA
Ans.
(2)
110.
Which of the following reaction (s) can be used for the preparation of alkyl halideds ?
anh. ZnCl2
(I) CH3CH2OH + HCl
(II) CH3CH2OH + HCl
(III) (CH3)3COH + HCl
anh. ZnCl2
(IV) (CH3)2CHOH + HCl
(1) (I), (III) and (IV) only
(3) (IV) only
(2) (I) and (II) only
(4) (III) and (IV) only
fuEufy f[kr esalsd kSulh vfHkf ;ka@ vfHkf ;k,a,sfYd y gSy kbM d sfojpu esami;ksx esay k ld rsgSa?
fut Zy ZnCl2
(I) CH3CH2OH + HCl
(II) CH3CH2OH + HCl
(III) (CH3)3COH + HCl
fut Zy ZnCl2
(IV) (CH3)2CHOH + HCl
Ans.
Sol.
(1) d s
oy (I), (III) rFkk(IV)
(2) d s
oy (I) rFkk(II)
(3) d s
oy (IV)
(1)
Lucas reagent in I & IV while SN1 in III
(4) d s
oy (III) rFkk(IV)
Page || 30
AIPMT-2015 |
111.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
The name of complex ion, [Fe(CN)6]3 is :
(1) Hexacyanoiron (III) ion
(2) Hexacyanitoferrate (III) ion
(3) Tricyanoferrate (III) ion
(4) Hexacyanidoferrate (III) ion
lad qy vk;u [Fe(CN)6]3 d k uke gS:
(1) gs
Dlklk;uks
v k;ju (III) vk;u
(2) gs
DlkbfuVks
Qs
js
V (III) vk;u
(3) V
kbZ
l k;uks
Qs
js
V (III) vk;u
(4*) gs
Dlklk;ukbMks
Qs
js
V (III) vk;u
Ans.
(4)
112.
Assuming complete ionization, same moles of which of the following compounds will require the least amount
of acidified KMnO4 for complete oxidation
iw.kZvk;uhd j.kd ksekursgq, fuEufy f[kr esalsd kSuls;kSfxd d siw.kZvkWDlhd j.kesalclsd e ek=kkesavEy h; KMnO4 d h
vko';d rkgksxh \
Ans.
(1*) FeSO4
(1)
(2) FeSO3
(3) FeC2O4
113.
In which of the following pairs, both the species are not isostructural ?
(4) Fe(NO2)2
fuEufy f[kr ;qXeksaesalsd kSulh nksuksaLih'kht lelajpukRed ughagS\
Ans.
114.
Ans.
Sol.
115.
Ans.
116.
(1) SiCl4, PCl4
(2) diamond, silicon carbide (ghjk]flfy d kW
u
(3) NH3, PH3
(4)
(4*) XeF4, XeO4
d kckZbM )
Caprolactum is used for the manufacture of :
(1) Nylon - 6
(2) Teflon
(4) Nylon - 6,6
d Sizksy SDVe d k mi;ksx fuEu esalsfd ld smRikn
(1) ukby kW
u-6
(2) Vs
Q y kWu
(4) ukby kW
u - 6,6
(3) Terylene
esagksrk gS:
(3) Vs
fjy hu
(1)
Caprolactum is used for the manufacturing of Nylon-6
The hybridization involved in complex [Ni(CN)4]2 is (At. No. Ni = 28)
lad qy [Ni(CN)4]2 esalad j.k gS% (i-la-Ni = 28)
(1) dsp2
(2) sp3
(3) d2sp2
(1)
(4) d2sp3
What is the mass of precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8%
NaCl solution ?
t c 50 mL , 16.9% AgNO3 d sfoy ;u d ks50 mL ,5.8% NaCl d sfoy ;u d slkFk fefJr fd ;k t krk gSrkscuusoky s
vo{ksi d k Hkkj D;k gS?
Ans.
117.
(Ag = 107.8, N = 14, O = 16, Na = 23, Cl = 35.5)
(1) 28 g
(2) 3.5 g
(3) 7 g
(3)
(4) 14 g
Gadolinium belongs to 4f series. It's atomic number is 64. Which of the following is the correct electronic
configuration of gadolinium ?
(XI)
xSM ksfy fu;e 4f JS.khlslacaf/kr gSA bld hijek.kqla[;k64 gSA fuEufy f[kr esalsxSaM ksfy fu;e d kd kSulklghby sDVksfud
foU;kl gS?
Ans.
(1) [Xe]4f86d2
(3)
(2) [Xe]4f 95s 1
(3) [Xe] 4f 75d 16s 2
(4) [Xe] 4f 65d 26s 2
Page || 31
AIPMT-2015 |
118.
| DATE: 25-07-2015
Which of the following is not the product of dehydration of
fuEufy f[kr esalsd kSulk mRikn]
(1)
Ans.
CODE - (D)
CHEMISTRY
d sfut Zy hd j.k d k ughagS
(2)
(3)
(4)
(2)
H O
2
* According to question (2) option is
probable answer
119.
A gas such as carbon monoxide would be most likely to obey the ideal gas law at :
(1) high temperatures and low pressures.
(2) low temperatures and high pressures.
(3) high temperatures and low pressures.
(4) low temperatures and low pressures.
,d xSl t Sl sd kcZu eksuksDlkbM vkn'kZxSl fu;e d k iky u fd l n'kk esad jsxk :
(1) mPp
rkiksa,oafuEc nkcksaij
(2) fuEu rkiks
a,oamPp nkcksaij
(3) mPp rkiks
a,oamPp nkcksaij
(4) fuEu rkiks
a,oafuEu nkcksaij
Ans.
(1)
120.
The stability of +1 oxidation state among Al, Ga, In and Tl increases in the sequence :
+1 vkW
Dlhd j.k voLFkk d k LFkkf;Ro Al, Ga, In ,oaTl esavuq e esac<+rk gS:
(1) Ga < In < Al < Tl
(2*) Al < Ga < In < Tl
(3) Tl < In < Ga < Al
(3) In < Tl < Ga < Al
(2)
Ans.
121.
Ans.
What is the pH of the resulting solution when equal volumes of 0.1 M NaOH and 0.01 M HCl are mixed ?
0.1 M NaOH ,oa0.01 M HCl d sleku vk;ru d ksfefJr d jusij cuusoky sfoy ;u d h pH D;k gS?
(XI)
(1*) 12.65
(2) 2.0
(3) 7.0
(4) 1.04
(1)
Page || 32
AIPMT-2015 |
122.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
Strong reducing behaviour of H3PO4 is due to :
(1) Presence of one OH group and two PH bonds
(2) High electron gain enthalpy of phosphourus
(3) High oxidation state of phosphorus
(4) Presence of two OH groups and one PH bond.
H3PO4 d siz
cy vipk;d xq.k d k d kj.k gS:
(1) ,d OH lew
g
,oanksPH vkca/kksad hmifLFkfr
d hmPp by sDVkWu xzkgh,sUFkSYihls
(3) Q kW
LQ ksjl d hmPp vkWDlhd j.kvoLFkk
(4) nksOH lew
gksa,oa,d PH vkca/kd h mifLFkfr
(2) Q kW
LQ ksjl
Ans.
(1)
123.
The number of structural isomers possible from the molecular formula C3H9N is :
v.kql w=k C3H9N lscuusoky slaHkkfor lajpukRed leko;oh;ksad h la[;kgS:
(1) 4
(2) 5
(3) 2
(4) 3
(1)
Structure isomers of C3H9N are
Ans.
Sol.
CH3CH2CH2NH2 ,
124.
, CH3NHCH2CH3 ,
Total = 4
Which of the following statements is not correct for a nucleophile ?
(1) Nucleophile is a Lewis acid
(2) Ammonia is a nucleophile
(3) Nucleophiles attack low e density sites
(4) Nucleophiles are not electron seeking.
fuEufy f[kr esalsd kSulk d Fku ukfHkd Lusgh d sfy ;slgh ughagS?
(1) ukfHkd Lus
ghy qbZl
vEy gSA
(2) veks
fu;k,d ukfHkd LusghgSA
(3) ukfHkd Lus
gh d e by sDVkWu ?kuRo LFkku ij vk e.k d jrk gSA
(4) ukfHkd Lus
gh by sDVkWu d h ry k'k easughajgrk gSA
Ans.
(1)
125.
Number of possible isomers for the complex [Co(en)2Cl2]Cl will be
(en = ethylenediamine)
lad qy [Co(en)2Cl2]Cl d sla
Hkkfor leko;oh;ksad hla[;kgks
xh
Ans.
(en = bZ
fFkyhuMkbZ
,ehu)
(1) 2
(3)
126.
Which is the correct order of increasing energy of the listed orbitals in the atom of titanium ?
(2) 1
(3) 3
(4) 4
VkbZVsfu;e ijek.kqd sfn;sx;sd {kd ksad h t kZd k c<+rk gqv k lgh e d kSu lk gS\
Ans.
(1) 3s 4s 3p 3d
(3)
(2) 4s 3s 3p 3d
(3) 3s 3p 3d 4s
(4) 3s 3p 4s 3d
Page || 33
AIPMT-2015 |
127.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
In an SN1 reaction on chiral centres there is :
(1) 100 % racemization
(2) inversion more than retention leading to partial racemization
(3) 100 % retention
(4) 100 % inversion
d kbjy d sUnzij SN1 vfHkf ;k esagksrk gS:
(1) 100 % js
flehd j.k
(2) /kkj.klsT;knkiz
fry kseu
d s}kjkvkaf'kd jsflehd j.k
(3) 100 % /kkj.k
Ans.
Sol.
128.
Ans.
129.
(4) 100 % iz
fry kseu
(2)
Inversion product will be more than retention product due to close ion pair formation.
The vacant space in bcc lattice cell is :
bcc t ky d ,d d d ks
f"Bd k esafjDr LFkku gksrk gS%
(1) 26 %
(2) 48 %
(4)
(3) 23 %
(4) 32 %
the heat of combustion of carbon to CO2 is 393.5 kJ/mol. The heat released upon formation of 35.2 g of CO2
from carbon and oxygen gas is
d kcZu lsd kcZu MkbZv kWDlkbM d sfy ;sngu "ek 393.5 kJ/mol. gSA d kcZu ,oavkWDlht u ls35.2 g cuusij mRlft Zr
"ek gS%
Ans.
130.
(1) 315 kJ
(1)
(2) +315kJ
(3) 630 kJ
(4) 3.15 kJ
Aqueous solution of which of the following compounds is the best conductor of electric current ?
(1) Acetic acid, C2H4O2
(2*) Hydrochloric acid, HCl
(3) Ammonia, NH3
(4) Fructose, C6H12O6
fuEu ;kSfxd ksaesalsfd ld k rt y h; foy ;u fo|qr /kkjk d k lclsvPNk lqp ky d gS\
(1) ,s
l hfVd vEy , C2H4O2
(2) gkbM
ksDyks
fjd vEy , HCl
(3) veks
fu;k, NH3
(4) DVks
l , C6H12O6
Ans.
(2)
131.
The oxidation of benzene by V2O5 in presence of air produces :
(1) benzoic anhydride
(2) maleic anhydride
(3) benzoic acid
(4) benzaldehyde
gok d h mifLFkfr esacsUt hu d k vkWDlhd j.k V2O5 d s}kjk nsrk gS:
(1) cs
Ut kW
bd
,ugkbM
kbM
(3) cs
Ut kb
Wd vEy
Ans.
(2) es
y Sbd
,ugkbMkbM
(4) cs
Ut fsYMgkbM
(2)
Page || 34
AIPMT-2015 |
132.
CODE - (D)
| DATE: 25-07-2015
CHEMISTRY
Reaction of a carbonyl compound with one of the following reagents involves nucleophilic addition followed by
elimination of water. The reagent is :
(1) a Grignard reagent
(2) hydrazine in presence of feebly acidic solution
(3) hydrocyanic acid
(4) sodium hydrogen sulphite
d kckZ
fsuy ;kS
fxd d hvfHkf ;kes
afuEu es
alsd kS
u&lkvfHkd eZ
d ukfHkd Lus
gh;kxs d si'pkr t y d kfoykisu gkrskgS
A vfHkd eZ
d
gS:
(1) fxz
xukMZvfHkd eZ
d
(2) vEy h; foy ;u es
agkbMkt hu
(3) gkbM
ks
l k;fud vEy
(4) lks
fM;e gkbMks
t u lYQ kbV
Ans.
Sol.
(2)
With Ammonia derivation carbonyl compounds give addition followed by elimination reaction. Sligtly acidic
medium will generate a nucleophilic centre for weak base like ammonia derivatives.
133.
Method by which Aniline cannot be prepared is :
(1) hydrolysis of phenylisocyanide with acidic solution
(2) degradation of benzamide with bromine in alkaline solution
(3) reduction of nitrobenzene with H2/Pd in ethanol
(4) potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH
solution.
fd l fof/k ls,suhfy u d ksughacuk;k t k ld rk gS:
(1) Q s
fuy
vkblksl k;ukbM d k vEy h; foy ;u esat y vi?kVu }kjk
(2) {kkjh; foy ;u es
acsUt kekbM d kfuEud j.kczksehu d slkFk
(3) ,s
FksukWy esaukbVkscsUt hu d kvip;u H2/Pd d slkFkA
(4) Fks
y sekbM d sikSVSf'k;e y o.k d h Dy ksjkscsUt hu d slkFk f ;k d jd srRi'pkr NaOH d st y h; foy ;u esavi?kvu
Ans.
Sol.
(4)
Chlorine of chlorobenzene is inert towards nucleophile, which is phthalimide ion.
134.
Two possible stereo-structures of
CH3CHOH.COOH, which are optically active, are called.
(1) Diastereomers
(2) Atropisomers
(3) Enantiomers
(4) Mesomers
CH3CHOH.COOH, d ksnksla
Hkkfor f=kfoe lajpuk;sat ksfd /kqzo.k?kw.kZd
(1) vojDr
fd j.ksa
(3) iz
frfcEc: i
gSa]d gy krhgSa:
(2) ,fV
ivkblkesj
(4) es
l ks
ej
Ans.
(3)
135.
The correct statement regarding defects in crystalline solid is :
(1) Schottky defects have no effect on the density of crystalline solids
(2) Frenkel defects decrease the density of crystalline solids
(3*) Frenkel defect is a dislocation defect.
(4) Frenkel defect is found in halides of alkaline metals.
f LVy h; Bksl ksaesanks"kksad slaca/k esalgh d Fku gS%
(1) f LVy h; Bks
l ksad s?kuro ij 'kkWVd hnks"kksad kd ksbZizHkko ughagksrkgSA
(2) S
ad sy nks"k f LVy h; Bksl ksad s?kuRo d ksd e d j nsrsgSa
(3*) S
ad sy nks"k,d LFkku Hkaz'knks"k gSA
(4) {kkj /kkrq
v ksad sgSy kbMksaesa Sad sy nks"k ik;kt krkgSA
Ans.
(3)
Page || 35
AIPMT-2015 |
CODE - (D)
| DATE: 25-07-2015
PHYSICS
PART C : PHYSICS
136.
The position vector of a particle R as a function of time is given by:
R = 4sin(2t) i + 4cos(2t) j
Where R is in meters, t is seconds and i and j denote unit vectors along x-and y-directions, respectively..
Which one of the following statements is wrong for the motion of particle?
(1) Magnitude of acceleration vector is
v2
, where v is the velocity of particle
R
(2) Magnitude of the velocity of particle is 8 meter/second
(3) path of the particle is a circle of radius 4 meter.
(4) Acceleration vector is along - R
le; d sQ y u d s: i esafd lh d .k d k fLFkfr lfn'kR fn;k x;k gS%
R = 4sin(2t) i + 4cos(2t) j
t gkR ehVj esarFkkt lsd .M esagSvkSj i RkFkk j e'k%x-RkFkky-fn'kkvksad svuqfn'k ,d kad lfn'k gSaA bl d .k d h xfr d s
fy ;sfuEukafd r esalsd kSulk d Fku lgh ughagS\
v2
(1) Roj.k&lfn'kd kifjek.k]
R
gS]t gkv d .kd kosx gSA
(2) d .k d sos
x
d k ifjek.k8 m/s gSA
(3) d .k d k iFk4 EkhVj f=kT;k d k o`
k gSA
Sol.
137.
(4) Roj.k&lfn'k]- R d svuq
fn'kgSA
(2)
x = 45 m 2t, y = 4 cos (2t)
Squiring and adding
x2 + y2 = 42
Circular motion
V = R = (2) (4) = 8
So, Ans. is (2)
R=4
The energy of the em waves is of the oder of 15 ke V. To which part of the spectrum does it belong?
(1) Infra-red rays
(2) Ultraviolet rays
(3) -rays
(4) X-ray
fd lh fo|qr pqEcd h; rjax d h t kZd h d ksfV 15 keV. gSA ;g LiSDVEk d sfd l Hkkx d k lnL; gS\
(1) vojDr
fd j.ksa
(3) xkekfd j.ks
a
Sol.
(4)
E of x-ray
(2) ijkoS
xuhfd j.ks
a
(4) ,Dl&fd j.ks
a
E (100 ev to 100 kev)
Page || 36
AIPMT-2015 |
138.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
A beam of light consisting of red, green and blue colours is incident on a right angled prism. The refractive
index of the material of the prism for the above red, green and blue wavelengths are 1.39, 1.44 and 1.47,
respectively.
,d iz
d k'kfd j.kiw
t ]yky]gjsrFkkuhysja
a
xks
alscukgS
A ;g fd j.kiq
t fd lhled ks
a
.khfiz
Te ij vkifrr gks
rkgSvkjs
[kns
f[k;s
fizTe d sinkFkZd k viorZukad ]y ky ]gjs]o uhy sjax d sfy ;s e'k%1.39, 1.44 RkFkk1.47 gSaA rks&
The prism will:
;g fizTe
(1) separate all the three colours from one another
(2) not separate the three colours at all
(3) separate the red colour part from the green and blue colours
(4) separate the blue coloure part from the red and green colours
(1) fd j.kiq
at d srhuksajaxksd ks,d nwl jslsi`Fkd d j nsxkA
(2) rhuks
ajaxksd ksfcYd qy
Hkhi`Fkd ughad jsxkA
(3) fd j.kiq
at
d sy ky jax Hkkx d ksvU; jaxksalsi`Fkd d j nsxkA
(4) fd j.kiq
at d suhy sjax Hkkx d ksvU; jaxkslsi`Fkd d j nsxkA
Sol.
(3)
For TIR
i > ic
so sin i > sin ic
> 1.414
2
Since of green and voilet are greater than 1.414 so they will total internal refrected. But red colour will be
vetracted
So Ans. is (3)
sin 450 >
Page || 37
AIPMT-2015 |
139.
CODE - (D)
PHYSICS
| DATE: 25-07-2015
Two particles A and B, move with constant velocities v 1 and v 2 . At the initial moment their position vector are
r1 and r2 respectively. The condition for particles A and B for their collision is:
nksd .k A RkFkkB fLFkj os e'k%v 1 rFkk v 2 lsxfr d j jgsgSA izkjafHkd {k.k easmud s lfn'k e'k% r1 rFkkr2 gSaA
rks] A RkFkkB d sla?kV~V gksusd sfy ;sizfrca/k gSfd %
Sol.
(1) r1 . v 1 r2 . v 2
(2) r1 v 1 r2 v 2
(3) r1 r2 v 1 v 2
r1 r2
v 2 v1
(4) r r
v 2 v1
1
2
(4)
For collision V B / A should be along B A ( rA / B )
r1 r2
V2 V1
So,
= |r r |
| V2 V1 |
1
2
140.
At the first minimum adjacent to the central maximum of a single-slit diffraction pattern, the phase difference
between the Huygen's wavelet from the edge of the slit and the wavelet from the midpoint of the slit is :
,d y f>jh foorZu iSVuZesa]d sUnzh; mfPp"B d sfud VorhZizFke fufEUk"B ij]f>jh d sfd ukjsrFkk mld se/; fcUnq
lsmRiUu gkbxsUl&rjafxd kvksad schp iFkkUrj gksrk gS%
(1)
Sol.
radian js
fM;u
2
(2) radian js
fM;u
(3)
radian js
fM;u
8
(4)
radian js
fM;u
4
(2)
For first minima
AP - BP =
AP - MP =
So phase difference =
2
2
Page || 38
AIPMT-2015 |
141.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
A proton and an alpha particle both enter a region of uniform magnetic field B, moving at right angles to field B.
If the radius of circular orbits for both the particles is equal and the kinetic energy acquired by proton is 1 MeV
the energy acquired by the alpha particle will b:
,d izksVhu rFkk ,d ,sYQ k d .k]fd lh ,d leku pqEcd h; {ks=k B d sizns'k esaizos'k d jrsgSaA bud h xfr d h fn'kk
{ks=kB d sy Ecor~gSA ;fn nksuksd .kksad sfy , o`kkd kj d {kkvksad hf=kT;kvkil esacjkcj gSA vksj izksVksu }kjkvft Zr
d h xbZxfrt t kZ1 MeV gSrks,sYQ k d .k }kjk vft Zr t kZgksxh
Sol.
(1) 0.5 MeV
(3)
R=
mV
qB
(2) 1.5 MeV
(3) 1 MeV
(4) 4 MeV
2m(kE)
qB
Since R is same so KE
So KE of particle will be
q2
m
( 2) 2
= same = 1 MeV
4
Ans. is (3)
142.
A circuit contains an ammeter, a batter of 30 V and a resistance 40.8 ohm all connected in series. If the
ammeter has a coil of resistance 480 ohm and a shunt of 20 ohm, the reading in the ammeter will be:
fd lhifjiFkesa]30 V d h,d cS
Vjh40.8 vkse d k,d izfrjks/krFkk,d ,ehVj lHkhJs
.kh e es
at q
M+sgS
A ;fn ,ehVj
d h d qaMy h d k izfrjks/k 480 vkse gSvksj bllst qM+slaV d k izfrjks/k 20 vkse gSrks],ehVj d k ikB~;kad gksxk&
Sol.
(1) 0.25 A
(4)
Resitance of ammeter =
i=
(2) 2A
(3) 1 A
(4) 0.5 A
480 20
= 19.2 .
480 20
30
= 0.5 A
40.8 19.2
Ans. is (4)
Page || 39
AIPMT-2015 |
143.
PHYSICS
| DATE: 25-07-2015
The value of coefficient of volume expansion of glycerin is 5 104 K1. The fractional chage in the density of
glycerin for a rise of 40C in its temperature, is:
fXy ljhu d k vk;ru izl kj xq.kkad
esavkaf'kd ifjorZu gksxkA
Sol.
CODE - (D)
(1) 0.020
(1)
= 0 (1 - t)
5 104 K1. gS
A
(2) 0.025
rc fXy ljhu d srki e esa40C o`f+) d jusij mld s?kUkRo
(3) 0.010
(4) 0.015
4
0 = T = (5 10 ) (40) = 0.02
Ans. is (1)
144.
An ideal gas is compressed to half its initial volume by measns of several processes. Which of the process
results in the maximum work done on the gas?
fd lhvkn'kZxS
l d ksxbZiz eksa}kjkbld sizkja
fHkd vk;ru d svk/ksvk;ru rd laihfMr fd ;kt krkgSA fd l iz e
xSl ij vf/kd re d k;Zd juk gksxk\
Sol.
(1) Isobaric
(2) Isochoric
(1) lenkches
a
(4)
(2) levk;rfud
esa
(3) Isothermal
(4) Adiabatic
(3) lerkihes
a
(4) : ) ks
"e
esa
Since area under the curve is max for adiabatic process so work done on the gas will be max for adiabatic
process.
Page || 40
AIPMT-2015 |
145.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
A series R-C circuit is connected to an alternating voltage source. Consider two situation:
(a) When capacitor is air filled.
(b) When capacitor is mica filled.
Current through resistor is i and voltage across capacitor is V then :
,d Js.kh R-C ifjiFk fd lh izR;korhZoksYVrk d sL=kksr lst qM+k gSA nksfLFkfr;ksa(a) RkFkk (b) ij fopkj d hft ,
(a) t c la
/kkfj=k ok;qlaifjr Hkjk gS
(b) t c] la
/kkfj=k ekbd k laifjr gSA
bl ifjiFk esaizfrjks/kd lsizokfgr fo|qr /kkjk gSrFkk la/kkfj=k d sfljkasd schp fooHkkUrj V gSrks
Sol.
(1) Va > Vb
(1)
(2) ia > ib
(3) Va = Vb
(4) Va < Vb
i=
1
R
c
R2
c
2
VC =
VC =
(Rc) 2 1
If we fill a di-electric material
C VC
Ans is (1)
Page || 41
AIPMT-2015 |
146.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
Light of wavelength 500 nm is incident on a metal with work function 2.28 eV. The de Broglie wavelength of the
emitted electron is:
fd lh/kkrqd kd k;ZQ y u 2.28 eV. gSA bl ij 500 nm rjaxnS/;Zd kizd k'kvkifrr gksrkgSrks]mRlft Zr by sDVkWu
d h nsczkWXy h rjaxnS/;ZgkxhSA
Sol.
(1) < 2.8 109 m
(2)
KEmax =
hc
KEmax =
1240
2.82
500
(2) 2.8 109 m
(3) 2.8 1012 m
(4) < 2.8 1010 m
KEmax = 2.48 2.28 = 0.2 ev
20
10 34
3
min = 2m(KE)
=
max
min =
2 9 10 31 0.2 1.6 10 19
25
10 9 = 2.80 109 nm
9
so 2.8 109 m
Ans. (2)
147.
Two metal wires of identical dimension are connected in series. If 1 and 2 are the conductivities of the metal
wires respectively, the effective conductivity of the combination is :
loZl e foLrkj ekid s/kkrqd snksrkj Js.kh e esat qM+
sgSaA ;fn bu rkjksad hpky d rk e'k%1 rFkk2 gSrks
]bud s
bl la;kst u d h pky d rk gksxh
1 2
(1) 2
1 2
Sol.
1 2
(2)
1 2
1 2
(3)
1
2
21 2
(4)
1
2
(4)
eq
Rec = A A = A
eq eq
1
2
2
1 2
=
eq A
A 1 2
eq =
21 2
1 2
Ans. (4)
Page || 42
AIPMT-2015 |
148.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
An automobile moves on a road with a speed of 54 km h1. The radius of its wheels is 0.45 m and the moment
of inertia of the wheel about its axis of rotation is 3 kg m2. If the vehicle is brought to rest in 15s, the magnitude
of average torque transmitted by its brakes to the wheel is :
,d eksVj okgu fd lh lM+d ij 54 km h1. d h pky lspy jgk gSA bld sifg;ksad h f=kT;k0.45 m gSvkSj ?kw.kZu
v{k d sifjr%ifg;sd k t M+Ro vk?kw.kZ3 kg m2. gSA ;fn czsd }kjk ifg;sij y xk vkSl r cy vk?kw.kZd k eku gksxk%
Sol.
(1) 8.58 kg m2 s2
(4)
i =
15
100
=
0.45
3
(2) 10.86 kg m2 s2
(3) 2.86 kg m2 s2
(4) 6.66 kg m2 s2
f = 0
f = i + t
0=
100
+ () (15)
3
= (I) () = 3
149.
100
45
100
= 6.66 N.m.
45
A source of sound S emitting waves of frequency 100 Hz and an observor O are located at some distance from
each other. The source is moving with a speed of 19.4 ms1 at an angle of 60 with the source observer line as
shown in the figure. The observor is at rest. The apparent frequency observod by the observer (velocity of sound
in air 330 ms1) is:
100 Hz vko`
fk d h /kofu
mRiUu d jrk gqv k ,d /ofu L=kksr S RkFkk ,d izs{kd O, ,d nwl jslsd qN nwjh ij fLFkr
gSa
A ;g /ofu L=kks
r]19.4 ms1 d hpky lspy jgkgSa
A mld spy usd hfn'kk]L=kks
r rFkkisz
{kd d hfLFkfr;ksad ksfeykus
oky h ljy js[kk ls60 d k d ks.k cukrh gSvkjs[k nsf[k;s;fn]isz{kd viuh fLFkfr ij gh : d k jgrk gSA rks]izs{kd
}kjk lquh xbZ/ofu d h vkHkklh vko`fk gok esa/ofu d k osx 330 ms1 gksxh %
Sol.
(1) 103 Hz
(1)
vv
t1 = f0 v v
s
(2) 106 Hz
(3) 97 Hz
(4) 100 Hz
v 0
f1 = 100
v 9.7
v
f1 = 100 v 1 9.7
v
9 .7
= 103 Hz
f1 = 100 1
330
Page || 43
AIPMT-2015 |
150.
CODE - (D)
PHYSICS
| DATE: 25-07-2015
On a frictionless surface a block of mass M moving at speed v collides elastically with another block of same
mass M which is initially at rest. After collision the first block moves at an angle to its initial direction and has
a speed
v
. The second block's speed after the collision is :
3
fd lh ?k"kZ.kghu i`"B ij v pky lspy rk gqv k M nzO;eku d k ,d Cy kWd mlh nzO;eku M d sfojkekoLFkk esafLFkr ,d vU;
Cy kWd lsVd jkrkgSA VDd j d si'pkr igy kCy kWd
v
3
pky lsviuhizkjfEHkd xfr d hfn'kklsd ks.kij py usy xrkgSA
rksVDDj d si'pkr~nwl jsCy kWd d h pky gksxhA
(1)
Sol.
3
v
4
(2)
3
2
(3)
3
v
2
(4)
2 2
v
3
(4)
Pi Pf
2
Pi = Pf = mV =
151.
2
V
m 3 mV2
V2 =
2 2
V
3
Ans.
Point masses m 1 and m 2 are placed at the opposite ends of a rigid of length L, and negligible mass. The
rod is to be set rotating about an axis perpendicular to it. The position of point P on this rod through
which the axis should pass so that the work required to set the rod rotating with angular velocity 0 is
minimum, is given by :
fd lh n`< NM+d h y EckbZL gSvkSj mld k nzO;eku ux.; gSA bld snksfoijhr fljksij e'k%m 1 rFkk m 2 nzO;eku d snks
fcUnqfiaM j[ksx;sgSA bl NM+d ksmld sLoa; d sy Ecor~v{k d sifjr%?kw.kZu d jkuk gS]t ksNM+ij fLFkr fd lh fcUnqP ls
gks
d j xq
t jrhgS(vkjs
[kns
f[k;s
)A rksfcUnqP d hog fLFkfr ft ld sfy;sNM+d ksd ks
.kh; os
x 0 ls?kw
.kZ
u d jkusd sfy;svko';d
d k;ZU;wure gksxk]gSA
m1
(1) x m L
2
Sol.
m2
(2) x m L
1
m2L
(3) x m m
1
2
m1L
(4) x m m
1
2
(3)
1
I 2
2
I is min. about the centre of mass
So. (m1) (x) = (m2) (Lx)
K.E. =
m 2L
x = m m
1
2
Page || 44
AIPMT-2015 |
152.
Sol.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
A ball is thrown vertically downwards from a height of 20 m with an initial velocity v0. It collides with the ground
loses 50 percent of its energy in collision and rebounds to the same height. The initial velocity v0 is :
(Take g = 10 ms2)
,d xksy k 20 m d h p kbZlsizkjfEHkd osx v0 }kjklh/ks( /okZ/kj) uhpsd hvksj Q sad k t krkgSA ;g xksy k Hkwry lsVd jkrk
gS
]bl VDd j lsbld h50 iz
fr'kr t kZ{kf;r gkst krhgSA Hkw
& ry lsVd jkusd sckn ;g xksy kmlh p kbZrd mNy t krk
gSA ;fn g = 10 ms2 gSrksxksy k d k izkjfEHkd osx gS:
(1) 20 ms1
(2) 28 ms1
(3) 10 ms1
(4) 14 ms1
(1)
KE f 1
KEi 2
Vf
1
Vi
2
2gh
2
0
V 2gh
1
2
V0 = 20 m sec
153.
A nucleus of uranium decays at rest into nuclei of thorium and helium. Then :
(1) The helium nucleus has less momentum than the thorium nucleus.
(2) The helium nucleus has more momentum than the thorium nucleus.
(3) The helium nucleus has less kinetic energy than the thorium nucleus.
(4) The helium nucleus has more kinetic energy than the thorium nucleus.
fojkekoLFkk esa;wjsfu;e d k,d ukfHkd Fkksfj;e rFkk ghfy ;e d sukfHkd ksaesa{kf;r gksrk gSA rks%
(1) ghfy;e&ukfHkd d kla
osx Fkks
fj;e&ukfHkd klsd e gksrkgSA
(2) ghfy ;e&ukfHkd d kla
osx]Fkksfj;e&ukfHkd lsvf/kd gksrkgSA
(3) ghfy ;e ukfHkd d hxfrt t kZ
]Fkksfj;e&ukfHkd lsd e gksrhgSA
(4) ghfy ;e&ukfHkd d hxfrt t kZFkks
fj;e&ukfHkd lsvf/kd gksrhgSA
Sol.
(4)
U
Th +
KE Th
P2
P2
KE
,
2m Th
2m
sinc m is less so KE will be mone
Page || 45
AIPMT-2015 |
154.
CODE - (D)
| DATE: 25-07-2015
PHYSICS
An electron moves on a straight line path XY as shown. The abcd is a coil adjacent to the path of electron.
What will be the direction of current if any, induced in the coil.
(1) adcb
(2) The current will reverse its direction as the electron goes past the coil
(3) No current induced
(4) abcd
,d by sDVkWu ljy js[kh; iFk]XY ij xfeku gSA ,d d qaM y h abcd bl by sDVkWu d sekxZd sfud VorhZgS(vkjs[k nsf[k;s) A
rks]bl d qaM y h esaizsfjr /kkjk (;fn d ksbZgksrks) d h fn'kk D;k gksxh\
Sol.
(1) adcb fn'kk es
aA
(2) by s
DVkWu d sd qaM y h d sikl
(3) /kkjkiz
sfjr ughgksxhA
(4) abcd fn'kkes
aA
(2)
lsfud y t kusij /kkjk d h fn'kk foijhr gkst k;sxhA
When e comes closer the induced current will be anticlockwise
When e comes farther induced current will be clockwise
Page || 46
AIPMT-2015 |
155.
(2)
(3)
(4)
2
2
(3)
2 A =
A =
=
T=
156.
PHYSICS
| DATE: 25-07-2015
A particle is executing a simple harmonic motion. Its maximum acceleration is and maximum velocity is .
Then its time period of vibration will be :
ljy vkorZxfr d jrsgq, fd lh d .k d k vf/kd re Roj.k rFkk vf/kd re osx gSaA rks]bld sd Eiu d k vkorZd ky gksxk%
(1)
Sol.
CODE - (D)
2 2
Two slits in Youngs experiment have widths in the ratio 1 : 25. The ratio of intensity at the maximum and
max
minima in the interference pattern,
is :
min
;ax d sfd lh f}f>jh iz;ksx esa]nksf>fj;ksad h pkSM kb;ksaesavuqikr 1 : 25 gSA rksO;frd j.k iSVuZesamfPp"B rFkk fuEu"B d h
max
rhozrkvksad k vuqikr,
gksxk%
min
(1)
Sol.
121
49
(2)
49
121
(3)
4
9
(4)
9
4
(4)
1 25
A1 5
2
1
A2 1
A max 5 1
6 3
A min 5 1 = 4 2
max 3 2 9
min = 2
4
Page || 47
AIPMT-2015 |
157.
Sol.
CODE - (D)
| DATE: 25-07-2015
If potential (in volts) in a region is expressed as V(x, y, z) = 6 xy y + 2yz, the electric field (in N/C) at point
(1, 1, 0) is :
;fn fd lh {ks=k esafoHko (oksYV esa) d ksV(x, y, z) = 6 xy y + 2yz, lsfufnZ"V fd ;k t k;srksfcUnq(1, 1, 0) ij fo|qr {ks=k
(N/C es
a) gS%
(1) (6i 5 j 2k)
(1)
V = 6xy y + 24z
(2) (2i 3j k)
(3) (6i 9 j k)
(4) (3i 5j 3k)
V V V
E
I
j
k
y
z
x
E 6y I 6x 1 2z j 2y k
E
= (6i 5 j 2k)
(1,1,0)
158.
A parallel plate air capacitor has capacity 'C' distance of separation between plates is 'd' and potential difference 'V' is applied between the plates. Force of attraction between the plates of the parallel plate air capacitor
is :
,d lekUrj Iy sV ok;qla/kkfj=k d hnksifd kvksad schp d h nwjh'd' rFkkbud schp foHkokUrj 'V' gSA ;fn bl la/kkfj=kd h/
kkfjrk 'C' gSrks]bld h ifd kvksad schp vkd "kZ.k cy gksxk%
(1)
Sol.
CV 2
2d
(2)
CV 2
d
(3)
C2 V 2
2
2d
(4)
C2 V 2
2d2
(1)
Attraction between the plates
F=
q2
0 A
where q = CV and C =
2A0
d
F=
C2 V 2 CV 2
2Cd
2d
Page || 48
AIPMT-2015 |
159.
CODE - (D)
| DATE: 25-07-2015
A plank with a box on it at one end is gradually raised about the other end. As the angle of inclination with the
horizontal reaches 30 the box starts to slip and slides 4.0 m down the plank in 4.0s. The coefficients of static
and kinetic friction between the box and the plank will be, respectively :
fd lhr[rsd s,d fljsij ,d cDlkj[kkgSA r[rsd sml fljsd ks/khjs& /khjs ij d hvksj mBk;kt krkgSA r[rsd s{kSfrt
ls30 d ks.kcukusij cDlk uhpsd h vksj fQ ly uk izkjEHkd jrk gSvkSj 4.0 s esa4.0m nwjhr; d j y srk gSA rks]cDlsrFkk
r[rsd schp LFkSfrd rFkk xfrd ?k"kZ.k xq.kkad ksad k e'k%eku gksxk%
Sol.
(1) 0.6 rFkk0.5
(1)
s = tan 30 =
(2) 0.5 rFkk0.6
1
3
(3) 0.4 rFkk0.3
(4) 0.6 rFkk0.6
0.5
s = 0.57 = 0.6
S = ut +
1 2
at
2
1
1
a(4)2 a =
= 0.5
2
2
a = gsin k (g) cos
4=
K =
160.
0.9
3
= 0.5
In the spectrum of hydrogen, the ratio of the longest wavelength in the Lyman series to the longest wavelength
in the Balmer series is
(1)
9
4
(2)
27
5
(3)
5
27
(4)
4
9
gkbMkst u d sLisDVe esa]y kbeu rFkk ckej Jsf.k;ksad h nh?kZre rjaxns/;ksaZd k vuqikr gksrk gSA
(1)
Sol.
9
4
(2)
27
5
(3)
5
27
(4)
4
9
(3)
1
1
1
Re 2 2
1
2
1
1
1
1
Re 2 2
2
3
2
1
5
2 27
Page || 49
AIPMT-2015 |
161.
CODE - (D)
| DATE: 25-07-2015
In the given figure, a diode D is connected to an external resistance R = 100 and an e.m.f. of 3.5 V. If the
barrier potential developed across the diode is 0.5 V ,the current in the circuit will be:
(1) 40 mA
(2) 20 mA
(3) 35 mA
(4) 30 mA
;gkifjiFkesa,d Mk;ksM D d k,d ckg; izfrjks/kR = 100 rFkk3.5 V bZ,e.,Q d hCkSVjhlst ksM kx;kgSA ;fn Mk;ksM
esanksuksa{ks=kksad hlaf/kd svkjikj mRiUUkjksf/kd kfoHko0.5 V gSrksifjiFkesa/kkjkgksxh:
Sol.
(1) 40 mA
(4)
Current =
162.
(2) 20 mA
(3) 35 mA
(4) 30 mA
( 3 .5 0 .5 )
A
100
3
A 30mA
100
A satellite S is moving in an elliptical orbit around the earth. The mass of the satellite is very small compared
to the mass of the earth. Then,
(1) the total mechanical energy of S varies periodically with time.
(2) the linear momentum of S remains constant in magnitude.
(3) the acceleration of S is always directed towards the centre of the earth.
(4) the angular momentum of S about the centre of the earth changes in direction, but its magnitude remains
constant.
,d mixzg S nh?kZo`rh; d {kkesai`Fohd kifj eksaesad j jgkgSA mixzg d knzO;eku i`Fohd snzO;eku d hrqy ukesacgqr d e
gSA rks
(1) S d h d q
y ;kaf=kd t kZd k eku le; d slkFk vkorhZ: i esaifjofrZr gksrh jgrh gSA
(2) S d sjs
[kh; laosx d k ifjek.k ekufLFkj jgrk gSA
(3) S d k Roj.k lnS
o i`Foh d sd sUnzd h vkjsgksxkA
(4) i`
Foh d sd sUnzd sifjr%S d sd ks.kh; losax d h fn'kk esaifjorZu gksrk jgrk gS]fd Urqbld k ifjek.k leku jgrk gSA
Sol.
(3)
The gravitation force on the satelite will be aiming toward the centre of earth so aceleration of the satelite will
also be aiming toward the centre of earth
Page || 50
AIPMT-2015 |
163.
CODE - (D)
| DATE: 25-07-2015
A force F i 3j 6k is acting at a point r 2i 6j 12k . The value of for which angular momentum
about origin is conserved is :
(1) 2
(2) zero
(3) 1
(4) 1
fd lhfcUnqr 2i 6j 12k ij ,d cy F i 3j 6k yx jgkgS
A rks d sfd l eku d sfy, ew
y fcUnqd sifjr%d ks
.kh;
laosx ljf{kr jgsxk :
Sol.
(1) 2
(2) 'kw
U;
(4)
If If L cons tan t then 0
(3) 1
(4) 1
3
6
so r F 0 F should be parallel to r so coefficient should be in same ratio.So
2 6 12
So Ans (4)
164.
A potentiometer wire of length L and a resistance r are connected in series with a battery of e.m.f. E0 and a
resistance r1. An unknown e.m.f. E is balanced at a length l of the potentiometer wire. The e.m.f. E will be given
by :
E0r l
(1) (r r ) . L
1
(2)
LE0r
(3) (r r )l
1
E0 l
L
(4)
LE0r
lr1
L y EckbZd s,d
foHkoekihrkj rFkk,d izfrjks/khr d ksJs.kh e esaE0 bZ,d ,Q d h,d cSVjhrFkkizfrjks/kr1 lst ksM kx;k
gSbl foHkoekih d h l y EckbZij fd lh vKkr bZ,e ,Q E d sfy ;slarqy u fcUnqizkIr gksrk gSrksE d k eku gS:
E0r l
(1) (r r ) . L
1
Sol.
(2)
LE0r
(3) (r r )l
1
E0 l
L
(4)
LE0r
lr1
(1)
E0 r 1
K = potential gradient = r r L
1
so E = K =
165.
Sol.
E0r
(r r1)L
4.0 g of a gas occupies 22.4 litres at NTP. The specific heat capacity of the gas at constant volume is
5.0JK1. IF the speed of sound in this gas at NTP is 952 ms1, then the heat capacity at constant pressure is
(Take gas constant R = 8.3 JK1 mol1)
(1) 7.5 JK1 mol1
(2) 7.0 JK1 mol1
(3) 8.5 JK1 mol1
(4) 8.0 JK1 mol1
lkekU; rki rFkk nkc ij fd lh xSl d s4.0 g nzO;eku d k vk;ru 22.4 fy Vj gSA fLFkj vk;ru ij bld h fof'k"V "ek /
kkfjrk5.0JK1 mol1 gSA ;fn bl xSl esalkekU; rki o nkc ij /ofu d kosx 952 ms1 gSrksbl xSl d hfLFkj nkc fof'k"V
"ek /kkfjrk gS%
(Take gas constant R = 8.3 JK1 mol1)
(1) 7.5 JK1 mol1
(2) 7.0 JK1 mol1
(3) 8.5 JK1 mol1
(4) 8.0 JK1 mol1
(4)
No. of mole of gas = 1 so molar mass = 4g/mole
V=
RT
m
CP 8
CV 5
952 952
os CP =
3.3 273
4 10
=1.6 =
16 8
10 5
85
8 jk 1mole 1
5
Page || 51
AIPMT-2015 |
166.
CODE - (D)
| DATE: 25-07-2015
r
and the lighter
2
one in radius r. The tangential speed of lighter stone is n times that of the value of heavier stone when they
experience same centripetal forces. The value of n is :
(1) 3
(2) 4
(3) 1
(4) 2
Two stones of masses m and 2 m are whirled in horizontal circles the heavier one in radius
r
2
nksiRFkjksd snzO;eku m rFkk2 m gS]HkkjhiRFkj d ks f=kT;k d srFkk bYd siRFkj d ksr f=kT;k d so`rkd kj {kSfrt iFkksaesa//
kqek;k t krk gSA t c ;siRFkj ,d leku vfHkd sUnzh; cy vuqHko d jrsgSrc gYd siRFkj d k js[kh; osx Hkkjh iRFkj d sjs[kh;
osx d k n xquk gSA n d k eku gS:
Sol.
(1) 3
(4)
(2) 4
(3) 1
(4) 2
FC =
mv 1
2mv 22 4mv 22
r
(r / 2 )
r
so v1 = 2v2
167.
Sol.
A remote - sensing satellite of earth revolves in a circular orbit at a height of 0.25 106 m above the surface of
earth. If earth's radius is 6.38 106 m and g = 9.8 ms2, then the orbital speed of the satellite is :
(1) 8.56 km s1
(2) 9.13 km s1
(3) 6.67 km s1
(4) 7.76 km s1
6
,d lqnwj laosnhmixzg i`Fohd si`"B ls0.25 10 m pkbZij o`kkd kj d {kkesai`Fohd kpDd j y xkjgkgS;fn i`Fohd h
f=kT;k 6.38 106 m gSvkSj g = 9.8 ms2gSrksmixzg d h d {kh; pky gksxh:
(1) 8.56 km s1
(2) 9.13 km s1
(3) 6.67 km s1
(4) 7.76 km s1
(4)
V0
GM
GM R 2
.
R2 r
9.8 6.38 6.38
6.63 10
60 10 6 m / sec
= 7.76 km/sec
168.
Sol.
A string is stretched between fixed points separated by 75.0 cm. It is observed to have resonant frequencies
of 420 Hz and 315 Hz. There are no other resonant frequencies between these two. The lowest resonant
frequency for this string is :
(1) 205 Hz
(2) 10.5 Hz
(3) 105 Hz
(4) 155 Hz
,d MksjhnksfLFkj fcUnvks
ad schp f[kphgS
A bu fcUnqv ksad schp d hnqjh75.0 cm gSA bl Mksjhd hnksvuqukn vko`fk;k420
Hz rFkk315 Hz gS
A bu nksuksd schp esad ksbZvU; vuqukn vko`fk ughagSA rksbl Mksjh d sfy ;sU;wure vuqukn vko`fk gSA
(1) 205 Hz
(2) 10.5 Hz
(3) 105 Hz
(4) 155 Hz
(3)
Fundamental frequency = highest common factor = 105Hz
Page || 52
AIPMT-2015 |
169.
CODE - (D)
| DATE: 25-07-2015
The coefficient of performance of a refrigerator is 5 if the temperature inside freezer is 20C, is temperature of
the surroundings to which it rejects heat is
(1) 41C
(2) 11C
(3) 21C
(4) 31C
fd lhiz'kkard jsf t js
Vjd kfu"iknu xq.kkad 5 gS
A ;fn ht j iz
'khfr=kd kHkhrjhrki 20C gSrksiz
'khrd d sckgj pkjks
vksj t gk;g rki ckgj Q Sad rk gSd k rkieku gksxk%
Sol.
(1) 41C
(4)
cop
(2) 11C
(3) 21C
(4) 31C
TC
q1
q2
5
w q1 q2 TH T C
TC = 5TH 5TC
6TC = 5TH
TH =
170.
Sol.
6
253k = 303.6 k = 30.6C = 31C
5
Water rises to a height 'h' in capillary tube. If the length of capaillary tube above the surface of water is made
less than 'h' then :
(1) water rises upto the top of capillary tube and stays there without overflowing
(2) water rises upto a point a little below the top and stays there
(3) water does not rise at all.
(4) Water rises upto the tip of capillary tube and then starts overflowing like fountain.
fd lh d sf'kd k esat y 'h' p kbZrd p<+rk gSA ;fn t y d h lrg ls ij d sf'kd k d h y EckbZ'h' lsd e gksrks
(1) t y d s
f'kd k d s ijh fljsrd p< t krk gSogh : d k jgrk gSckgj ugh cgrkA
(2) t y d s
f'kd k d s ijh fljslsd qN uhpsrd p<+rk gSvkSj ogh cuk jgrk gSA
(3) t y d s
f'kd k esaugh p<rkA
(4) t y d s
f'kd k d s ijh fljsrd p<d j Q Ookjsd s: i esackgj cgusy xrk gSA
(1)
Water will not overflow but will change its radius of curvature.
Page || 53
AIPMT-2015 |
171.
CODE - (D)
| DATE: 25-07-2015
Two vessels separately contain two ideal gases A and B at the same temperature the pressure of A being twice
that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of
molecular weight of A and B is :
(1)
3
4
(2) 2
(3)
1
2
(4)
2
3
,d leku rkieku ij nksik=kksaesals,d esavkn'kZxSl A rFkk nwl jseasavkn'kZxSl B Hkjh gSA xSl A d k nkc xSl B d snkc
d knksxqukgSA bu n'kkvksad svUrxZr xSl A d k?kuRo xSl B d s?kuRo ls1.5 xqukik;kt krkgSrksA rFkkB d sv.kqHkkjksad k
vuqikr gksxk:
(1)
Sol.
3
4
(2) 2
1
2
(4)
2
3
(1)
PA
PA A MA
MA 3
A MA
BMB 3
PB
= 2M 2
P
M
RT
RT
2
B
B
B
B
so,
172.
(3)
MA 3
MB 4
Two Young's modulus of steel is twice that of brass. Two wires of same length and of same area of cross
section, one of steel and another of brass are suspended from the same roof. If we want the lower ends of the
wires to be at the same level, then the weights added to the steel and brass wires must be in the ratio of :
(1) 2 : 1
(2) 4 : 1
(3) 1 : 1
(4) 1 : 2
LVhy d k;ax izR;kLFkrkxq
.kka
d ]ihry lsnksxqukgSA ,d ghy EckbZrFkk,d ghvuqizLFkd kV d snksrkjksa],d LVhy d krFkk
,d ihry d kd ks,d ghNr lsy Vd k;kt krkgS
A ;fn Hkkj yVd kusij nks
uksrkjksd sfupy sfljs,d ghry ij gSrksLVhy
rFkk ihry d srkjkslsy Vd k;sHkkjksad k vuqikr gksukspkfg;s%
Sol.
(1) 2 : 1
(1)
(2) 4 : 1
(3) 1 : 1
(4) 1 : 2
W
.
A
so
w
AY
e1 e2
w1 w 2
AY1 AY2
w1 Y1
2
w 2 Y2
Page || 54
AIPMT-2015 |
173.
CODE - (D)
| DATE: 25-07-2015
The input signal given to a CE amplifier having a voltage gain of 150 is Vi = 2 cos 15 t . The corresponding
3
output signal will be :
2
(1) 75 cos 15 t
3
(2) 2 cos 15 t
6
(3) 300 cos 15 t
3
(4) 300 cos 15 t
3
fd lh CE mHk;fu"B mRlt Zd izo/kZd d h oksYVrk y fC/k 150 gSA bld k fuos'k flXuy lad sr,
Vi = 2 cos 15 t . gS
] rkslaxr
3
Sol.
(1) 75 cos 15 t
3
(3)
fuxZr flXuy gksxk%
(2) 2 cos 15 t
6
(3) 300 cos 15 t
3
(4) 300 cos 15 t
3
CE amplifier causes phase difference of (= 180) so Vout = 300 cos 1 5 t
3
174.
In an astronomical telescope in normal adjustment a straight black line of lenght L is drawn on inside part of
objective lens. The eye-piece forms a real image of this line. The length of this image is I. The magnification of
the telescope is :
(1)
L
1
I
(2)
LI
LI
(3)
L
I
(4)
(1)
L
1
I
(2)
LI
LI
(3)
L
I
(4)
L
1
I
lkekU; lek;kst u d hfLFkfr eas]fd lh[kxksy h; nwjn'kZd d svfHkn`';d y sal d sHkhrjhHkkx ij L y EckbZd s,d d ky hljy
js[kk f[kph xbZgSA usf=kd k bl ljy js[kk d k okLrfod izfrfcEc cukrh gSA izfrfcEc d h y EckbZ I gSrksnwjn'kZd d k
vko/kZu gS:
Sol.
L
1
I
(3)
Magnification by eyepiece
m
f
f u
fe
L fe ((f0 fe )
m.p.
fe
L f
0
f0 L
fe
Page || 55
AIPMT-2015 |
175.
Sol.
CODE - (D)
The heart of man pumps 5 litres of through the arteries per minute at a pressure of 150 mm of mercury. If the
density of mercury be 13.6 103 kg/m3 and g = 10m/s2 then the power of heart in watt is :
(1) 2.35
(2) 3.0
(3) 1.50
(4) 1.70
fd lh O;fDr d k n; ?kefu;ksals150 mm ikjn nkc ij 5 fy Vj jDr izfr feuV IkEi d jrk gSA ;fn ikjn d k ?kuRo
13.6 103 kg/m3 rFkkg = 10m/s2 gS
A rksn; d h 'kfDr okV esagS:
(1) 2.35
(2) 3.0
(3) 1.50
(4) 1.70
(4)
power = F.V PAV ghAV
= 13.6 103 10 150 103 0.5 103 / 60 watt
176.
| DATE: 25-07-2015
102
watt 1.70watt
60
If dimensions of critical velocity c of a liquid flowing through a tube are expressed as [ x yr x ] , where , and
r are the coefficient of viscosity of liquid, density of liquid and radius of the tube respectively, then the values of
x, y and z are given by :
(1) 1, 1, 1
(2) 1, 1, 1
(3) 1,1,1
(4) 1, 1, 1
fd lh ufy d k lscgusoky snzo d s kafrd osx c d h foekvksad ks[ x yr x ] ,lsfufn"V fd ;k t krk gSt gk, rFkkr e'k%
nzo d k ';kurk xq.kkad nzo d k ?kuRo rFkk ufy d k d h f=kT;k gSA rksx, y rFkkz d k e'k%eku gSA
(1) 1, 1, 1
Sol.
(2) 1, 1, 1
(4) 1, 1, 1
(4)
VC = xyrz
critical velocity is given by VC
so, x = 1
y = 1
177.
(3) 1,1,1
R
2r
z = 1
. If the
2
maximum kinetic energy of the emitted photoelectrons in the second case is 3 times that in the first case, the
work function of the surface of the material is :
(h = Planck's constant, c = speed of light)
A photoelectric surface is illuminated successively by monochromatic light of wavelength and
(1)
hc
(2)
2hc
(3)
fd lhizd k'koS| qr i`"B d ks e'k% rFkk
hc
3
(4)
hc
2
rja
xns/;Zd s,d o.khZizd k'klsiznhIr fd ;kt krkgSA ;fn mRlft Zr izd k'kfo|qr
by sDVkWuksd h vf/kd rk xfrt t kZd k eku nwl jh n'kk esaigy h n'kk 3 xquk gSrksbl i`"B d sinkFkZd k d k;ZQ y u gS%
(h = Iy ka
d fLFkjkd ac = izd k'k d k osx)
(1)
Sol.
hc
(2)
2hc
(3)
hc
3
(4)
hc
2
(4)
k1
hc
so 2
hc
k 2 3k1
so
2hc
3hc
3
hc
2
Page || 56
AIPMT-2015 |
178.
CODE - (D)
| DATE: 25-07-2015
The cylindrical tube of a spray pump has radius, R, one end of which has n fine holes, each of radius r. If the
speed of the liquid in the tube is V, the speed of the ejection of the liquid through the holes is :
(1)
VR 2
nr
(2)
VR 2
(3)
3 2
n r
V 2R
nr
(4)
VR 2
n 2r 2
fd lhLizsiEi d hcs
y ukd kj uy hd hf=kT;kR gS
A bl uy hd sfljsij n lw
{e fNnzgS
]ft uesaizR;sd d hf=kT;kr gS
A ;fn uy h
esanzo d h pky V gSrksbu fNnzksalsckgj fud y rsgq, nzo d h pky gksxh:
(1)
Sol.
VR 2
(2)
nr 2
(3)
n 3r 2
V 2R
nr
(4)
VR 2
n 2r 2
(1)
Volume inflow rate = volume anflow rate
R2V = nr2 (v)
179.
VR 2
R 2 V
nr 2
VR 2
nr 2
If vectors A cos t i sin t j and B cos t i sin t j are functions of time, then the value of t at
2
2
which they are orthogonal to each other is :
(1) t
(2) t
(4) t
(3) t = 0
;fn lfn'k A c o s t i s in t j rFkklfn'kB cos t i sin t j le; d sQ y u gS
]rkst gSog eku D;kgksxk
ft l ij ;slfn'k ijLij y acd ksf.k gksxh
(1) t
Sol.
(2) t
(3) t = 0
(4) t
(2)
A cos wt i sin wtj
wt
wt
B cos
i sin
j
2
2
for A.B 0
wt
wt
A.B 0 cos wt.cos
sin wt.sin
2
2
wt
wt
= cos wt
cos 2
2
so
wt
2
2
Page || 57
AIPMT-2015 |
180.
Sol.
CODE - (D)
| DATE: 25-07-2015
A rectangular coil of length 0.12 m and width 0.1 m having 50 turns of wire is suspended vertically in a uniform
magnetic field of strength 0.2 Weber/m2. The coil carries a current of 2A. If the plane of the coil is inclined at
an angle of 30 with the direction of the field, the torque required to keep the coil in stable equilibrium will be :
(1) 0.20 Nm
(2) 0.24 Nm
(3) 0.12 Nm
(4) 0.15 Nm
,d 0.12 m y Ech]0.1 m pkSM h d qaM y h esarkj d s50 Q sjsgSbld ks0.2 Weber/m2 d s,d leku pqEcd h; {ks=k esa /ok/kZj
yVd k;kx;kgS
Adq
My hesa2A fo|q
a
r/kkjkiz
okfgr gksjghgS
A ;fn d a
M yh]pEcd h; {ks=kls30 d ks.kcukrhgSrksbusjks
q
d sj[kus
d sfy , vko';d cy vk?kw.kZd k eku gksxk%:
(1) 0.20 Nm
(2) 0.24 Nm
(3) 0.12 Nm
(4) 0.15 Nm
(1)
M B MB sin 60
= Ni AB sin 60
= 50 2 0.12 0.1 0.2
3
2
= 12 3 10 2 Nm = 0.20784 Nm
Page || 58
Read the following instructions carefully
fuEufy f[kr funsZ'k /;ku l si<+s:
1.
The candidates should fill in the required particulars on the
Test Booklet and Answer Sheet (Side-1) with Blue/Black Ball
Point Pen.
1.
ijh{kkFkhZd ksijh{kk iqfLrd k vkSj mkj i=k (i`"B-1) ij okafNr fooj.k
uhy s@ d ky sckWy IokbaV isu lsgh Hkjuk gSA
2.
For writing/marking particulars on Side-2 of the Answer Sheet,
use Blue/Black Ball point Pen only.
2.
mkj i=k d si`"B-2 ij fooj.k fy [kus@ vafd r d jusd sfy , d soy
uhy s@ d ky sckWy IokbaV isu d k ;ksx d jsaA
3.
The candidates should not write their Roll Numbers anywhere
else (except in the specified space) on the Test Booklet/
Answer Sheet.
3.
ijh{kkiqfLrd k@mkj i=kij fu/kkZfjr LFkku d svy kokijh{kkFkhZviuk
vuq ekad vU; d ghaughafy [ksaA
4.
Out of the four options given for each question, only one
option is the correct answer.
4.
R;sd 'u d sfy ;sfn;sx;spkj fod Yiksaesalsd soy ,d fod Yi lgh
gS
A
5.
For each incorrect response, onefourth () of the total marks
allotted to the question would be deducted should be deducted
from the total score. No deduction from the total score,
however, will be made if no response is indicated for an item
in the Answer Sheet.
5.
R;sd xy r mkj d sfy , ml 'u d sfy , fu/kkZfjr d qy vad ksaesals
,d pkSFkkbZ() vad d qy ;ksx esalsd kV fy , t k,saxsA ;fn mkj i=k
esafd lh'u d kd ksbZmkj ughafn;kx;kgS]rksd qy ;ksx esalsd ksbZ
vad ughad kVst k,axsA
6.
Handle the Test Booklet and Answer Sheet with care, as under
no circumstances (except for discrepancy in Test Booklet
Code and Answer Sheet Code), another set will be provided.
6.
ijh{kkiqfLrd k ,oamkj i=kd k /;kuiwoZd ;ksx d jsaD;ksafd fd lhHkh
ifjfLFkfr esa(d soy ijh{kk iqfLrd k ,oamkj i=k d slad sr esafHkUurk
d h fLFkfr d ksNksM +d j), nwl jh ijh{kk iqfLrd k miy C/k ughad jk;h
t k,xhA
7.
The candidates are not allowed to do any rough work or
writing work on the Answer Sheet. All calculations/writing
work are to be done in the space provided for this purpose in
the Test Booklet itself, marked Space for Rough Work. This
space is given at the bottom of each page and in one at the
end of the booklet.
7.
mkj i=k ij d ksbZHkh jQ d k;Z;k fy [kkbZd k d ke d jusd h vuqefr
ughagSA lHkh x.kuk ,oafy [kkbZd k d ke] ijh{kk iqfLrd k esafu/kkZfjr
t xg t ksfd *jQ d k;Zd sfy , t xg* }kjk ukekafd r gS] ij gh fd ;k
t k,xkA ;g t xg R;sd i`"B ij uhpsd hvksj vkSj iqfLrd kd svUr esa
,d i`"B ij nh xbZgSA
8.
On completion of the test, the candidates must hand over the
Answer Sheet to the Invigilator on duty in the Room/Hall.
However, the candidates are allowed to take away this
Test Booklet with them.
8.
ijh{kklEiUu gksusij]ijh{kkFkhZd {k@gkWy NksM +uslsiwoZmkj i=kd {k
fujh{kd d ksvo'; lkSai nsaA ijh{kkFkhZv iusl kFk bl ijh{kk iqfLrd k
d ksy st k l d rsgSA
9.
Each candidate must show on demand his/her Admit Card to
the Invigilator.
9.
ekaxst kusij R;sd ijh{kkFkhZfujh{kd d ksviuk os'k d kMZfn[kk,A
10.
No candidate, without special permission of the Superintendent
or Invigilator, should leave his/her seat.
10.
v/kh{kd ;k fujh{kd d h fo'ks"k vuqefr d sfcuk d ksbZijh{kkFkhZviuk
LFkku u NksM +sA
11.
The candidates should not leave the Examination Hall without
handing over their Answer Sheet to the Invigilator on duty and
sign the Attendance Sheet second time will be deemed not to
have handed over the Answer Sheet and dealt with as an
unfair means case. The candidates are also required to
put their left hand THUMB impression in the space
provided in the Attendance Sheet.
11.
d k;Z
jr fujh{kd d ksviuk mkj i=k fn, fcuk ,oamifLFkfr i=k ij nq
ckjk
gLrk{kj fd , fcukd kbsZijh{kkFkhZijh{kkgkyW ughaNkMsxas+As ;fn fd lhijh{kkFkhZus
nw
l jhckj mifLFkfr i=kij gLrk{kj ughafd , rks;g ekuktk,xkfd mlus
mkj i=k ughaykVSk;k gSft lsvuq
fpr lk/ku ;kxs Js
.kh es
aekuk tk,xkA
ijh{kkFkhZviusck;asgkFkd sva
xBwsd kfu'kku mifLFkfr i=kes
afn, x, LFkku ij
vo'; yxk,
A
12.
Use of Electronic/Manual Calculator and any Electronic device
like mobile phone, pager etc. is prohibited.
12.
bysDV
kWfud @gLrpkfy r ifjd y d ,oaeks
ckby Q ksu]ist j bR;kfn t S
ls
fd lh by sDVkWfud mid j.k d k ;ksx oft Zr gSA
13.
The candidates are governed by all Rules and Regulations of
the JAB/Board with regard to their conduct in the Examination
Hall. All cases of unfair means will be dealt with as per Rules
and Regulations of the JAB/Board.
13.
ijh{kk gkWy esavkpj.k d sfy , ijh{kkFkhZt -,-c-@cksM Zd slHkh fu;eksa
,oafofu;eksa}kjk fu;fer gksaxsA vuqfpr lk/ku iz;ksx d slHkh ekey ksa
d k Q Sl y k t -,-c-@cksM Zd sfu;eksa,oafofue;ksad svuql kj gksxkA
14.
fd lh Hkh fLFkfr esaijh{kk iqfLrd k rFkk mkj i=k d k d ksbZHkh Hkkx
vy x ughafd ;k t k,xkA
15.
ijh{kkFkhZ}kjkijh{kkd {kgkyW @es
aos
'kd kMZd svykokfd lhHkhd kj d h
ikB~
; lkexz
h]eq
fnz
r ;kgLrfyf[kr]d kxt d hifpZ
;k
]is
t j]ekcskby Q kus
;k fd lh Hkh d kj d sbys
DV
kfWud mid j.kkas;k fd lh vU; d kj d h
lkexz
hd ksyst kus;kmi;kxs d jusd hvuq
efr ughagS
A
14.
No part of the Test Booklet and Answer Sheet shall be detached
under any circumstances.
15.
Candidates are not allowed to carry any textual material, printed
or written, bits of papers, pager, mobile phone, electronic
device or any other material except the Admit Card inside the
examination room/hall.
Anda mungkin juga menyukai
- Transportation in PlantsDokumen11 halamanTransportation in Plantsridwan100% (15)
- Companion Planting Chart SgaDokumen3 halamanCompanion Planting Chart Sgaapi-240710343Belum ada peringkat
- INTERMEDIATE SCIENCE-XII Isc 10+2 MODEL PAPER 2015 RSPVM PAIBIGHA GAYADokumen168 halamanINTERMEDIATE SCIENCE-XII Isc 10+2 MODEL PAPER 2015 RSPVM PAIBIGHA GAYAJohnny PooleBelum ada peringkat
- Vyaktitav Vikas Evam Adhyatm: Manovigyan Ki Drushti MeDari EverandVyaktitav Vikas Evam Adhyatm: Manovigyan Ki Drushti MeBelum ada peringkat
- Chanakya Niti: Suvevsthit Vekati Aur Samaj Ke Nirman Hetu Upyogi PustakDari EverandChanakya Niti: Suvevsthit Vekati Aur Samaj Ke Nirman Hetu Upyogi PustakBelum ada peringkat
- Neet 2016 Paper With Solution Code C R YDokumen79 halamanNeet 2016 Paper With Solution Code C R Yxanshah100% (1)
- NEET Solved Paper 2016Dokumen77 halamanNEET Solved Paper 2016vishnuttyBelum ada peringkat
- Electrical Asst Manager E01Dokumen18 halamanElectrical Asst Manager E01Siddharth RaiBelum ada peringkat
- Aimpt - 2015Dokumen41 halamanAimpt - 2015Vino PrabaBelum ada peringkat
- NEET Question Paper Code G1 ResonanceDokumen27 halamanNEET Question Paper Code G1 ResonanceTaher MohdBelum ada peringkat
- Practical Skills in Science and Technology-C-X-2007-RA6Dokumen20 halamanPractical Skills in Science and Technology-C-X-2007-RA6meeranismailBelum ada peringkat
- AIPMT 2015 Paper With Solutions PDFDokumen64 halamanAIPMT 2015 Paper With Solutions PDFdgkulkarniBelum ada peringkat
- Sample Test Paper: Class: Xi Stream: Science - MathsDokumen14 halamanSample Test Paper: Class: Xi Stream: Science - MathsNihalSingh1111Belum ada peringkat
- 7738diploma Community-Nutrition - OptDokumen4 halaman7738diploma Community-Nutrition - Optalinisamaryam1Belum ada peringkat
- Paper Code QQ v2Dokumen61 halamanPaper Code QQ v2Mir MusaibBelum ada peringkat
- STaRT 2015 Sample Test Paper Clsss 5 Stage-IIDokumen16 halamanSTaRT 2015 Sample Test Paper Clsss 5 Stage-IIajitpratapsinghntpccBelum ada peringkat
- 02 JE Electronics QP 001Dokumen13 halaman02 JE Electronics QP 001balajipeda100% (1)
- Introductory Agriculture and Principles of AgronomyDokumen10 halamanIntroductory Agriculture and Principles of AgronomyRishikesh MeenaBelum ada peringkat
- STaRT 2015 Sample Test Paper CLASS 9 Stage-IIDokumen19 halamanSTaRT 2015 Sample Test Paper CLASS 9 Stage-IIAryan Gupta100% (1)
- Agron - 111 (1200)Dokumen11 halamanAgron - 111 (1200)Rishikesh MeenaBelum ada peringkat
- NBCC JE CE 10 June 2018 (English)Dokumen15 halamanNBCC JE CE 10 June 2018 (English)My TubeBelum ada peringkat
- 10th Science Set - 3Dokumen24 halaman10th Science Set - 3sapna kainBelum ada peringkat
- SAIL2Dokumen17 halamanSAIL2Sunil SinghBelum ada peringkat
- Sample Test Paper: Le Feuv Egùke VadDokumen18 halamanSample Test Paper: Le Feuv Egùke VadFarhanBelum ada peringkat
- Sample Test Paper 2016 17 JA JF JDDokumen21 halamanSample Test Paper 2016 17 JA JF JDVishal MalikBelum ada peringkat
- Baed 02Dokumen6 halamanBaed 02Kundan DhurveBelum ada peringkat
- STaRT 2016 Sample Paper Clsss 7Dokumen12 halamanSTaRT 2016 Sample Paper Clsss 7PreyanshMitharwalBelum ada peringkat
- DMRC JE Electronics Engineering Question Papers 2015 PDF Download PDFDokumen23 halamanDMRC JE Electronics Engineering Question Papers 2015 PDF Download PDFjagdish singhBelum ada peringkat
- 522ADokumen32 halaman522Asuprita100% (1)
- (HKKX) : Iqflrdk LHJHT+Dokumen8 halaman(HKKX) : Iqflrdk LHJHT+शुभ्रम् ऽBelum ada peringkat
- Darshan Academy HOLIDAY HOMEWORK (2019-20) Class - Vi: EnglishDokumen5 halamanDarshan Academy HOLIDAY HOMEWORK (2019-20) Class - Vi: EnglishdharamBelum ada peringkat
- Fø Kdyki 11: Mís' Vko' D LkexzhDokumen25 halamanFø Kdyki 11: Mís' Vko' D LkexzhAbhinav BawaneBelum ada peringkat
- Hindi & English Versions: InstructionsDokumen22 halamanHindi & English Versions: Instructionscrazy about readingBelum ada peringkat
- Bihar Specia Testa - 7 (140424) QDokumen22 halamanBihar Specia Testa - 7 (140424) QAbhishek Prem MBelum ada peringkat
- Sail Pyp QuestionsDokumen14 halamanSail Pyp QuestionsBikesh KumarBelum ada peringkat
- 5 Geography II 16 FebDokumen6 halaman5 Geography II 16 FebAmandaBelum ada peringkat
- Answerkey NurtureDokumen27 halamanAnswerkey NurtureKaran DoshiBelum ada peringkat
- NK ) KJK HKJK Tkuk GS: To Be Filled in by The CandidateDokumen11 halamanNK ) KJK HKJK Tkuk GS: To Be Filled in by The CandidateRishikesh MeenaBelum ada peringkat
- Fci Assistant Grade III 2015 Paper 1 West Zone 3eff2785Dokumen19 halamanFci Assistant Grade III 2015 Paper 1 West Zone 3eff2785Lily KimBelum ada peringkat
- D (KK & 1 D (KK & 1 D (KK & 1 D (KK & 10 0 0 0oha Oha Oha OhaDokumen25 halamanD (KK & 1 D (KK & 1 D (KK & 1 D (KK & 10 0 0 0oha Oha Oha OhaAshay JainBelum ada peringkat
- Class Xii Summer HolidayDokumen4 halamanClass Xii Summer HolidaySaNkAlPBelum ada peringkat
- Distance Learning Programme: Pre-Medical: Leader Test Series / Joint Package CourseDokumen44 halamanDistance Learning Programme: Pre-Medical: Leader Test Series / Joint Package CourseMadhuri GautamBelum ada peringkat
- SAILOCTTDokumen15 halamanSAILOCTTpradeepmeena321Belum ada peringkat
- WWW - Gate2016.info: Code-05CLMLS02 (P-I)Dokumen15 halamanWWW - Gate2016.info: Code-05CLMLS02 (P-I)Santosh RaiBelum ada peringkat
- Test 03 Sept MCQDokumen8 halamanTest 03 Sept MCQAnuj ??Belum ada peringkat
- Biology 119Dokumen32 halamanBiology 119bihariz096gfBelum ada peringkat
- JE Electrical Paper IDokumen17 halamanJE Electrical Paper ISarvesh DahiyaBelum ada peringkat
- BL Iqflrdk Dks VKNS'K Feyus Ij GH (Kksysa: Ç'U Iqflrdk J'A (Kyk %& Ç'U Iqflrdk La ( K %&Dokumen13 halamanBL Iqflrdk Dks VKNS'K Feyus Ij GH (Kksysa: Ç'U Iqflrdk J'A (Kyk %& Ç'U Iqflrdk La ( K %&Rajesh KumawatBelum ada peringkat
- Sample Test Paper: Le Feuv Egùke VadDokumen17 halamanSample Test Paper: Le Feuv Egùke VadFarhanBelum ada peringkat
- 5 6075484956795076904 PDFDokumen36 halaman5 6075484956795076904 PDFSrihar PriyaBelum ada peringkat
- Biology Set 2, Model Papers of Madhya Pradesh Board of Secondary Education, XIIth ClassDokumen21 halamanBiology Set 2, Model Papers of Madhya Pradesh Board of Secondary Education, XIIth ClassAkshay PandeyBelum ada peringkat
- Foundation Course II YearDokumen3 halamanFoundation Course II Yeard.cBelum ada peringkat
- FCI AGM Question Paper 2015Dokumen33 halamanFCI AGM Question Paper 2015saswataroyBelum ada peringkat
- Class 8Dokumen12 halamanClass 8henilBelum ada peringkat
- Hpbose 10th HP Board Science Sample Papers 2016 Set 1 Question Paper PDFDokumen25 halamanHpbose 10th HP Board Science Sample Papers 2016 Set 1 Question Paper PDFmanBelum ada peringkat
- Pre Full Test-GSDokumen24 halamanPre Full Test-GSVivek KumarBelum ada peringkat
- NEET 2019 Question Paper With Solution R3Dokumen64 halamanNEET 2019 Question Paper With Solution R3Yash JainBelum ada peringkat
- Signal Tel Asst Manager E02Dokumen18 halamanSignal Tel Asst Manager E02Anand KumarBelum ada peringkat
- Esic Udc: Previous Year PaperDokumen14 halamanEsic Udc: Previous Year Paperrvshjha191Belum ada peringkat
- Bhautik Evam Rasyan Vigyan: Vigyaan ki anubhutiyo ka moolik prastutikaranDari EverandBhautik Evam Rasyan Vigyan: Vigyaan ki anubhutiyo ka moolik prastutikaranBelum ada peringkat
- Aadhunik Jeevan Jeene Ki Kala: Apni Soch Ko Modern BanaiDari EverandAadhunik Jeevan Jeene Ki Kala: Apni Soch Ko Modern BanaiBelum ada peringkat
- Oils From Herbs, Spices, and Fruit Seeds AaDokumen26 halamanOils From Herbs, Spices, and Fruit Seeds AaWendy FXBelum ada peringkat
- The Language and Meaning of Flowers.20140815.195322Dokumen3 halamanThe Language and Meaning of Flowers.20140815.195322shield6musicBelum ada peringkat
- Activity 3 Philippine LiteratureDokumen10 halamanActivity 3 Philippine LiteratureMaria Monica SanielBelum ada peringkat
- 2004 RPP PDFDokumen33 halaman2004 RPP PDFMustapha Hal BabaBelum ada peringkat
- Pakistan's California Has Some Ways To GrowDokumen3 halamanPakistan's California Has Some Ways To GrowSajjad AshrafBelum ada peringkat
- UjjwalDokumen87 halamanUjjwalGoni SethiBelum ada peringkat
- Plant TissuesDokumen3 halamanPlant TissuesHolofa Andrea LemanaBelum ada peringkat
- Junior Neet Weekly Test QP (11!10!2021)Dokumen19 halamanJunior Neet Weekly Test QP (11!10!2021)r3flexxBelum ada peringkat
- Kingdomfungi 170304171711Dokumen34 halamanKingdomfungi 170304171711trisha abadBelum ada peringkat
- Furniture Manufacturing Vol 1Dokumen51 halamanFurniture Manufacturing Vol 1sreeramtv9361Belum ada peringkat
- TreeDokumen23 halamanTreegraphicsgriffin100% (1)
- Effect of Plant Growth Regulators On Hard Wood Cuttings ofDokumen5 halamanEffect of Plant Growth Regulators On Hard Wood Cuttings ofFaten AbdallahBelum ada peringkat
- Mechanisms of Date Palm Resistance To Bayoud DiseaseDokumen8 halamanMechanisms of Date Palm Resistance To Bayoud DiseasemekaekBelum ada peringkat
- Landscape Design and Site Planning: Assignment-07: VegetationDokumen8 halamanLandscape Design and Site Planning: Assignment-07: VegetationSaleha FatimaBelum ada peringkat
- Dilcher 1974Dokumen158 halamanDilcher 1974Antonio NetoBelum ada peringkat
- Harris T.M. - The Yorkshire Jurassic Flora. II. Caytoniales, Cycadales & Pteridosperms. London - British Museum (Natural History) (1964)Dokumen214 halamanHarris T.M. - The Yorkshire Jurassic Flora. II. Caytoniales, Cycadales & Pteridosperms. London - British Museum (Natural History) (1964)Anahiti AtenaBelum ada peringkat
- Hogan Linda WalkingDokumen5 halamanHogan Linda WalkingRtoesBelum ada peringkat
- Lesson Plan: Plants & InsectsDokumen9 halamanLesson Plan: Plants & InsectsGemerlyn VallesBelum ada peringkat
- Bacterial Spot On AlmondsDokumen5 halamanBacterial Spot On Almondssallom1973Belum ada peringkat
- Table of Wood DensityDokumen9 halamanTable of Wood DensityAri WahyuBelum ada peringkat
- Difference Between Homologous and Analogous Structure - Major DifferencesDokumen3 halamanDifference Between Homologous and Analogous Structure - Major DifferencesArvind BoudhaBelum ada peringkat
- To Study The Natural Phenomenon of InheritanceDokumen1 halamanTo Study The Natural Phenomenon of InheritanceDhruv AroraBelum ada peringkat
- Photosynthetic Pathways 9Dokumen8 halamanPhotosynthetic Pathways 9Nathaly Mendoza MorenoBelum ada peringkat
- Traditional Knowledge of Kani Tribals in Kouthalai of Tirunelveli Hills, Tamil Nadu, IndiaDokumen10 halamanTraditional Knowledge of Kani Tribals in Kouthalai of Tirunelveli Hills, Tamil Nadu, IndiachanduBelum ada peringkat
- Basil: Growing GuideDokumen6 halamanBasil: Growing GuideAlmiansyahBelum ada peringkat
- Ethnobotanical Observations On The Tribals of Chinnar Wildlife SanctuaryDokumen8 halamanEthnobotanical Observations On The Tribals of Chinnar Wildlife SanctuarykavalapparaBelum ada peringkat
- Hort 221Dokumen90 halamanHort 221Voice AnonymousBelum ada peringkat
- 11 Biology Notes Ch02 Biological ClassificationDokumen8 halaman11 Biology Notes Ch02 Biological ClassificationabhiBelum ada peringkat