2009 A
Diunggah oleh
Popa ElenaJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
2009 A
Diunggah oleh
Popa ElenaHak Cipta:
Format Tersedia
THE CANADIAN CHEMISTRY CONTEST 2009
for high school and CEGEP students
PART A - MULTIPLE CHOICE QUESTIONS (60 minutes)
All contestants should attempt this part of the contest before proceeding to Part B (the CIC section) and/or Part C (the CCO section).
A CIC/CCO Periodic Table is required, but no other data may be given. Answers should be marked on the Answer Grid provided.
1. You have been asked to insert a thermometer through a rubber stopper.
There is resistance upon your first try. Which of the following actions
should you take (in the correct order)?
(i)
(ii)
(iii)
(iv)
A. i
Try to put the thermometer through again
Wrap the thermometer with rubber tubing or paper towel
Lubricate the bulb of the thermometer with water or glycerin
Get a friend to hold the stopper while you push in the thermometer
B. ii, i
C. iii, i
D. iii, iv
E. iii, ii, i
5.
In which one of the following molecules are the atoms NOT all in one
plane?
A. C2H2
B. C2H4
C. C2H6
D. C6H6
E. CH2O
6. The following graph shows the logarithm of successive ionization
1
energies (log I.E. in kJ mol ) as the first 14 electrons are removed from
atoms of one particular element.
6.00
5.50
Which one of the following molecules contains a pair of atoms that
share six electrons?
A. CO2
B. C2H2
C. C2H4
D. C2H6
5.00
log I.E.
2.
E. C6H6
B. KBr
C. Br2
D. HBr
4.00
3.50
3.00
3. In which of the following substances does the bonding have the most
ionic character?
A. K
4.50
2.50
2.00
E. H2
1 2
3 4
6 7
8 9 10 11 12 13 14 15
Electron number
4. When analysing water, each mole of dissolved oxygen (O2) liberates
two moles of iodine (I2) through a series of complex redox reactions
involving manganese and iodine. The amount of dissolved iodine is then
accurately determined by titration with standard sodium thiosulfate solution.
The balanced equation representing the titration is:
2 Na2S2O3 + I2 2 NaI + Na2S4O6
1
A 25.00-mL water sample required 18.64 mL of a 0.00113 mol L sodium
thiosulfate solution to titrate the amount of iodine present after the original
treatment. What is the concentration of dissolved oxygen in this sample (in
mg O2 per litre of water)?
A. 0.211 mg O2/L
B. 3.37 mg O2/L
D. 13.48 mg O2/L
E. 18.64 mg O2/L
C. 6.74 mg O2/L
Which one of the following elements could this be?
A. Ca
B. Mg
C. S
D. Sn
E. Si
7. A student reported that in the synthesis of an iodide of tin, 0.500 g of tin
and 2.00 g of iodine were completely consumed in the reaction. From these
data, you can conclude that the formula of the tin iodide is:
A.
B.
C.
D.
E.
SnI2, with an experimental error of more than 10%
SnI2, with an experimental error of less than 10%
SnI4, with no appreciable experimental error
SnI4, with an experimental error of more than 10%
SnI4, with an experimental error of less than 10%
CCC 2009 page 2 of 4
Given the following data:
Hydrogen halide
Boiling point, K
HF
293
HCl
188
HBr
206
HI
238
Which one of the following statements about the hydrogen halides can be
deduced from these data?
A.
B.
C.
D.
E.
HF has the strongest intramolecular bonding
HF has the strongest intermolecular bonding
HCl has the strongest intramolecular bonding
HCl has the strongest intermolecular bonding
HI has the strongest intramolecular bonding
B. 9.40 g Al2O3
D. 12.7 g Al2O3
E. 19.2 g Al2O3
Experimental data: Mass of flask and sample = 103.639 g
Mass of flask alone
= 94.604 g
1.00
9. Aluminium reacts with oxygen to produce aluminium oxide. If 6.00 g of
aluminium is reacted with 6.00 g of oxygen gas, what is the maximum mass
of aluminium oxide that can be produced?
A. 8.25 g Al2O3
12. In an experiment to determine the percentage of water in a mixture of
water and propanone (acetone), a 9.95-mL sample of a liquid mixture of
propanone and water was weighed:
Density, g/mL
8.
0.95
0.90
0.85
0.80
0.75
0
C. 11.3 g Al2O3
10
20 30
40
50
60
70 80
90 100
% Propanone by volume
10. A 2.0 cm length of magnesium ribbon was added to 100 mL of
1
2.0 mol L hydrochloric acid. All the magnesium reacted and the
o
temperature of the acid increased by 20 C.
1
From the experimental data and the calibration graph shown above, what
was the percentage of water by volume in the mixture?
A. 32%
B. 42%
C. 58%
D. 68%
E. 75%
What volume of 2.0 mol L hydrochloric acid would produce a temperature
o
rise of 10 C with a 1 cm length of magnesium ribbon?
13. Which of the following aqueous solutions has the highest numerical
value of pH?
A. 20 mL
A. Pure water
B. 0.01 M KBr
D. 0.01 M NH4Br
E. 0.01 M CH3COOK
B. 25 mL
C. 50 mL
D. 100 mL
E. 200 mL
C. 0.01 M HBr
11. A sample of baking soda of total mass 0.364 g is composed of sodium
hydrogen carbonate, NaHCO3, mixed with a non-volatile impurity. When
heated, the sodium hydrogen carbonate decomposes to sodium carbonate,
Na2CO3, and there is a loss in mass of 0.112 g. The purity of the NaHCO3
mixture was:
14. Zinc hydroxide is used in surgical dressings. Given that its solubility
17
product constant, KSP (Zn(OH)2) = 4.15 x 10 , what is the concentration of
hydroxide ions in a saturated solution of zinc hydroxide?
A. 30.8%
A. 2.2 x 10
mol L
D. 4.4 x 10
mol L
B. 50.0%
C. 69.2%
D. 83.3%
E. 90.3%
B. 1.5 x 10
mol L
E. 6.9 x 10
mol L
C. 3.5 x 10
mol L
CCC 2009 page 3 of 4
3+
15. Dinitrogen tetroxide is often a component in smog: it is corrosive,
highly toxic and oxidising. It undergoes three different types of self
dissociation according to the following equations:
N2O4 2 NO2 ;
at 303 K
at 303 K
Keq = 10
N2O4 NO + NO3 ; Keq = 10
N2O4 NO2 + NO2 ; Keq = 10
+
22
18. In aqueous solution the Fe ion is bound to six water molecules and
3+
forms the hydrated ion Fe(H2O)6 . Many salts of this ion form acidic
solutions, for example in red iron-containing soils. The cause of this acidity
is that the hydrated ion
A.
B.
C.
D.
E.
at 303 K
Assuming that no other reactions are taking place, which one of the
following species will be present in the greatest concentration at 303K?
A. NO
B. NO2
C. NO2
D. NO3
E. N2O4
16. The polymer sodium poly(aspartate) (used as an anti-scaling agent) is
manufactured by initially heating the amino acid known as aspartic acid at
180C, causing a condensation polymerization to occur. This is followed by
reaction with sodium hydroxide to form the final polymer, which has two
repeating units (structure below).
O
sodium poly(aspartate)
A. a polyamide
B. a polyester
D. polynucleotide
E. a polysaccharide
CH2
Pyrrolidine
N
H
C. 0
C. a rubber
D. +3
B. 4.4 x 10
C. 2.3 x 10
19
19
D. 6.7 x 10
E. 1.9
20. A test for hydrogen iodide (a colourless gas) is to insert a hot platinum
rod into the gas, which will then turn purple due to the formation of iodine
gas according to the following equation:
2 HI(g) H2(g) + I2(g)
17. In one type of breathalyser used to detect alcohol in a drivers breath, an
orange solution of potassium dichromate (K2Cr2O7) in dilute sulfuric acid
reacts with alcohol (if present) to form green Cr2(SO4)3, and the intensity of
the green colour is measured. In this reaction, what is the change in the
oxidation number of each chromium atom?
B. 3
CH2
H2C
A. 1.3 x 10
What type of polymer is sodium poly(aspartate)?
A. -6
H2C
O-Na+
H
N
O
19. Pyrrolidine is a weak base found in carrot leaves, with formula C4H9N
and structure as shown below.
The pH of a 1.00 x 10 mol/L solution of pyrrolidine in water is measured
to be 10.82. The value of the ionization constant K b of this base is
O
N
H
O-Na+
3+
can add a water molecule to form Fe(H2O)7
3+
can lose a water molecule to form Fe(H2O)5
4+
can lose a hydroxide ion from a water molecule to form FeH(H2O)5
2+
can lose a proton from a water molecule to form FeOH(H2O)5
2+
can lose a proton from the iron nucleus to form Mn(H2O)6
E. +6
Based on this information, which combination of the following statements
about the forward reaction is correct:
A.
B.
C.
D.
E.
Type of reaction
Endothermic
Endothermic
Exothermic
Exothermic
Neither exothermic nor endothermic
Enthalpy
Decreases
Increases
Decreases
Increases
Does not change
CCC 2009 page 4 of 4
21. A possible battery system for electric cars is the zinc-chlorine battery, in
which the overall reaction is:
Zn(s) + Cl2(g) ZnCl2(aq)
What is the overall voltage of each battery cell under standard conditions,
given the following reduction potentials:
B. 0.595 V
E = + 1.358 V
E = 0.763 V
Cl2(g) + 2e 2 Cl (aq) ;
2+
Zn (aq) + 2e Zn(s) ;
A. 0.168 V
24. Each of the following organic substances has the chemical formula
C5H12O2 and is a liquid at room temperature and pressure. Which one has
the highest boiling point?
C. 1.943 V
D. 2.121 V
A.
E. 3.469 V
4NH3(g) + 5O2(g) 4NO(g) + 6H2O(l) ; H = 1170 kJ
4NH3(g) + 3O2(g) 2N2 (g) + 6H2O(l) ; H = 1530 kJ
What is the value of H f (NO) as found from these equations?
1
B. 90 kJ mol
1
E. + 2700 kJ mol
HO
OH
22. The standard enthalpy change of formation of nitric oxide, H f (NO),
cannot be determined directly from the elements but it can be calculated
using the following thermodynamic equations:
A. + 90 kJ mol
1
D. 360 kJ mol
B.
O
HO
D.
OH
C.
O
E.
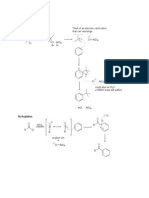
25. Farnesol (structure below) is an organic substance used in perfumery to
emphasise the odours of floral fragrances, and is a natural pesticide for
mites. How many different stereoisomeric (geometric) structures of farnesol
are possible?
C. + 360 kJ mol
OH
farnesol
23. Halomon (structure below) is a polyhalogenated hydrocarbon initially
isolated from Portieria hornemannii, a red algae. This substance has
cytotoxic properties and has important potential as an anti-cancer drug.
Br
A. 2
B. 3
C. 4
D. 6
Cl
halomon
Cl
Cl
Br
Which of the following represents the correct IUPAC name of halomon?
A. 6-bromo-3-(bromomethyl)-2,3,7-trichloro-7-methyloct-1-ene
B. 3-bromo-6-(bromomethyl)-2,6,7-trichloro-2-methyloct-7-ene
C. 6-bromo-3-(bromomethyl)-7-methyl-2,3,7-trichlorooct-1-ene
D. 6-bromo-3-(bromoethyl)-2,3,7-trichloro-7-methyloct-1-ene
E. 3,6-dibromo-2,6,7-trichloro-2-methyloct-7-ene
This is the end of Part A of the contest.
Now go back and check your work.
E. 8
Anda mungkin juga menyukai
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Pap Test and Bethesda 2014: ReviewDokumen12 halamanThe Pap Test and Bethesda 2014: ReviewpdianaBelum ada peringkat
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Cervicalcancerscreening ModuleDokumen45 halamanCervicalcancerscreening ModulePopa ElenaBelum ada peringkat
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Chemical Institute of Canada L'Institut de Chimie Du CanadaDokumen2 halamanThe Chemical Institute of Canada L'Institut de Chimie Du CanadaPopa ElenaBelum ada peringkat
- Olympiad Support Booklet - Full Text 2Dokumen60 halamanOlympiad Support Booklet - Full Text 2Popa ElenaBelum ada peringkat
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- Austrian National Chemistry Olympiad 1998Dokumen21 halamanAustrian National Chemistry Olympiad 1998Popa ElenaBelum ada peringkat
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- The Chemical Institute of Canada L'Institut de Chimie Du CanadaDokumen2 halamanThe Chemical Institute of Canada L'Institut de Chimie Du CanadaPopa ElenaBelum ada peringkat
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Fibroza 1Dokumen8 halamanFibroza 1Popa ElenaBelum ada peringkat
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- 99prepare SolDokumen53 halaman99prepare SolPopa ElenaBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Articol DR PopaDokumen5 halamanArticol DR PopaPopa ElenaBelum ada peringkat
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Friedel Crafts NotDokumen3 halamanFriedel Crafts NotPopa ElenaBelum ada peringkat
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Practical Problems: Safety RulesDokumen9 halamanPractical Problems: Safety RulesPopa ElenaBelum ada peringkat
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Olympiad 2013 R1 Mark SchemeDokumen9 halamanOlympiad 2013 R1 Mark SchemePopa ElenaBelum ada peringkat
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- C3L6 Teachers Mark Scheme 2011Dokumen8 halamanC3L6 Teachers Mark Scheme 2011Popa ElenaBelum ada peringkat
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- Physical ExaminationDokumen2 halamanPhysical ExaminationPopa Elena0% (1)
- Carotenoids-Birkhäuser Basel (1971)Dokumen934 halamanCarotenoids-Birkhäuser Basel (1971)feghbalibBelum ada peringkat
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Linkage 2 Lab ReportDokumen25 halamanLinkage 2 Lab Reportapi-25176084883% (6)
- WEEK 3 LAB EXERCISE - Cell Structures and Functions - UY-OCODokumen4 halamanWEEK 3 LAB EXERCISE - Cell Structures and Functions - UY-OCOBianca LouiseBelum ada peringkat
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Ronnel Del RioDokumen5 halamanRonnel Del Rioamity balweg100% (2)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- CW Catalogue Cables and Wires A4 En-2Dokumen1.156 halamanCW Catalogue Cables and Wires A4 En-2Ovidiu PuieBelum ada peringkat
- Passive ROMDokumen3 halamanPassive ROMCzarina FayeBelum ada peringkat
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- PED16 Foundation of Inclusive Special EducationDokumen56 halamanPED16 Foundation of Inclusive Special EducationCHARESS MARSAMOLO TIZONBelum ada peringkat
- 08 163 4 JPL ScheickDokumen50 halaman08 163 4 JPL ScheickSaqib Ali KhanBelum ada peringkat
- Classical and Operant Conditioning ExamplesDokumen6 halamanClassical and Operant Conditioning ExamplesPersephone355100% (1)
- Question Bank Chemistry (B.Tech.) : Solid StateDokumen10 halamanQuestion Bank Chemistry (B.Tech.) : Solid StatenraiinBelum ada peringkat
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- DoDough FriedDokumen7 halamanDoDough FriedDana Geli100% (1)
- Good Laboratory Practice GLP Compliance Monitoring ProgrammeDokumen17 halamanGood Laboratory Practice GLP Compliance Monitoring ProgrammeamgranadosvBelum ada peringkat
- Gas Piping Building Services 1Dokumen21 halamanGas Piping Building Services 1abinayaBelum ada peringkat
- OPzS Solar - Power En0213Dokumen2 halamanOPzS Solar - Power En0213janiankoBelum ada peringkat
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- 2 Avaliação Edros 2023 - 7º Ano - ProvaDokumen32 halaman2 Avaliação Edros 2023 - 7º Ano - Provaleandro costaBelum ada peringkat
- MCQSDokumen25 halamanMCQSAsifa Liaqat0% (1)
- Radiador y Sus Partes, Motor Diesel 504BDTDokumen3 halamanRadiador y Sus Partes, Motor Diesel 504BDTRamón ManglesBelum ada peringkat
- ANNEX I of Machinery Directive 2006 - 42 - EC - Summary - Machinery Directive 2006 - 42 - CE - Functional Safety & ATEX Directive 2014 - 34 - EUDokumen6 halamanANNEX I of Machinery Directive 2006 - 42 - EC - Summary - Machinery Directive 2006 - 42 - CE - Functional Safety & ATEX Directive 2014 - 34 - EUAnandababuBelum ada peringkat
- GEC PE 3 ModuleDokumen72 halamanGEC PE 3 ModuleMercy Anne EcatBelum ada peringkat
- Congenital Malformation of The Lung and AirwaysDokumen48 halamanCongenital Malformation of The Lung and AirwaysrubyniBelum ada peringkat
- Sadcas TR 14 - Sadcas Policy - Iso Iec 17025-2017 TransitionDokumen16 halamanSadcas TR 14 - Sadcas Policy - Iso Iec 17025-2017 TransitionSuresh KumarBelum ada peringkat
- Recognizing Fractures and Dislocations: Corpuz, Rachella Nicole PDokumen46 halamanRecognizing Fractures and Dislocations: Corpuz, Rachella Nicole PRachella Nicole CorpuzBelum ada peringkat
- Public Health Interventions: Applications For Public Health Nursing PracticeDokumen249 halamanPublic Health Interventions: Applications For Public Health Nursing PracticeJemimah AdaclogBelum ada peringkat
- Fermentation Media: Agustin Krisna WardaniDokumen27 halamanFermentation Media: Agustin Krisna WardaniYosiaBelum ada peringkat
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Report On Marketing of ArecanutDokumen22 halamanReport On Marketing of ArecanutsivakkmBelum ada peringkat
- Cardiovascular SystemDokumen47 halamanCardiovascular SystemAtnasiya GadisaBelum ada peringkat
- Barangay Clearance SampleDokumen1 halamanBarangay Clearance SampleBarangay Onse Malaybalay100% (3)
- Development of Elevator Ropes: Tech Tip 15Dokumen2 halamanDevelopment of Elevator Ropes: Tech Tip 15أحمد دعبسBelum ada peringkat
- 134.4902.06 - DM4170 - DatasheetDokumen7 halaman134.4902.06 - DM4170 - DatasheetVinicius MollBelum ada peringkat
- Ens TecDokumen28 halamanEns TecBorja CanalsBelum ada peringkat
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactDari EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactPenilaian: 5 dari 5 bintang5/5 (5)