Sistema Nanotubos de Carbono
Diunggah oleh
secateHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Sistema Nanotubos de Carbono
Diunggah oleh
secateHak Cipta:
Format Tersedia
Simultaneous Production of Hydrogen and Carbon Nanotubes
in a Conventional Microwave Oven
S. Nomura1, H. Yamashita2, H.Toyota1, S.Mukasa1, Y. Okamura2
1
Department of Mechanical Engineering, Ehime University, Ehime, Japan
2
Department of Applied Chemistry, Ehime University, Ehime, Japan
Abstract: Hydrogen and carbon nanotubes (CNTs) were produced by generating plasma in an
organic solvent. The 2.45GHz microwave in-liquid plasma is generated by using a conventional microwave oven. The hydrogen generation efficiency using the in-plasma method is
approximately 34% that of the electrolysis of water. When cyclohexane is used as the source
liquid, multi-walled carbon nanotubes (MWCNTs) having a diameter of approximately 30 nm
can be grown on porous silica supported by Mo and Co dip coating technique. This process
enables hydrogen and MWCNTs to be simultaneously manufactured by the in-liquid plasma
method.
Keywords: in-liquid plasma, plasma in liquid, hydrogen, CNT, microwave, bubble
1. Introduction
In-liquid plasma by irradiating High-frequency (HF) or
microwave (MW) is generated in the bubbles in the liquid
[1,2]. The temperature of the gas inside the bubbles can
reach several thousand K, however, since the liquid temperature does not rise above the saturation temperature [3,
4], the insides of the bubbles in the liquid become a
plasma field that can be used in a low-temperature environment. It is theorized that large amounts of activated
species exist inside the bubbles due to the raw material
being supplied to the insides of the bubbles by the evaporation of the liquid itself. If the in-liquid plasma can be
used as a chemical reactor, we expect that it will become
possible to attain much higher reaction rates than in conventional gas-phase plasma. The authors have been proposed applying in-liquid plasma as a replacement for
gas-phase plasma.
Up to now the authors have revealed that when 2.45
GHz microwave plasma is generated in an organic solvent
hydrogen gas with a purity of 70 to 80% can produced [5].
Since the in-liquid plasma method can directly decompose the liquid itself, hydrogen can be extracted from
waste oils, such as engine oil or cooking oil. If flammable
gases such as hydrogen can be synthesized while simultaneously solidifying the carbon, it will be possible to
create flammable gases without emitting CO2. With further advancements, if the residual carbon is transformed
into nanotech materials at the same time the flammable
gases such as hydrogen from waste liquids are produced,
it can be expected an in-liquid plasma zero emission system.
On the other hand, the catalyst chemical vapor deposition (CCVD) method is one method for synthesis of CNT
[6, 7]. With this method, methane, ethylene, acetylene,
benzene and other such chemicals serve as the source of
carbon supply. Since these are originally liquids, they can
easily be applied to the in-liquid plasma method for use
organic solvent source material. The authors applied the
CCVD method in a liquid, CNTs can be synthesized in
benzene solution dispersed a metal-supported catalyst [9].
Large-volume CNT creation can be expected by using the
in-liquid plasma method.
The purpose of this research is to develop a process that
will produce gas by using the in-liquid plasma method
while simultaneously solidifying the carbon and synthesizing useful carbonized materials, such as CNTs or activated charcoal, at high speed and low cost. In this study,
the simultaneous production of hydrogen and CNT inside
a conventional microwave oven is carried out.
2. Experimental apparatus and procedure
Figure 1 shows the experimental apparatus. With the
exception of the piping used for extracting exhaust gases,
which is mounted on the top of the device, this reactor has
nearly the same design as a commercially available microwave oven (with a frequency of 2.45.GHz). The magnetron, which is the device on the right side, irradiated the
inside of the reactor with 2.45 GHz microwaves. These
microwaves were received by an antenna positioned in the
reactor vessel, generating plasma at the tip of the antenna.
In order to avoid the microwave energy from being absorbed by items inside the reactor, such as the reaction
vessel, reactor platform and piping were made using
heat-resistant gas and silicone rubber. The power consumption of the reactor when the device was in operation
was 1,260 W, of which 750 W was microwave output.
A schematic of the receiving antenna is shown in Fig. 2.
Seven antennas were positioned on a copper sheet. One
copper rod having a diameter of 1.5 mm was placed at the
center of the copper plate and the six antennas were positioned at even spacing perpendicularly around a circumference that is 1/4 the wavelength of the microwave pass-
ing through the liquid. The length of the antenna is 21 mm,
which is 1/4 the wavelength of the microwave in consideration of the dielectric constant of the liquid
(n-dodecane: 2.01), so that the electric field will be the
strongest at the tip of the electrode. The antenna unit is
installed on a Teflon base so that the reactor vessel will
not be damaged by the heat when the plasma is being
generated.
When the tip of the electrode is in the liquid, plasma
will be generated inside the bubbles inside the liquid,
however, since the surface of liquid will go down as the
liquid is decomposed, the electrode will protrude beyond
the surface of the liquid and plasma will be generated at
the gas phase side above the surface of the liquid. Hence,
the experiment is conducted with the electrode completely
submerged in the liquid.
The production gas is collected by the water substitution method for the gas that is expelled from the pipe
connected to the reactor vessel.
n-dodecane was used as the experimental liquid in this
experiment to hydrogen production. The receiving antenna for the microwaves is placed on the bottom of the
reactor, then, 500 ml n-dodecane is poured into the reactor.
The inside of the reactor and the piping used for extracting the exhaust gas are filled with argon gas. In this condition, microwaves are irradiated and plasma was generated. 1000 ml of generated gas was recovered using the
water substitution method.
3. Production of Hydrogen Using a Microwave Oven
Table 1 shows the results of the gas chromatography
Fig.1 In-liquid plasma reactor using a
conventional microwave oven.
the 1000 ml of gas. When the plasma is generated in
analysis of the recovered gas. It took 28 seconds to collect
n-dodecane, low-grade flammable hydrocarbon gases,
such as methane, ethylene and acetylene are generated in
addition to the 74% hydrogen. When only four types of
generated gases are examined, the ratio of hydrogen atoms and carbon atoms comprising the gas is 1:0.225.
The ratio of these atoms in the n-dodecane is 10.462.
The ratio of the number of carbon atoms declined after the
plasma is generated. There are carbon atoms that have not
been included in the generated gas. The following shows
the chemical reaction formula for what is assumed to be
when these carbon atoms are all changed into graphite.
aC12H26(l)
nH2H2 + nCH4CH4 + nC2H4C2H4 + nC2H2C2H2 + bC(s)
(1)
where,
a = (2nH2 + 4nCH4 + 4nC2H4 + 2nC2H2)/26
(2)
b = 12a (nCH4 + 2nC2H4 + 2nC2H2)
(3)
Here, it is supposed that nH2 :nCH4 : nC2H4 : nC2H2 are the
molecular ratio as shown in Table 1. The molarity of these
mixed gases is expressed as ngas and become
nH12
0.74/0.98ngas
(4)
nCH4 0.02/0.98ngas
(5)
n C2H4 0.74/0.98ngas
(6)
n C2H2 0.20/0.98ngas
(7)
The enthalpy of formation per 1 mol of gas in the reaction in eq.(1) is shown below.
n-dodecane:12C(s) + 13H2 C12H26(l)
HC12H26= 350.9 kJ/mol
(8)
Methane: C(s) + 2H2 CH4(g)
HCH4 = 74.87 kJ/mol
(9)
Ethylene: 2C(s) + 2H2 C2H4(g)
HC2H4 = 52.47 kJ/mol
(10)
Acetylene:2C(s) + H2 C2H2(g)
HC2H2 = 226.73 kJ/mol
(11)
If these four chemical reaction formulas are substituted
in eq. (1),
aHC12H26+ nCH4 HCH4+ nC2H4HC2H4
+ nC2H2HC2H273.9ngas
(12)
is obtained. The enthalpy of formation per 1 mol of gas in
the reaction in eq. (1) is 73.9 kJ/mol.
Present experiment that was performed by consuming
1260 W of electricity, it took 28 seconds to produce the
1000ml of gas. When the reactor enthalpy in eq. (1) is
Table 1 Contents of gas generated by MW in-liquid plasma in
n-dodecane
Fig.2 Microwave receiving antenna.
Product
H2
CH4
C2H4
C2H2
Vol. %
74
20
4. Experiments in the synthesis of CNTs
Carbons remain in the liquid after the hydrogen has
been generated. In our previous work [8], a metal supported catalyst was dispersed into a benzene solution and
synthesis of carbon nanotubes in liquid was successful,
showing that is possible to recover the residual carbons as
CNT. Based on this knowledge, in this research we used
cyclohexane (dielectric constant = 2.02), which has the
same basic structure as CNT. This is because CNT could
easily be formed by decomposing and then reconnecting
the cyclohexane when the plasma is generated. Porous
silica that is supported by metallic catalyst (Mo and Co)
dip coating technique on the antenna was mounted as a
catalyst for growing the CNT. The porous silica has an
average pore diameter of 1.12m, that is tube shaped
with an interior diameter of 0.86 cm and length of 10 cm.
This tube is cut in half. As shown in Fig. 1, the silica of
this half cylinder was placed in a reactor vessel.
Fig. 3 (a) shows a SEM image. It is confirmed the
presence of CNT on the porous silica that had been coated
with the metal catalyst. The CNT had a diameter of approximately 30nm and a length of approximately 1m. It
believes that CNT growth is dependent on the diameter of
the supported metal catalyst inside the narrow pores.
From the TEM image shown in Fig. 3(b), several vertical
stripes on the CNT can be observed. Based on these vertical stripes it means that the tube shaped over-lapping
MWCNT were created.
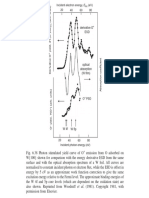
Figure 4 shows the results of the Raman spectrum
measurement. The ratio of the D band and G band is approximately 2.5. This value is small compared to other
SWCNTs because the present nanotubes are layered several times.
The amount of hydrocarbons remaining in the liquid
was measured after the generation of plasma. The time
was recorded until 1000 ml of gas was generated and
performed a quantitative analysis of the carbides remain
(a) SEM image
(b) TEM image
Fig. 3 CNTs synthesized by plasma in cyclohexane
Intensity / arb. unit
converted to a per liter of gas ratio, it is 3.3 kJ/L. Hence,
the maximum 9.4% of electric power consumption is the
amount of energy consumed in the chemical reaction of
eq. (1).
When ratio of generated hydrogen is estimated, we see
that the amount of energy needed to generate 1 mol of
hydrogen converts to 1070 kJ/mol. On the other hand,
from eq. (8) we found that the enthalpy for creating 1 mol
of hydrogen in the reaction where all of the n-dodecane,
which is the liquid that gives the most ideal chemical reaction, breaks down into hydrogen and graphite, is 27
kJ/mol. From this experiment it was found that the creation of acetylene is the main cause for the increase in energy.
The standard enthalpy of formation of acetylene is 227
kJ/mol, which is high in comparison to others, hence, in
the in-liquid production of hydrogen process, there is a
need to find reaction conditions that suppress the formation of acetylene.
Here, by comparing this method with the electrolysis of
alkaline water, the actual production efficiency of hydrogen is calculated. Since the required enthalpy for creating
1 mol of hydrogen from water is 286 kJ/mol (H2OH2 +
1/2O2), if it is supposed that the common value of 80%
for the energy conversion ratio for creating hydrogen
from the electrolysis is used, the required amount of energy to create 1 mol of hydrogen is approximately 360
kJ/mol. This means that the energy conversion ratio of
this equipment is 34% that of the electrolysis of alkaline
water. Even when the energy consumption of the magnetron (750W) is converted, it is 54%, which makes it expensive at the present time when it is used only for creating hydrogen. Also, the actual production of hydrogen by
in-liquid plasma method corresponds to approximately
1% of that by natural gas steam reforming method.
At the present time, the cost is high if the process is
limited to only creating hydrogen. However, we can use
the in-liquid plasma method to reduce CO2 emissions to
zero and use it on a wide variety of waste liquids, such as
cyclohexane, benzene, engine oil and vegetable oil.
Moreover, since the internal gas temperatures of the
in-plasma method reach approximately 2,000 to 5,000 K
[9], this process offers the merit of being able to create
hydrogen by processing chlorinated lubricants and other
such liquids that are otherwise difficult to break down.
500
1000
1500
Raman Shift / cm -1
2000
Fig. 4 Raman spectrum of CNTs grown on porous
silica
ing in the liquid after the generation of plasma. The liquid
was passed through a filter and the mass of the carbides
was measured. These results are shown in Fig.5 .The
maximum amount of carbides produced was at 30 s, after
which the values become approximately 0.1 g.
The amount of carbide experimentally produced is approximately 15% of the production amount that could be
theoretically estimated in chemical reaction process.
However, the recovering carbide is the mass remaining
inside the reactor and excludes the materials that adhered
to the piping and the container for the water substitution
method.
5. Simultaneous production and CNTs
The gas analysis were performed by gas chromatography when the plasma was generated in cyclohexane. Just
as with n-dodecane, when the plasma is generated in
cyclohexane, flammable hydrocarbon gases of low molecular weights such as hydrogen, methane, ethylene and
acetylene are generated. The purity of the hydrogen gas
falls to approximately 40 % with the simultaneously producing CNTs by using metallic catalyst. The reason for
this difference between hydrogen purity of n-dodecane
and that of simulations production of cyclohexane is not
clear at this moment. The effect of catalyst on hydrogen
production will be the subject to further study. As a result,
the type and amount of the catalyst plays a major role in
the production of not only the CNTs, but in the production
of hydrogen.
While only a slight amount of CNTs were attained, the
simultaneous production of CNTs and hydrogen is successful in a simple apparatus that uses a conventional microwave oven. The residual carbon from the simultaneous
production was not only in the reactor vessel, but also
attached to other pieces of equipment, such as the piping
and the container for the water substitution. In consideration of the results of this experiment, we would like to
conduct further research, including research into the design of the apparatus that will enable methods that will
Quantity of carbide [g]
0.15
0.1
0.05
0
25
30
35
40
45
50
Plasm a outbreak tim e [s]
Fig. 5 Quantity of carbide remaining in liquid
after plasma generation producing 1000 ml gas.
solidify a considerable amount of the carbon and collect it
for use as nanotech materials.
5. Conclusion
The experiments in producing hydrogen and CNTs using a conventional microwave oven were conducted and
the following results were obtained.
(1) The generation of 74% pure hydrogen gas in
n-dodecane is confirmed through in-liquid plasma
generation inside a commercially available microwave oven.
(2) In this experiment, the ratio of hydrogen generation is
approximately 34% that of electrolysis of water.
(3) In cyclohexane, CNT can be grown on porous silica
supported by Mo and Co dip coating technique.
(4) When cyclohexane is used as a solvent, the CNTs and
hydrogen could be simultaneously produced. The use
of in-liquid plasma to simultaneously create hydrogen and form CNTs by using a conventional microwave offers the new usefulness in technologies for
disposing of waste oils.
Acknowledgements
This work was partially supported by Grants-in Aid
from the Ministry of Education, Culture, Sports, Science
and Technology of Japan (No.20360098).
References
[1] S. Nomura, H. Toyota, S. Mukasa, Y. Takahashi, T.
Maehara, A. Kawashima, H. Yamashita, Appl. Phys.
Express, Vol.1,No.4(2008).
[2] T. Maehara, I. Miyamoto, et al., Plasma Chem.
Plasma Process, Vol. 28, No.4, 467(2008).
[3] Y. Hattori, S. Mukasa, S. Mukasa, S. Nomura, H.
Toyota, Thermal Science & Engineering, Vol.16,
No.4, 131(2008) [in Japanese].
[4] Y. Hattori, S. Mukasa, H. Toyota, S. Nomura, The
2nd Inter. Fourm on Heat transfer(IFHT2008, Tokyo),
CD-ROM 238 (2008).
[5] S. Nomura, H. Toyota, M. Tawara, and H. Yamashita,
K. Matsumoto, Appl. Phys. Lett., Vol.88, No.23,
231502(2006).
[6] S. Maruyama, R. Kojima, Y. Miyauchi, S. Chiashi
and M. Kohno, Chem. Phys. Lett., (2002), Vol. 360,
No. 3-4, 229 (2002).
[7] H. Dai, A. G. Rinzler, P. Nikolaev, A. Thess, D. T.
Colbert and R. E. Smalley, Chem. Phys. Lett. Vol.
260, No.3, 471 (1996).
[8] S. Nomura, H. Toyota, S. Mukasa, H. Yamashita, T.
Maehara, M. Kuramoto, Appl. Phys. Lett.,
Vol.88 ,No.21, 211503 (2006).
[9] P. Bruggeman, C. Leys, J. Phys. D. Appl. Phys.,
Vol.42, No.5, 053001 (2009).
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5795)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Lambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundDokumen1 halamanLambda 365 UV/Vis Spectrophotometer With UV Lab Software: Image Not Found Image Not FoundsecateBelum ada peringkat
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- HRSG Water Chemistry Control OverviewDokumen5 halamanHRSG Water Chemistry Control OverviewRahul ChoubeyBelum ada peringkat
- Je5b00789 Si 001Dokumen7 halamanJe5b00789 Si 001secateBelum ada peringkat
- Extraction Titanium Dioxide (Tio,) From Ilmenite and Titaniferous SlagDokumen6 halamanExtraction Titanium Dioxide (Tio,) From Ilmenite and Titaniferous SlagsecateBelum ada peringkat
- Surfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodDokumen4 halamanSurfactant-Assisted Synthesis of Batio Nanoparticles by Micro-Emulsion MethodsecateBelum ada peringkat
- AGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1Dokumen8 halamanAGENDA ITEM: 650-472 Load Combinations 8 Ballot: P Determined in F.4.1secateBelum ada peringkat
- Improved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypesDokumen4 halamanImproved Temperature Compensation For in Situhumic-Like and Tryptophan-Like Fluorescence Acquisition in Diverse Water TypessecateBelum ada peringkat
- E-Books: PublicationsDokumen4 halamanE-Books: PublicationssecateBelum ada peringkat
- Copper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzerDokumen2 halamanCopper in Drinking Water by Anodic Stripping Voltammetry at The Sctrace Gold Using The 946 Portable Va AnalyzersecateBelum ada peringkat
- Accepted Manuscript: RSC - Li/pccpDokumen11 halamanAccepted Manuscript: RSC - Li/pccpsecateBelum ada peringkat
- Ab-416 3 enDokumen20 halamanAb-416 3 ensecateBelum ada peringkat
- Surface Interaction Energy Simulation of Ceramic Materials With Epoxy ResinDokumen4 halamanSurface Interaction Energy Simulation of Ceramic Materials With Epoxy ResinsecateBelum ada peringkat
- Pancreatic JournalDokumen4 halamanPancreatic JournalsecateBelum ada peringkat
- Zinc Oxide Nanostructures For Optoelectronic and Energy DevicesDokumen3 halamanZinc Oxide Nanostructures For Optoelectronic and Energy DevicessecateBelum ada peringkat
- Quattro ESEM DatasheetDokumen4 halamanQuattro ESEM DatasheetsecateBelum ada peringkat
- Journal of Colloid and Interface ScienceDokumen8 halamanJournal of Colloid and Interface SciencesecateBelum ada peringkat
- Prestressed Concrete Developments in Japan: Ben C. Gerwick, JRDokumen11 halamanPrestressed Concrete Developments in Japan: Ben C. Gerwick, JRsecateBelum ada peringkat
- Fig. 6.38Dokumen1 halamanFig. 6.38secateBelum ada peringkat
- Meso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatteryDokumen8 halamanMeso-Porous Silicon-Coated Carbon Nanotube As An Anode For Lithium-Ion BatterysecateBelum ada peringkat
- AaaaaaaaaaaaaaaaaaaaaaaaaaaaDokumen1 halamanAaaaaaaaaaaaaaaaaaaaaaaaaaaasecateBelum ada peringkat
- High Temperature NTC Batio - Based Ceramic Resistors: Ying Luo, Xinyu LiuDokumen4 halamanHigh Temperature NTC Batio - Based Ceramic Resistors: Ying Luo, Xinyu LiusecateBelum ada peringkat
- Boon Lak Horn 2016Dokumen5 halamanBoon Lak Horn 2016secateBelum ada peringkat
- Sonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodsDokumen6 halamanSonochemical and Microwave-Assisted Synthesis of Linked Single-Crystalline Zno RodssecateBelum ada peringkat
- Ceramics International: Gao-Yu Zhao, Yu Zhang, Lin Jiang, Hong-Mei ZhangDokumen5 halamanCeramics International: Gao-Yu Zhao, Yu Zhang, Lin Jiang, Hong-Mei ZhangsecateBelum ada peringkat
- 10.1016/j.tsf.2016.12.036: Thin Solid FilmsDokumen34 halaman10.1016/j.tsf.2016.12.036: Thin Solid FilmssecateBelum ada peringkat
- A) From W. J. Stark, L. Mädler, M. Maciejewski, S. E. Pratsinis, and A. Chem. Comm., 588-589 (2003) - Reproduced by Permission of The RoyalDokumen1 halamanA) From W. J. Stark, L. Mädler, M. Maciejewski, S. E. Pratsinis, and A. Chem. Comm., 588-589 (2003) - Reproduced by Permission of The RoyalsecateBelum ada peringkat
- Kebersihan, Fungsi Sanitasi Dan Drainase - BAHASA INGGRIS - VII - Semester IDokumen5 halamanKebersihan, Fungsi Sanitasi Dan Drainase - BAHASA INGGRIS - VII - Semester IRiska AyuBelum ada peringkat
- Hydraulic Calculation For A Dam and Its PowerDokumen7 halamanHydraulic Calculation For A Dam and Its PowerMotasem AbushanabBelum ada peringkat
- Power Transformer FailureDokumen5 halamanPower Transformer FailureNguyen Xuan TungBelum ada peringkat
- Purification of GraphiteDokumen30 halamanPurification of GraphiteSyeda Ammara AnwarBelum ada peringkat
- Solar Net-Metering and Grid Tie SystemDokumen30 halamanSolar Net-Metering and Grid Tie SystemBilly Twaine Palma Fuerte100% (1)
- Colors of BiotechnologyDokumen66 halamanColors of Biotechnologyasdfgh0% (1)
- ENVS2000 Written Assignment - Marissa CrosswhiteDokumen7 halamanENVS2000 Written Assignment - Marissa CrosswhiteTony LindenBelum ada peringkat
- Climatology Kingsley Take HomeDokumen5 halamanClimatology Kingsley Take HomePrince SiawBelum ada peringkat
- Energy Can Neither Be Created Nor Destroyed, Only Altered in FormDokumen2 halamanEnergy Can Neither Be Created Nor Destroyed, Only Altered in FormKalpBelum ada peringkat
- Chapter 11 - WavesDokumen25 halamanChapter 11 - Wavesapi-275374056Belum ada peringkat
- Ch. 4 - Living in The Environment 17thDokumen57 halamanCh. 4 - Living in The Environment 17thGabrielBelum ada peringkat
- Threats of BiodiversityDokumen2 halamanThreats of BiodiversityArkisha BuenafeBelum ada peringkat
- M. N. Macrossan - Scaling Parameters For Hypersonic Flow: Correlation of Sphere Drag DataDokumen6 halamanM. N. Macrossan - Scaling Parameters For Hypersonic Flow: Correlation of Sphere Drag DataFraosmBelum ada peringkat
- Green Kalam: Bring To Know What Is EnvironmentDokumen12 halamanGreen Kalam: Bring To Know What Is Environmentshanmugaraja85Belum ada peringkat
- Q2-Earth Science For STEM-WK3Dokumen7 halamanQ2-Earth Science For STEM-WK3queancy euleBelum ada peringkat
- Chem Chapter 11 - 12 NotesDokumen9 halamanChem Chapter 11 - 12 NotesKavya PandyaBelum ada peringkat
- Analysis of Gravity Dam - Advance Engineering Mathematics ReviewDokumen3 halamanAnalysis of Gravity Dam - Advance Engineering Mathematics ReviewimrancenakkBelum ada peringkat
- The Air PollutionDokumen14 halamanThe Air PollutionEiman UzmiBelum ada peringkat
- Geologi SejarahDokumen88 halamanGeologi SejarahFaizin Mulia RizkikaBelum ada peringkat
- Examen Por ResolverDokumen7 halamanExamen Por ResolverDante TenBelum ada peringkat
- 4.0 Solid-State Nucleation and Growth PDFDokumen17 halaman4.0 Solid-State Nucleation and Growth PDFLEONARD NYIRONGOBelum ada peringkat
- EEE-5101 Power System EngineeringDokumen19 halamanEEE-5101 Power System EngineeringASIF NEOWAZBelum ada peringkat
- Circular FashionDokumen17 halamanCircular FashionElizaveta MaryntsevaBelum ada peringkat
- Assignment 2Dokumen2 halamanAssignment 2Faiz TaimuriBelum ada peringkat
- Insulation of High-Voltage Equipment: V.Y.UshakovDokumen4 halamanInsulation of High-Voltage Equipment: V.Y.UshakovathenssBelum ada peringkat
- QM 1 Tutorial 1Dokumen10 halamanQM 1 Tutorial 1Mister SinisterBelum ada peringkat
- JEE Main 2023 January Session 1 Shift-1 (DT 31-01-2023) PhysicsDokumen12 halamanJEE Main 2023 January Session 1 Shift-1 (DT 31-01-2023) PhysicsResonance EduventuresBelum ada peringkat
- Chemical Engineering: A Level/IB Requirements by College For 2021 EntryDokumen9 halamanChemical Engineering: A Level/IB Requirements by College For 2021 EntryJames BondBelum ada peringkat
- THERMOFLUIDS EXIT TERMS COMPILATION Updated PDFDokumen1.659 halamanTHERMOFLUIDS EXIT TERMS COMPILATION Updated PDFAlteaAlBelum ada peringkat