Spectros 6
Diunggah oleh
Kamal KishoreJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Spectros 6
Diunggah oleh
Kamal KishoreHak Cipta:
Format Tersedia
B.Sc.
III (Vth Semester)
a
CH3
a
CH3
c
b
CH CH2
a
CH3
Br
1-Bromo-2-methylpropane
a
CH3
C
a
CH3
Br
2-Bromo-2- methylpropane
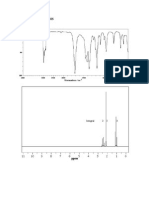
The only structure that satisfies the PMR spectrum for the compound corresponds to 1-Bromo-2methylpropane.
Solved Problem 5. An aromatic hydrocarbons (molecular mass 134) exhibits the following
PMR data: (i) a doublet at -088 (6H) ; (ii) a multiplet at -186 (1H), (iii) a doublet at -245
(2H) ; (iv) a singlet at -712 (5H). Assign a suitable structure to the hydrocarbon.
Solution. From the PMR data it is clear that the given compound has 14 hydrogen atoms. This
means out of the total mass of the molecule i.e. 134, 14 units come from hydrogens. The remaining
mass i.e. 134 14 = 120 comes from carbons. Since the atomic mass of carbon is 12 units therefore the
number of carbons in the compound will be 120/12 = 10. This means the molecular formula of the
compound will be C10H14.
As there are four signals this means the compound has four sets of equivalent protons.
The first signal (-088) which is a doublet and its area correspond to 6 H must be due to two
methyl groups attached to methine carbon i.e. CH(CH3)2. These are marked as a.
The second signal (-186) which is a multiplet and its area correspond to 1 H must be due to one
methine carbon having two methyl groups and one methylene group attached i.e. CH2CH(CH3)2.
This is marked as b.
The third signal (-245) which is a doublet and its area correspond to 2 H must be due to one
methylene attached to benzene ring on one side and methine group on the other side i.e. C6H5CH2
CH. These are marked as c.
The fourth signal (-712) which is a singlet and its area correspond to 5 H must be a phenyl group
i.e. C6H5. These are marked as d.
On combining these four fragments we can assign the following structure to the compound :
Hd
d
Hd
c
CH2
H
dH
Hd
b
a
CH CH3
a
CH3
SOLVED PROBLEMS BASED ON UV, IR, PMR AND ANALYTICAL DATA
Problem 1. An organic compound contain 666% carbon, 111% hydrogen and the rest
being oxygen. In its UV spectrum, it showed a characteristic band at 274 nm ( 17). In the IR
spectrum, it showed bands at 2941 - 2827 (m), 1715 (s) and 1460 (m) cm1. The PMR spectrum of
the compound exhibits three signals at -248, quartet (2H), 212, singlet (3H) and 107 triplet
(3H). Determine the structure of the compound.
PDF Creator - PDF4Free v3.0
http://www.pdf4free.com
Anda mungkin juga menyukai
- NMR 1Dokumen3 halamanNMR 1amitBelum ada peringkat
- Group 1 CationsDokumen5 halamanGroup 1 CationsJoann Justiniane H100% (2)
- Annotated-Atomic Structure Bohr Models-1Dokumen2 halamanAnnotated-Atomic Structure Bohr Models-1Ivania Joselina Lobo MontoyaBelum ada peringkat
- Chem 20024 - Lab No. 6 GRP ReportDokumen4 halamanChem 20024 - Lab No. 6 GRP ReportOrangeIsLemonBelum ada peringkat
- Spectral For PrintingDokumen15 halamanSpectral For PrintingChandra ReddyBelum ada peringkat
- Chapter12 Organic Chemistry Some Asic Principles and TechniquesDokumen32 halamanChapter12 Organic Chemistry Some Asic Principles and TechniquesJamunadevi RajkumarBelum ada peringkat
- Chemistry Oo Kashqeysan Imtixaanka Dowlada 2022Dokumen6 halamanChemistry Oo Kashqeysan Imtixaanka Dowlada 2022cazmi AndirahmanBelum ada peringkat
- Chemguide - Answers: H-1 NMR: High ResolutionDokumen2 halamanChemguide - Answers: H-1 NMR: High ResolutionKhondokar TarakkyBelum ada peringkat
- Spectroscopic Solutions of StructureDokumen21 halamanSpectroscopic Solutions of StructureKassimBelum ada peringkat
- 14 CHEMISTRY Organic Chemistry Some Basic Principles & TechniquesDokumen3 halaman14 CHEMISTRY Organic Chemistry Some Basic Principles & TechniquesHasan shaikhBelum ada peringkat
- IUPAC TH E O0bBdNyDokumen21 halamanIUPAC TH E O0bBdNyChutiyaBelum ada peringkat
- (3935) Sheet Iupac and Structu Isomerism Theory eDokumen28 halaman(3935) Sheet Iupac and Structu Isomerism Theory eRAJDEEP DASBelum ada peringkat
- Tutorial Letter 203/2/2017: General Chemistry 1BDokumen18 halamanTutorial Letter 203/2/2017: General Chemistry 1BLeigh MakanBelum ada peringkat
- Wade Ch.4 QuestionsDokumen8 halamanWade Ch.4 QuestionsAlexGeorgeBelum ada peringkat
- Chem CHPT 6 Learning Module 2Dokumen57 halamanChem CHPT 6 Learning Module 2Patrick Joshua GregorioBelum ada peringkat
- 2013 Alkane Tutorial (Solutions)Dokumen7 halaman2013 Alkane Tutorial (Solutions)Pinzhen ChenBelum ada peringkat
- A (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Dokumen1 halamanA (2e, 4e) B (2Z, 4Z) C (2Z, 4e) D (2e, 4Z)Agatha chilesheBelum ada peringkat
- Spectra ProblemsDokumen111 halamanSpectra ProblemsChandra Reddy100% (1)
- 2240 Exam1 Practice Sp03Dokumen9 halaman2240 Exam1 Practice Sp03Romil PatelBelum ada peringkat
- Jms-3 Paper - 1 SolDokumen15 halamanJms-3 Paper - 1 SoljanmanchiBelum ada peringkat
- Chemguide - Answers: H-1 NMR: Low ResolutionDokumen2 halamanChemguide - Answers: H-1 NMR: Low ResolutionKhondokar TarakkyBelum ada peringkat
- XI Mid Term QPDokumen3 halamanXI Mid Term QPtechnical SiteBelum ada peringkat
- CHM 102 Past Test QuestionsDokumen15 halamanCHM 102 Past Test QuestionsCharlie StonesBelum ada peringkat
- WS Class 11 Org ChemDokumen4 halamanWS Class 11 Org ChemJavedBelum ada peringkat
- Topic 20 Answers To ExercisesDokumen4 halamanTopic 20 Answers To ExercisesSiti NuraqidahBelum ada peringkat
- A5 - Spectroscopic IdentificationDokumen1 halamanA5 - Spectroscopic IdentificationAlyasin FrougaBelum ada peringkat
- 235practice Exam 2 AnswerDokumen9 halaman235practice Exam 2 Answernbobs7Belum ada peringkat
- Chapter 12Dokumen21 halamanChapter 12NurdianAsriBelum ada peringkat
- OCHEM Practice FinalsDokumen13 halamanOCHEM Practice FinalsNoleBelum ada peringkat
- Kathmandu University Dhulikhel, Kavre: Course: Genomics & Proteomics (BIOT 414) Assignment: 02Dokumen7 halamanKathmandu University Dhulikhel, Kavre: Course: Genomics & Proteomics (BIOT 414) Assignment: 02Reeha NeupaneBelum ada peringkat
- A18 - Spectroscopic IdentificationDokumen1 halamanA18 - Spectroscopic IdentificationAlyasin FrougaBelum ada peringkat
- Class 11 - Chemistry - Organic Chemistry Some Basic PrinciplesDokumen30 halamanClass 11 - Chemistry - Organic Chemistry Some Basic PrinciplesSachit GuptaBelum ada peringkat
- OrganicDokumen6 halamanOrganicTrent SwordsBelum ada peringkat
- 1 Answer:4: Quick Revision Test Integer Type QuestionsDokumen41 halaman1 Answer:4: Quick Revision Test Integer Type QuestionsSanthosh SenthilBelum ada peringkat
- A19 - Spectroscopic IdentificationDokumen1 halamanA19 - Spectroscopic IdentificationAlyasin FrougaBelum ada peringkat
- 11 Chemistry23 24sp 01Dokumen13 halaman11 Chemistry23 24sp 01AbhishekBelum ada peringkat
- CHEM 331 Kraus Ihazlett 1 Chapter8Dokumen9 halamanCHEM 331 Kraus Ihazlett 1 Chapter8Ahmed Sideeg100% (2)
- Organic Chemistry: Section A: Straight Objective TypeDokumen26 halamanOrganic Chemistry: Section A: Straight Objective TypeAmarBelum ada peringkat
- Tutorial Letter 203/1/2016: General Chemistry 1BDokumen22 halamanTutorial Letter 203/1/2016: General Chemistry 1BLeigh MakanBelum ada peringkat
- Pectros 4Dokumen1 halamanPectros 4Kamal KishoreBelum ada peringkat
- Alkanes Alkenes AlkynesDokumen10 halamanAlkanes Alkenes AlkynesPanda Boy100% (2)
- XM 9 V J7 HL KZFF TJDPK OFFDokumen34 halamanXM 9 V J7 HL KZFF TJDPK OFFKrish JaiswalBelum ada peringkat
- Ncert Solutions For Class 11 Chemistry Jan11 Chapter 12 Organic Chemistry Some Basic Principles and TechniquesDokumen32 halamanNcert Solutions For Class 11 Chemistry Jan11 Chapter 12 Organic Chemistry Some Basic Principles and TechniquesSachit GuptaBelum ada peringkat
- Ii) A Six-Proton Singlet at The Highest Field (: C CH CHDokumen1 halamanIi) A Six-Proton Singlet at The Highest Field (: C CH CHKamal KishoreBelum ada peringkat
- NMR - Multiple Choice QuestionsDokumen71 halamanNMR - Multiple Choice QuestionsOmSilence265171% (31)
- CHM 102 Past Test QuestionsDokumen15 halamanCHM 102 Past Test Questionsalexapierre08Belum ada peringkat
- Organic Chemistry 2: Number of C ×Dokumen5 halamanOrganic Chemistry 2: Number of C ×Trung VõBelum ada peringkat
- General Organic Chemistry-02 - Solved ProblemsDokumen10 halamanGeneral Organic Chemistry-02 - Solved ProblemsRaju SinghBelum ada peringkat
- NMR Multiple Choice Questions PDFDokumen71 halamanNMR Multiple Choice Questions PDFDeepak SinghBelum ada peringkat
- Solving HNMR ProblemsDokumen7 halamanSolving HNMR ProblemsJorge Eneas DosoliBelum ada peringkat
- Chem52 Su13 PracticeExam1ADokumen11 halamanChem52 Su13 PracticeExam1Aamarka01Belum ada peringkat
- CLS Aipmt-18-19 XII Che Study-Package-7 SET-2 Chapter-12 PDFDokumen44 halamanCLS Aipmt-18-19 XII Che Study-Package-7 SET-2 Chapter-12 PDFJagnesh BhardwajBelum ada peringkat
- Organic Chemistry Some Basic Principles and Techniques 100 Questions With Solution 0 2023 01 10 095917Dokumen31 halamanOrganic Chemistry Some Basic Principles and Techniques 100 Questions With Solution 0 2023 01 10 095917rashmiBelum ada peringkat
- Ijcb 44B (6) 1239-1242Dokumen4 halamanIjcb 44B (6) 1239-1242Sarathkumar PathivadaBelum ada peringkat
- Chapter 12 Organic Chemistry Some Basic Principles and TechniquesDokumen34 halamanChapter 12 Organic Chemistry Some Basic Principles and TechniquesAnonymous 8VJhV1eI2yBelum ada peringkat
- January HW Sec 6Dokumen3 halamanJanuary HW Sec 6Freya SawBelum ada peringkat
- Topic 11 - Measurement and Data Processing End of Topic Questions (Page 289)Dokumen1 halamanTopic 11 - Measurement and Data Processing End of Topic Questions (Page 289)DeepakBelum ada peringkat
- Ariketak Ebazteko GomendioakDokumen4 halamanAriketak Ebazteko Gomendioakjosune ramirez romeroBelum ada peringkat
- Organic Chem. NotesDokumen80 halamanOrganic Chem. NoteselcarlsansBelum ada peringkat
- PDFDokumen16 halamanPDFMary Rose AllinegBelum ada peringkat
- Matriculation Chemistry (Introduction To Organic Compound) Part 1Dokumen24 halamanMatriculation Chemistry (Introduction To Organic Compound) Part 1ridwan71% (7)
- Iupac Nomenclature & Structural Isomerism: Section (A) : Fundamental of Organic ChemistryDokumen21 halamanIupac Nomenclature & Structural Isomerism: Section (A) : Fundamental of Organic ChemistrynandiniBelum ada peringkat
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDari EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionPenilaian: 5 dari 5 bintang5/5 (1)
- Pectros 4Dokumen1 halamanPectros 4Kamal KishoreBelum ada peringkat
- Solutions, Phase Equilibrium, Conductance, Electrochemistry & Functional Group Organic Chemistry-IiDokumen1 halamanSolutions, Phase Equilibrium, Conductance, Electrochemistry & Functional Group Organic Chemistry-IiKamal KishoreBelum ada peringkat
- Ii) A Six-Proton Singlet at The Highest Field (: C CH CHDokumen1 halamanIi) A Six-Proton Singlet at The Highest Field (: C CH CHKamal KishoreBelum ada peringkat
- Solutions DineshDokumen110 halamanSolutions DineshKamal KishoreBelum ada peringkat
- What Do We Mean by Black Body Radiation ?Dokumen2 halamanWhat Do We Mean by Black Body Radiation ?Kamal KishoreBelum ada peringkat
- CN CH CH CL CH CH CoochDokumen1 halamanCN CH CH CL CH CH CoochKamal KishoreBelum ada peringkat
- Synthetic RubbersDokumen3 halamanSynthetic RubbersKamal KishoreBelum ada peringkat
- Natural and Vulcanized RubberDokumen2 halamanNatural and Vulcanized RubberKamal KishoreBelum ada peringkat
- Date Relaxation For Attending Orientation and Refresher CoursesDokumen4 halamanDate Relaxation For Attending Orientation and Refresher CoursesKamal Kishore100% (2)
- Synthesis, Characterization, and Catalytic Applications of Porous Transition-Metal Oxide Systems For The Conversion of BiomassDokumen38 halamanSynthesis, Characterization, and Catalytic Applications of Porous Transition-Metal Oxide Systems For The Conversion of BiomassKamal KishoreBelum ada peringkat
- Relative Stabilities of CycloakanesDokumen8 halamanRelative Stabilities of CycloakanesKamal KishoreBelum ada peringkat
- B. Sc. IDokumen266 halamanB. Sc. IKamal KishoreBelum ada peringkat
- Need and Scope of Food ScienceDokumen26 halamanNeed and Scope of Food ScienceKamal Kishore100% (1)
- Nitrogen Family QuesDokumen2 halamanNitrogen Family QuesKamal KishoreBelum ada peringkat
- Isoprene Derivatives As Natural Pesticides For Better Yields and Lesser HarmsDokumen1 halamanIsoprene Derivatives As Natural Pesticides For Better Yields and Lesser HarmsKamal KishoreBelum ada peringkat
- R K Sharma Coordination Chemistry 10-30Dokumen23 halamanR K Sharma Coordination Chemistry 10-30Kamal KishoreBelum ada peringkat
- CAS Guidelines For Sixth PayDokumen66 halamanCAS Guidelines For Sixth PayKamal KishoreBelum ada peringkat
- Promotion From Associate Professor To ProfessorDokumen21 halamanPromotion From Associate Professor To ProfessorKamal KishoreBelum ada peringkat
- QUESTION ASSIGNMENT SK015 20212022 - Edited - 3 - 9 - 2021Dokumen6 halamanQUESTION ASSIGNMENT SK015 20212022 - Edited - 3 - 9 - 2021BM10622P Nur Ain Aisyah binti RosliBelum ada peringkat
- Chemical AnalysisDokumen7 halamanChemical AnalysisSaher BashirBelum ada peringkat
- Essay Question AnswersDokumen30 halamanEssay Question AnswersHo Quan Xiu100% (7)
- Physical Science Lesson 2 TransmutationDokumen45 halamanPhysical Science Lesson 2 TransmutationCarl BuglosaBelum ada peringkat
- Grade 8 Unit 4 - Science Sri LankaDokumen6 halamanGrade 8 Unit 4 - Science Sri LankaSCIENCE BUDDHIKA punchihewaBelum ada peringkat
- Chemistry The Molecular Nature of Matter 7th Edition Jespersen Solutions Manual DownloadDokumen28 halamanChemistry The Molecular Nature of Matter 7th Edition Jespersen Solutions Manual DownloadSean Bates100% (23)
- HL Paper 1: Two Species Are Isotopes of The Same Element?Dokumen8 halamanHL Paper 1: Two Species Are Isotopes of The Same Element?fuduBelum ada peringkat
- Matter PacketDokumen6 halamanMatter PacketDon King EvangelistaBelum ada peringkat
- API 598 Valves Inspection and TestingDokumen457 halamanAPI 598 Valves Inspection and TestingSenthamizhselvan SelvanBelum ada peringkat
- Predicting Scale Formation: BARON Chemicals & Systems (P) LTDDokumen2 halamanPredicting Scale Formation: BARON Chemicals & Systems (P) LTDdalton2003Belum ada peringkat
- SOLUTION of Mole Concept Sheet 1630318949510Dokumen35 halamanSOLUTION of Mole Concept Sheet 1630318949510AkBelum ada peringkat
- Pathway C+D BookletDokumen33 halamanPathway C+D BookletH ChowdreyBelum ada peringkat
- Molybdenum Species in Aqueous Solution - A Brief SummaryDokumen8 halamanMolybdenum Species in Aqueous Solution - A Brief SummaryJuan Ignacio Gonzalez CabreraBelum ada peringkat
- Alcohol and EtherDokumen24 halamanAlcohol and EtherXyzBelum ada peringkat
- Science Quiz ShowDokumen48 halamanScience Quiz ShowYashwanth SrinivasaBelum ada peringkat
- Coordination ChemistryDokumen19 halamanCoordination ChemistryPrityyyBelum ada peringkat
- Soalan Kimia Pertengahan Tahun Form 4Dokumen11 halamanSoalan Kimia Pertengahan Tahun Form 4Ridzuan Mohd AliBelum ada peringkat
- Qualitatile Inorganic AnalysisDokumen9 halamanQualitatile Inorganic AnalysisRamanBelum ada peringkat
- 2013 H2 Chemistry Prelim Paper 2 AnswersDokumen5 halaman2013 H2 Chemistry Prelim Paper 2 AnswersMelinda BowmanBelum ada peringkat
- CHEMDokumen15 halamanCHEMsalman pradhanBelum ada peringkat
- Oxygen CycleDokumen11 halamanOxygen CycleMarc Jonas DiazBelum ada peringkat
- Vsepr TheoryDokumen3 halamanVsepr TheoryZakaria Azam100% (1)
- Nitrogen Family QuesDokumen2 halamanNitrogen Family QuesKamal KishoreBelum ada peringkat
- Science: Quarter 2, WK 8 - Module 8Dokumen27 halamanScience: Quarter 2, WK 8 - Module 8Ericha Solomon67% (9)
- (2094) Lecture Notes Ionic Equilibrium eDokumen40 halaman(2094) Lecture Notes Ionic Equilibrium eKartikey SharmaBelum ada peringkat
- Acids Bases and Salts Notes PDFDokumen7 halamanAcids Bases and Salts Notes PDFMoghanram JBelum ada peringkat
- Sodium Lauryl Sulfate USPDokumen2 halamanSodium Lauryl Sulfate USPDebahis BoseBelum ada peringkat