P H
Diunggah oleh
andreyJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
P H
Diunggah oleh
andreyHak Cipta:
Format Tersedia
pH
pH measurement reveals the hydrogen ion concentration in water. It is used to determine both the
deposition and corrosion tendency of a water. The most widely used type of pH measurement is the
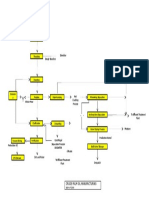
electrode method. The assembly in Figure 36-2 shows the necessary elements that make up a typical
pH sensor: a glass pH electrode, a reference cell, a temperature compensation element, a preamplifier,
and a sensor body. Because of the difficulty of maintaining good pH control, manual systems are being
replaced by continuous monitoring and automatic control of pH in many water treatment applications. In
cooling tower systems, pH has been particularly difficult to control manually because the response curve
of pH to acid addition is not linear. Figure 36-3 shows the variation of pH in a cooling tower system with
manually adjusted feed of sulfuric acid. Results of random plant tests were plotted to show the number of
occurrences of each test value.
pH controllers use much of the same technology as the conductivity controllers discussed above. The
Betz Accutrak pH controller (Figure 36-4) uses microprocessor-based electronics, programmable alarm
and control modes (such as time proportional control, PID control, self-diagnostics, and a display of
electronic or sensor fault conditions), and analog and RS-485 signals for computer interface for data
acquisition and communication. pH sensor technology has advanced significantly to overcome many of
the problems encountered in the past, such as rapid fouling and chemical attack of the pH electrodes,
contamination and rapid depletion of reference cells and electrolytes, and electrical noise and
environmental interference with the low-level pH signal.
Several variations of pH sensor assemblies are available for different applications. For relatively clean
water, where extensive fouling is not a problem (such as in most cooling towers), a combination pH
sensor assembly is normally used. The single, molded body sensor assembly shown in Figure 36-2
combines all of the elements.
Glass electrode deterioration, reference junction plugging, and electrolyte depletion (which occurs in all
pH sensor applications) proceed at approximately the same rate. This progression is slow enough in
clean water to provide an acceptable economic life. When the combination sensor is worn out, it is
discarded.
A rugged, modular pH assembly (Figure 36-5) is used in processes such as metal treatment baths and
waste systems, where fouling or chemical attack of glass electrodes, reference junctions, and other
elements is a problem. The modular assembly allows periodic maintenance and replacement of
individual components
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Getting it Dunn with the Learning Styles ModelDokumen21 halamanGetting it Dunn with the Learning Styles ModelandreyBelum ada peringkat
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- SQL Server DBA Daily ChecklistDokumen4 halamanSQL Server DBA Daily ChecklistLolaca DelocaBelum ada peringkat
- What Is A Political SubjectDokumen7 halamanWhat Is A Political SubjectlukaBelum ada peringkat
- Renato Cristin Heiddegger and LeibnizDokumen10 halamanRenato Cristin Heiddegger and Leibnizaida100% (1)
- Palmoil 1Dokumen5 halamanPalmoil 1andreyBelum ada peringkat
- Crude Palm Oil Manufacturing Process: Dilution WaterDokumen1 halamanCrude Palm Oil Manufacturing Process: Dilution WaterandreyBelum ada peringkat
- P HDokumen1 halamanP HandreyBelum ada peringkat
- Free TrialDokumen1 halamanFree TrialandreyBelum ada peringkat
- Elements of A Test of HypothesisDokumen5 halamanElements of A Test of HypothesisNadia AlamBelum ada peringkat
- Fisica Básica 3er Año SantillanaDokumen66 halamanFisica Básica 3er Año SantillanaElifrnak91% (22)
- Fractions, Decimals and Percent Conversion GuideDokumen84 halamanFractions, Decimals and Percent Conversion GuideSassie LadyBelum ada peringkat
- Affine CipherDokumen3 halamanAffine CipheramitpandaBelum ada peringkat
- Learning by LivingDokumen5 halamanLearning by LivingPaul SchumannBelum ada peringkat
- Data Capture Form Environmental ManagementDokumen1 halamanData Capture Form Environmental ManagementDonavel Nodora JojuicoBelum ada peringkat
- RSBACDokumen166 halamanRSBACtradersanBelum ada peringkat
- Impact of K-Pop Music On The Academic PDokumen29 halamanImpact of K-Pop Music On The Academic Pdave tayron paggao100% (1)
- Political Philosophy of J S MillDokumen9 halamanPolitical Philosophy of J S MillRajkumar SunnyBelum ada peringkat
- Digital Image Processing System AbstractDokumen4 halamanDigital Image Processing System AbstractTelika RamuBelum ada peringkat
- 2 Reason Why I Like DoraemonDokumen2 halaman2 Reason Why I Like Doraemonpriyanka shafiraBelum ada peringkat
- Laser Telemetric SystemDokumen2 halamanLaser Telemetric Systemdellibabu509Belum ada peringkat
- Fox Hunting - The Art of Dating and SeductionDokumen93 halamanFox Hunting - The Art of Dating and SeductionEdward Ashley Latimore100% (1)
- The Letter and The Cosmos How The Alphabet Has Shaped The Western View of The WorldDokumen285 halamanThe Letter and The Cosmos How The Alphabet Has Shaped The Western View of The Worldmarnekib100% (3)
- E F Schumacher - Small Is BeautifulDokumen552 halamanE F Schumacher - Small Is BeautifulUlrichmargaritaBelum ada peringkat
- Importance of AC & Design for Minimizing Use in Offices & MallsDokumen2 halamanImportance of AC & Design for Minimizing Use in Offices & MallsRitz BernalBelum ada peringkat
- Equipment BrochureDokumen60 halamanEquipment BrochureAmar BeheraBelum ada peringkat
- Me-143 BcmeDokumen73 halamanMe-143 BcmekhushbooBelum ada peringkat
- Devki N Bhatt01240739754Dokumen10 halamanDevki N Bhatt01240739754menuselectBelum ada peringkat
- Cricket Bat Thesis - 2006 SymesDokumen297 halamanCricket Bat Thesis - 2006 SymesAnonymous unj3NHW82vBelum ada peringkat
- WBI06 01 Rms 20190124Dokumen17 halamanWBI06 01 Rms 20190124Imran MushtaqBelum ada peringkat
- PESTEL Team Project (Group)Dokumen9 halamanPESTEL Team Project (Group)Yadira Alvarado saavedraBelum ada peringkat
- Notes On Unit - 4 - Employees Roles in Service MarketingDokumen3 halamanNotes On Unit - 4 - Employees Roles in Service MarketingSridhar Gowda67% (3)
- SplunkCloud-6 6 3-SearchTutorial PDFDokumen103 halamanSplunkCloud-6 6 3-SearchTutorial PDFanonymous_9888Belum ada peringkat
- AufEx4 02 02Dokumen28 halamanAufEx4 02 02BSED SCIENCE 1ABelum ada peringkat
- Proposal LayoutDokumen11 halamanProposal Layoutadu g100% (1)
- (Genus - Gender in Modern Culture 12.) Segal, Naomi - Anzieu, Didier - Consensuality - Didier Anzieu, Gender and The Sense of Touch-Rodopi (2009)Dokumen301 halaman(Genus - Gender in Modern Culture 12.) Segal, Naomi - Anzieu, Didier - Consensuality - Didier Anzieu, Gender and The Sense of Touch-Rodopi (2009)Anonymous r3ZlrnnHcBelum ada peringkat