Aseptic Meningitis
Diunggah oleh
Cheng XinvennDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Aseptic Meningitis
Diunggah oleh
Cheng XinvennHak Cipta:
Format Tersedia
The Child With Aseptic
Meningitis
Jason G. Newland, MD,* Samir S. Shah, MD, and Theoklis E. Zaoutis, MD
EDUCATIONAL OBJECTIVES
To realize that partially treated bacterial
meningitis may present with similar
cerebrospinal fluid findings as viral
meningitis.
To realize that the course of enteroviral
meningitis usually is self-limited and benign.
To realize that enteroviruses are the most
common cause of aseptic meningitis and may
be detected within 24 hours using RT-PCR.
Key Questions
1 What is meant by the term aseptic meningitis?
2 What are the most common causes of aseptic
meningitis?
3 What clinical and laboratory features suggest
enteroviruses as the most likely cause of this
toddlers illness?
4 What laboratory test(s) can be performed to
confirm diagnosis?
5 What is the prognosis for aseptic meningitis?
The child with fever, headache, stiff neck, and photophobia requires prompt evaluation for meningitis.
Bacterial meningitis mandates rapid initiation of antimicrobial therapy whereas aseptic meningitis is often selflimited and needs no specific therapy. Because discrimination between these two entities at the time of
presentation may be difficult, children with these symptoms and cerebrospinal fluid (CSF) pleocytosis are frequently admitted to the hospital to receive broad-spectrum antibiotics pending bacterial culture results. CSF
From the *Department of Pediatrics, University of Nebraska Medical Center/
Creighton University Medical Center, Omaha, NE and the Divisions of
General Pediatrics and Infectious Diseases, The Childrens Hospital of
Philadelphia, Philadelphia, PA.
Address correspondence and reprint requests to Dr. Samir S. Shah, 2nd
floor, Division of General Pediatrics, The Childrens Hospital of
Philadelphia, 34th Street and Civic Center Boulevard, Philadelphia, PA
19104. E-mail: shahs@email.chop.edu
218
cell counts have wide areas of overlap between aseptic
and bacterial meningitis, and the administration of antibiotics before lumbar puncture may hinder accurate diagnosis. Advances in the use of polymerase chain reaction techniques may alter our management of the child
with meningitis. In this issue, we discuss the diagnostic
and therapeutic approaches to the child with aseptic
meningitis, with a focus on enteroviral infection, which
is the most common identifiable cause in children.
Case Presentation
In August, a previously healthy 3-year old boy presented to the general pediatric clinic with a 2-day
history of fever as high as 38.9C. He has experienced nonbilious emesis, decreased activity, and anorexia. He has not traveled recently and does not
have any pets. He has not received antibiotics before
the evaluation. Ill contacts include his mother and
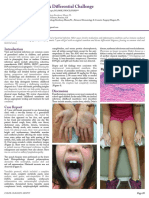
brother, who both have fever, cough, and rhinorrhea. On examination, the child was alert and responded appropriately. There was marked photophobia and nuchal rigidity. The tympanic
membranes were normal in appearance and mobility. There was no lymphadenopathy, abdominal tenderness, or petechiae. The remainder of the examination was normal.
A complete blood count revealed 10,000 white
blood cells/mm3 with 55% neutrophils, 3% bands,
37% lymphocytes, 3% monocytes, and 2% eosinophils. CSF analysis showed 3 red blood cells/mm3
and 110 white blood cells/mm3 with 23% neutrophils, 58% lymphocytes, and 19% monocytes. The
CSF protein and glucose were 55 mg/dl and 50
mg/dl, respectively. The serum glucose was 90 mg/
dl. No organisms were identified on CSF Gram stain.
What is meant by the term aseptic
meningitis?
Aseptic meningitis syndrome refers to meningeal
inflammation in the absence of a readily identifiable
bacterial cause.1 Although viruses are most commonly implicated, no cause can be identified in up to
40% of cases. If the patient has received antibiotics
Pediatric Case Reviews Volume 3 Number 4 October 2003
before lumbar puncture, a negative CSF culture does
not exclude a bacterial etiology.
Clinical Pearl: Partially treated bacterial meningitis may present with similar CSF findings as viral
meningitis. This possibility should be considered in
every patient presenting with aseptic meningitis.
What are the most common causes of
aseptic meningitis?
The diverse common causes of aseptic meningitis
include infectious agents (Table 1) and noninfectious
conditions (Table 2).2 Non-polio enteroviruses (EV),
including coxsackieviruses A and B, echoviruses, and
numbered enteroviruses, account for approximately
95% of all cases of aseptic meningitis in which a causative agent is identified.1 In the United States, enteroviral disease occurs during the summer and early autumn. In humans, the only known reservoir,
transmission of the virus occurs via the fecal oral
route.
Other infectious disease considerations during the
summer and autumn include tick-borne illnesses (e.g.,
Rocky Mountain spotted fever, ehrlichiosis, and
Lyme disease) and arbovirus infections. Arboviruses
(arthropod-borne viruses) primarily cause encephalitis but are also associated with aseptic meningitis, particularly during epidemic periods. The most common
arthropod vector-transmitted causes of aseptic meningitis in the United States are St. Louis encephalitis
virus (SLE), California encephalitis virus (LaCrosse
strain), Eastern equine encephalitis, Western equine
encephalitis, and West Nile virus (WNV). SLE virus
caused 60% of childhood cases of aseptic meningitis
during one epidemic period.3 During 2002, West Nile
virus, another flavivirus, was responsible for the largest arboviral meningoencephalitis epidemic documented in the Western hemisphere. However, only 31
(1%) of the 2,354 reported cases occurred in children
younger than 9.4
Unlike EV, tick-borne illness, and arboviruses,
lymphocytic choriomeningitis (LCM) virus is primarily seen during the winter months. The reservoirs for
this virus include house mice, guinea pigs, and hamsters. Cases of LCM virus disease are more common
in the winter as a result of the migration of mice indoors.5 Influenza A and B may also cause aseptic meningitis during the winter months.1 Other causes of
aseptic meningitis such as herpes simplex virus do not
have such a clear seasonal distribution.
Mycobacterium tuberculosis should also be considered in the child with aseptic meningitis, especially
if the patient has a subacute onset, modest lymphocytic pleocytosis, and protein and glucose abnormalities similar to those seen in bacterial meningitis (low
CSF glucose and elevated CSF protein). Fungi and
parasites are rare causes.
What clinical and laboratory features
suggest enteroviruses as the most likely
cause of this toddlers illness?
Pediatric Case Reviews Volume 3 Number 4 October 2003
TABLE 1 Differential Diagnosis for
Aseptic Meningitis
Syndrome
Enteroviruses
Echoviruses
Group A and B coxsackieviruses
Poliovirus
Numbered enteroviruses
Herpes viruses
Herpes simplex virus 1 and 2
Varicella-zoster virus
Cytomegalovirus
EpsteinBarr virus
Human herpes virus 6
Respiratory viruses
Influenza A and B
Parainfluenza viruses
Adenoviruses
Arboviruses
St. Louis encephalitis virus
Eastern equine encephalitis virus
Western equine encephalitis virus
LaCrosse virus
West Nile virus
Other Viruses
Human immunodeficiency virus (HIV)
Mumps virus
Lymphocytic choriomeningitis virus
Rabies virus
Spirochetes
Leptospira species (leptospirosis)
Borrelia burgdorferi (Lyme)
Treponema pallidum (syphilis)
Rickettsiae
Rickettsia rickettsii (Rocky Mountain spotted fever)
Rickettsia typhi (typhus)
Bacteria
Partially treated meningitis
Mycobacteria tuberculosis
Mycoplasma pneumoniae
Brucella species
Bartonella species (cat-scratch disease)
Parameningeal infection (e.g., mastoiditis, brain abscess)
Fungi
Cryptococcus neoformans
Coccidioides immitis
Histoplasma capsulatum
Parasites
Taenia solium (cysticercosis)
Toxoplasma gondii
Baylisascaris procyonis
Plasmodium falciparum (malaria)
Enteroviral meningitis is primarily seen in children, with those younger than 1 year affected most
frequently.6 The illness begins with fever (38 40C),
anorexia, and vomiting.7 Headache, a common symptom in children old enough to comprehend and communicate this as a symptom, develops 2 to 3 days
later. In children older than 1 year, nuchal rigidity and
photophobia are common.7 The presence of other
219
TABLE 2 Noninfectious Causes of
Aseptic Meningitis
Vascular Disease
Subarachnoid hemorrhage
Cerebral venous thrombosis
Primary vasculitis (e.g., systemic lupus erythematosus)
Malignancy
Primary central nervous system tumor
Metastatic spread
Systemic Disease
Behcet disease
Kawasaki disease
Sarcoid
Status epilepticus
Others
Iatrogenic Conditions
Intrathecal medications
Ventricular shunts
Spinal anesthesia
identifiable enteroviral syndromes in the community,
such as herpangina and hand-foot-mouth disease,
supports the diagnosis, because outbreaks tend to be
epidemic and seasonal.8 CSF analysis in children with
enteroviral meningitis reveals a white blood cell count
(WBC) typically between 100 and 500 cells/mm3, but
CSF WBC counts as high as 2,000 cells/mm3 occasionally occur. In the first 1 or 2 days of illness neutrophils predominate, but transition to a lymphocytic
pleocytosis may occur within several days.9 Normally, the CSF protein concentration is mildly increased whereas the CSF glucose is normal.
What laboratory test(s) can be performed
to confirm diagnosis?
In the past, isolation of EV in cell culture was the
mainstay of laboratory diagnosis. Although cell culture allows for accurate diagnosis, the time to virus
isolation by cell culture ranges from 4 to 8 days.10
This makes it unlikely that culture results will affect
clinical decision-making, especially if the patients
bacterial cultures remain negative and the symptoms
have resolved. Additionally, some EVs are difficult to
grow in cell culture. For example, group A coxsackieviruses require inoculation into suckling mice for
detection. This test is rarely used today.
Reverse transcription-polymerase chain reaction
(RT-PCR), a new diagnostic modality, has improved
the ability to detect EV. The EV RT-PCR assay amplifies the highly conserved 5' nontranslated region of
the EV genome, allowing for the detection of all EV
serotypes. More importantly, the sensitivity and specificity of this test are high, ranging 86% to 100% and
92% to 100%, respectively.11 In some tertiary care
medical centers, RT-PCR provides the clinician a result within 24 hours of specimen collection, offering
the potential for affecting patient management. Ram220
ers et al. showed that routine use of an enteroviral
RT-PCR assay on CSF samples decreased the length of
stay, duration of antibiotic use, and the use of ancillary tests in children with viral meningitis.12
Clinical Pearl: RT-PCR, the diagnostic test of
choice for EV meningitis, provides a rapid diagnosis,
allowing a clinician to discontinue antibiotics and
discharge the patient from the hospital sooner than
if cell culture methods were used.
What is the appropriate management for
this patient?
The management of EV meningitis is primarily
supportive. Children may require antipyretics, analgesia, and intravenous fluid hydration. The risk associated with bacterial meningitis makes it appropriate
to consider empirical therapy with an antibiotic active
against Streptococcus pneumoniae and Neisseria
meningitidis, the most likely bacterial pathogens. In
this case, the child received ceftriaxone and vancomycin. However, with the aid of RT-PCR it was possible
to discontinue these antibiotics once the EV genome
was amplified from the CSF.
What is the prognosis for aseptic
meningitis?
Overall prognosis in young children with EV meningitis is good,1 but serious complications occur in
approximately 10% of cases. Febrile seizures are the
most common complication.1 Rare complications include increased intracranial pressure, encephalitis,
and the syndrome of inappropriate antidiuretic hormone secretion.13 Most children with EV meningitis
will have a self-limited illness that lasts approximately
1 week. Therefore, no special follow-up is needed in
patients with uncomplicated EV meningitis.
Some patient populations are at increased risk for
prolonged and potentially severe EV disease. Because
humoral immunity is important in fighting EV infection, patients with antibody-deficient states such as
X-linked agammaglobulinemia, severe combined immune deficiency, and bone marrow transplant, are at
increased risk for having chronic EV disease.14 These
patients may receive the investigational antiviral
agent, pleconaril, for treatment. Pleconaril prevents
viral replication by inhibiting viral uncoating and
blocking viral attachment to host-cell receptors.15 Patients with certain antibody-deficient conditions
should also receive monthly immune globulin for prophylaxis against chronic EV meningoencephalitis.16
Conclusion
On the second day of hospitalization, the EV
genome was amplified from the CSF. The patients
symptoms improved and he was discharged with the
diagnosis of EV meningitis. Isolation by cell culture
did not occur until 5 days after the CSF was
collected.
Pediatric Case Reviews Volume 3 Number 4 October 2003
Summary of Key Points
are the most common cause of
Enteroviruses
aseptic meningitis and may be detected within
24 hours using RT-PCR.
treated bacterial meningitis may
Partially
present with CSF findings similar to those
seen with enterovirus infection. This possibility
should be considered in every patient
presenting with aseptic meningitis.
course of enteroviral meningitis usually is
The
self-limited and benign.
REFERENCES
1. Rotbart HA. Viral meningitis. Semin Neurol. 2000;20:
277292.
2. Seay A, Vivo DD. Viral infections of the central nervous
system. In: Rudolph C, et al. eds. Rudolphs Pediatrics.
New York: McGraw-Hill; 2003:23052322.
3. Tsai TF, Canfield MA, Reed CM, et al. Epidemiological
aspects of a St. Louis encephalitis outbreak in Harris
County, Texas. J Infect Dis. 1988;1986:157:351356.
4. Centers for Disease Control and Prevention. Provisional surveillance summary of the West Nile virus epidemicUnited States, JanuaryNovember. MMWR
Morb Mortal Wkly Rep. 2002;51:1129 1133.
7. Singer JI, Maur PR, Riley JP, et al. Management of central
nervous system infections during an epidemic of enteroviral aseptic meningitis. J Pediatr. 1980;96:559 563.
8. Cherry JD. Enteroviruses. Coxsackieviruses, enteroviruses, and polioviruses. In: Feigin RD, Cherry JD, eds.
Textbook of Pediatric Infectious Diseases. Philadelphia: Saunders; 1998:17871839.
9. Amir J, Harel L, Frydman M, et al. Shift of cerebrospinal polymorphonuclear cell percentage in the early
stage of aseptic meningitis. J Pediatr. 1991;119:938
941.
10. Rotbart H. Enteroviruses. In: Murray P, et al. eds. Manual of Clinical Microbiology. Washington, D.C.: ASM
Press; 1999:990 998.
11. Romero JR. Reverse-transcription polymerase chain reaction detection of the enteroviruses. Arch Pathol Lab
Med. 1999;123:11611169.
12. Ramers C, Billman G, Hartin M, et al. Impact of a
diagnostic cerebrospinal fluid enterovirus polymerase
chain reaction test on patient management. JAMA.
2000;283:2680 2685.
13. Chemtob S, Reece ER, Mills EL. Syndrome of inappropriate secretion of antidiuretic hormone in enteroviral
meningitis. Am J Dis Child. 1985;139:292294.
14. McKinney RE Jr., Katz SL, Wilfert CM. Chronic enteroviral meningoencephalitis in agammaglobulinemic
patients. Rev Infect Dis. 1987;9:334 356.
5. Meyer H, Johnson R, Crawford I, et al. Central nervous
system syndromes of viral etiology: A study of 713
cases. Am J Med. 1960:334 347.
15. Rotbart HA, Webster AD. Treatment of potentially
life-threatening enterovirus infections with pleconaril.
Clin Infect Dis. 2001;32:228 235.
6. Rantakallio P, Leskinen M, von Wendt L. Incidence
and prognosis of central nervous system infections in a
birth cohort of 12,000 children. Scand J Infect Dis.
1986;18:287294.
16. Webster AD, Rotbart HA, Warner T, et al. Diagnosis of
enterovirus brain disease in hypogammaglobulinemic
patients by polymerase chain reaction. Clin Infect Dis.
1993;17:657 661.
Pediatric Case Reviews Volume 3 Number 4 October 2003
221
Anda mungkin juga menyukai
- Karen - S Notes-Clinical ExaminationDokumen11 halamanKaren - S Notes-Clinical ExaminationGanesh Namasivayam100% (3)
- Improving Patient and Worker SafetyDokumen171 halamanImproving Patient and Worker SafetylaggantigganBelum ada peringkat
- Tracheostomy Care Reflective EssayDokumen2 halamanTracheostomy Care Reflective EssayAnjae Gariando100% (3)
- Essential Elements of Communication in Medical.21 PDFDokumen4 halamanEssential Elements of Communication in Medical.21 PDFdr_mksinhaBelum ada peringkat
- History Taking in ObgynDokumen17 halamanHistory Taking in Obgynselvie87100% (1)
- PIDSR Manual of ProceduresDokumen55 halamanPIDSR Manual of ProceduresOrlea FranciscoBelum ada peringkat
- 4.management of Vertical Discrepancies (2) 2Dokumen97 halaman4.management of Vertical Discrepancies (2) 2Arun Joy100% (2)
- Sensation MethodDokumen5 halamanSensation MethodDr. Nancy MalikBelum ada peringkat
- 3 Intraoperative PhaseDokumen28 halaman3 Intraoperative Phaseada_beer100% (2)
- Tendon TransferDokumen1 halamanTendon TransferPandi Smart VjBelum ada peringkat
- Sexual History TakingDokumen3 halamanSexual History TakingCheng XinvennBelum ada peringkat
- Procedures in Critical Obstetrics PDFDokumen268 halamanProcedures in Critical Obstetrics PDFRatan YadavBelum ada peringkat
- Physiological Adaptation Q&ADokumen17 halamanPhysiological Adaptation Q&AFilipino Nurses CentralBelum ada peringkat
- Medical Tourism in IndiaDokumen2 halamanMedical Tourism in IndiaDipak Rana0% (1)
- AKP Paper 2 PDFDokumen54 halamanAKP Paper 2 PDFMostafa Mahmoud Elsebey100% (2)
- Infectious Diseases CasesDokumen30 halamanInfectious Diseases Casestaliya. shvetzBelum ada peringkat
- Recurrent MeningitisDokumen14 halamanRecurrent Meningitisidno1008Belum ada peringkat
- Pulmonary Tuberculosis Nclex QuestionsDokumen3 halamanPulmonary Tuberculosis Nclex Questionssheen100% (6)
- Review Article: Etiology and Risk Factors of Febrile Seizure - An UpdateDokumen10 halamanReview Article: Etiology and Risk Factors of Febrile Seizure - An UpdateResty Rahmiliah RahimBelum ada peringkat
- Faktor Resiko Kejang PDFDokumen10 halamanFaktor Resiko Kejang PDFHamtaroHedwigBelum ada peringkat
- Meningitis PDFDokumen8 halamanMeningitis PDFrizeviBelum ada peringkat
- Crux 05Dokumen8 halamanCrux 05Rajkishor YadavBelum ada peringkat
- Meningitis Clinical PresentationDokumen10 halamanMeningitis Clinical PresentationAniwat NillakarnBelum ada peringkat
- Case Study of Neurological DisordersDokumen9 halamanCase Study of Neurological DisordersSkyerexBelum ada peringkat
- Cerebro Spinal Fluid Analysis in Childhood Bacterial MeningitisDokumen2 halamanCerebro Spinal Fluid Analysis in Childhood Bacterial MeningitisPinto D. R PiliangBelum ada peringkat
- Meningitis (Physical Exam)Dokumen6 halamanMeningitis (Physical Exam)MohammadAwitBelum ada peringkat
- Immunodeficiency 2017 FinalA - 304963 - 284 - 2104 - v3Dokumen6 halamanImmunodeficiency 2017 FinalA - 304963 - 284 - 2104 - v3Muhammed Hashim MBelum ada peringkat
- Background: Pneumonia Arthritis Osteomyelitis SepsisDokumen53 halamanBackground: Pneumonia Arthritis Osteomyelitis SepsisWuwun NurulhidayatiBelum ada peringkat
- A Chilly FeverDokumen5 halamanA Chilly FeverChangBelum ada peringkat
- Fever and PetechiaeDokumen9 halamanFever and PetechiaeJohn Carlo PizarraBelum ada peringkat
- A Chilly Fever: Clinical Problem-SolvingDokumen5 halamanA Chilly Fever: Clinical Problem-SolvingJuan Carlos Carbajal SilvaBelum ada peringkat
- North America. 2002 49: 1009-1025.: ND THDokumen5 halamanNorth America. 2002 49: 1009-1025.: ND THJim MayorgaBelum ada peringkat
- Incidence and Risk Factors For Seizures in Central Nervous System Infections in ChildhoodDokumen6 halamanIncidence and Risk Factors For Seizures in Central Nervous System Infections in ChildhoodLiani Toniaço BellesBelum ada peringkat
- Febrile ChildDokumen43 halamanFebrile Childdagnenegash19Belum ada peringkat
- Babesiosis in Pregnancy: An Imitator of HELLP SyndromeDokumen6 halamanBabesiosis in Pregnancy: An Imitator of HELLP Syndromemade dharmaBelum ada peringkat
- Acute Viral Encephalitis - NEJMDokumen17 halamanAcute Viral Encephalitis - NEJMFredghcBelum ada peringkat
- 1) - Oral Exam Revealed A White Strawberry Tongue: Ecker, Habashy, SkopitDokumen5 halaman1) - Oral Exam Revealed A White Strawberry Tongue: Ecker, Habashy, SkopitAngela DuangchitBelum ada peringkat
- Investigatory Project On MeningitisDokumen15 halamanInvestigatory Project On MeningitisMaanya PrithianiBelum ada peringkat
- Level of Evidence 3Dokumen4 halamanLevel of Evidence 3andamar0290Belum ada peringkat
- Final Infectious Pe - 1-EditDokumen59 halamanFinal Infectious Pe - 1-EditAhmad SobihBelum ada peringkat
- Bacterial Meningitis in Infants Over 3 Months of AgeDokumen8 halamanBacterial Meningitis in Infants Over 3 Months of AgedrirrazabalBelum ada peringkat
- Approachtocommonbacterial Infections:: Community-Acquired PneumoniaDokumen17 halamanApproachtocommonbacterial Infections:: Community-Acquired PneumoniaFátima MartínezBelum ada peringkat
- Recurrent Aseptic Meningitis in A Child: Case ReportDokumen3 halamanRecurrent Aseptic Meningitis in A Child: Case ReportAprimadhansari FarzimBelum ada peringkat
- Complications of Infectious Mononucleosis in Children: PediatricsDokumen10 halamanComplications of Infectious Mononucleosis in Children: PediatricsOrhan ErBelum ada peringkat
- "Neonatal Infections" Lecture 1: Pediatrics Dr. Sawsan AliDokumen5 halaman"Neonatal Infections" Lecture 1: Pediatrics Dr. Sawsan AliAmmarBelum ada peringkat
- 278 977 1 SMDokumen2 halaman278 977 1 SMMohamed MukhrizBelum ada peringkat
- Aseptic Meningitides, Is Caused by A Systemic Viral Infection Whose Infectivity WithinDokumen9 halamanAseptic Meningitides, Is Caused by A Systemic Viral Infection Whose Infectivity WithinEveline YuniartiBelum ada peringkat
- Istory: Pneumoniae MeningitisDokumen2 halamanIstory: Pneumoniae MeningitisMohammadAwitBelum ada peringkat
- Etiologies of Fever of Unknown Origin in Adults - UpToDate (2019) PDFDokumen17 halamanEtiologies of Fever of Unknown Origin in Adults - UpToDate (2019) PDFMoisés León RuizBelum ada peringkat
- Two Cases and A Review of Streptococcus Pyogenes Endocarditis in ChildrenDokumen6 halamanTwo Cases and A Review of Streptococcus Pyogenes Endocarditis in ChildrenShigellaKimBelum ada peringkat
- Typhoid Fever/Paratyphoid Fever (Enteric Fever) : Disease PlanDokumen16 halamanTyphoid Fever/Paratyphoid Fever (Enteric Fever) : Disease PlanBayu Surya DanaBelum ada peringkat
- Meningitis, AAFPDokumen8 halamanMeningitis, AAFPCarlos Danilo Noroña CBelum ada peringkat
- Patho Unit 5Dokumen37 halamanPatho Unit 5Shafiya ShaikBelum ada peringkat
- Meningitis: Author InformationDokumen10 halamanMeningitis: Author Informationakbar011512Belum ada peringkat
- Republic of The Philippines Tarlac State University College of Nursing Graduate Studies ProgramDokumen9 halamanRepublic of The Philippines Tarlac State University College of Nursing Graduate Studies ProgramCorinthiaMaeMarceloBelum ada peringkat
- Anatomy & Medicine MCQs April 14Dokumen21 halamanAnatomy & Medicine MCQs April 14sb medexBelum ada peringkat
- Atypical MeaslesDokumen2 halamanAtypical MeaslesBagusPranataBelum ada peringkat
- Subacute and Chronic MeningitisDokumen8 halamanSubacute and Chronic MeningitisLucky PuspitasariBelum ada peringkat
- Assessment: Infection May Progress To A Life-Threatening Illness If Antibiotic Treatment Is Not GivenDokumen28 halamanAssessment: Infection May Progress To A Life-Threatening Illness If Antibiotic Treatment Is Not GivenCabdiBelum ada peringkat
- Case Report: Fatal Staphylococcal Infection Following Classic Dengue FeverDokumen4 halamanCase Report: Fatal Staphylococcal Infection Following Classic Dengue FeverRia Septi HarmiaBelum ada peringkat
- Aseptic MeningitisDokumen24 halamanAseptic Meningitisidno1008100% (1)
- Acute Bacterial and Viral MeningitisDokumen16 halamanAcute Bacterial and Viral MeningitisMaharani MahaBelum ada peringkat
- Antibioticoterapia Na Pneumonia Pediátrica 2006Dokumen7 halamanAntibioticoterapia Na Pneumonia Pediátrica 2006Tainah__100% (1)
- Dengue CurrentDokumen6 halamanDengue CurrentDani BurgosBelum ada peringkat
- Group 1 - Case PresentationDokumen70 halamanGroup 1 - Case PresentationVeejay CervantesBelum ada peringkat
- Bacterial Meningitis in ChildrenDokumen10 halamanBacterial Meningitis in ChildrenAnny AryanyBelum ada peringkat
- Evaluation of The Febrile Patient A Case-Based Approach: Fevers and Fevers of Unknown OriginDokumen97 halamanEvaluation of The Febrile Patient A Case-Based Approach: Fevers and Fevers of Unknown Originkrish vjBelum ada peringkat
- Dorsett2016 PDFDokumen26 halamanDorsett2016 PDFSirGonzBelum ada peringkat
- Epstein-Barr Virus Infectious Mononucleosis: MARK H. EBELL, M.D, M.S., Athens, GeorgiaDokumen9 halamanEpstein-Barr Virus Infectious Mononucleosis: MARK H. EBELL, M.D, M.S., Athens, GeorgiaMagda MariaBelum ada peringkat
- MononucleosisDokumen9 halamanMononucleosisLucas TobingBelum ada peringkat
- Diagnostics of Acute Rheumatic Liquorad (Literature Review)Dokumen4 halamanDiagnostics of Acute Rheumatic Liquorad (Literature Review)Central Asian StudiesBelum ada peringkat
- Infectious Disease Sample QuestionsDokumen7 halamanInfectious Disease Sample QuestionsPAUL JASPER RONQUILLOBelum ada peringkat
- Meningitidis Consists of The Sudden Onset of Fever, Nausea, Vomiting, HeadacheDokumen9 halamanMeningitidis Consists of The Sudden Onset of Fever, Nausea, Vomiting, HeadacheEduardo Romero StéfaniBelum ada peringkat
- A Doctors Duty To Help in A Medical EmergencyDokumen4 halamanA Doctors Duty To Help in A Medical EmergencyCheng XinvennBelum ada peringkat
- Enxm, e Linked Immunosorbant Assay, Elisa, Detectantiboties To Hic Sensitive But Not Specific, Estern Blot Fone Elisan Opstive, Polymerase Chain Reaction PCR, Alternatibe M, Eans Fo TetsingDokumen1 halamanEnxm, e Linked Immunosorbant Assay, Elisa, Detectantiboties To Hic Sensitive But Not Specific, Estern Blot Fone Elisan Opstive, Polymerase Chain Reaction PCR, Alternatibe M, Eans Fo TetsingCheng XinvennBelum ada peringkat
- Jwehgrjwhegrjwehgrjhk 4 UhDokumen1 halamanJwehgrjwhegrjwehgrjhk 4 UhCheng XinvennBelum ada peringkat
- Aid To Multiple Choice Questions in Surgery 20161013Dokumen166 halamanAid To Multiple Choice Questions in Surgery 20161013Cheng Xinvenn100% (1)
- Interview For Med Officer at BDH 210721Dokumen1 halamanInterview For Med Officer at BDH 210721Cheng XinvennBelum ada peringkat
- Urehcny, Upposed Detrusonr Ocntraction, CoDokumen1 halamanUrehcny, Upposed Detrusonr Ocntraction, CoCheng XinvennBelum ada peringkat
- Hpwriteup ExampleDokumen6 halamanHpwriteup ExampleCheng XinvennBelum ada peringkat
- Floor Plan CFCSDokumen1 halamanFloor Plan CFCSCheng XinvennBelum ada peringkat
- Nbme Block 1 1. Adenocarcinoma of Endometrium 2. Amitriptyline 3. DJ 4. D 5. C 6. A 7. DCDCCDC 8. A 9. A 10. XCXC 11. XCXC 12. XCXC 13. XCXC 14. DDokumen1 halamanNbme Block 1 1. Adenocarcinoma of Endometrium 2. Amitriptyline 3. DJ 4. D 5. C 6. A 7. DCDCCDC 8. A 9. A 10. XCXC 11. XCXC 12. XCXC 13. XCXC 14. DCheng XinvennBelum ada peringkat
- Sample-Case Report - Sem 5Dokumen4 halamanSample-Case Report - Sem 5Cheng XinvennBelum ada peringkat
- AndgfhgvhDokumen1 halamanAndgfhgvhCheng XinvennBelum ada peringkat
- GINA Pocket 2014 Jun11Dokumen32 halamanGINA Pocket 2014 Jun11Gilbert Petrus Richard SamsBelum ada peringkat
- Pathogenesis of Erectile DysfunctionDokumen4 halamanPathogenesis of Erectile DysfunctionCheng XinvennBelum ada peringkat
- Montelukast SodiumDokumen3 halamanMontelukast SodiumCheng Xinvenn0% (1)
- Nbme Block 1 1. Adenocarcinoma of Endometrium 2. Amitriptyline 3. DJ 4. D 5. C 6. A 7. DCDCCDC 8. A 9. A 10. XCXC 11. XCXC 12. XCXC 13. XCXC 14. DDokumen1 halamanNbme Block 1 1. Adenocarcinoma of Endometrium 2. Amitriptyline 3. DJ 4. D 5. C 6. A 7. DCDCCDC 8. A 9. A 10. XCXC 11. XCXC 12. XCXC 13. XCXC 14. DCheng XinvennBelum ada peringkat
- PancreasDokumen11 halamanPancreasCheng XinvennBelum ada peringkat
- Anti-Fungal Drugs SushDokumen31 halamanAnti-Fungal Drugs SushCheng XinvennBelum ada peringkat
- Causes of Vaginal DischargeDokumen2 halamanCauses of Vaginal DischargeCheng XinvennBelum ada peringkat
- Pituitary GlandDokumen18 halamanPituitary GlandCheng XinvennBelum ada peringkat
- Alcohol HistoryDokumen11 halamanAlcohol HistoryCheng XinvennBelum ada peringkat
- Adaptive Changes of Remaining and Transplanted KidneyDokumen2 halamanAdaptive Changes of Remaining and Transplanted KidneyCheng XinvennBelum ada peringkat
- Semester Three ME113Dokumen36 halamanSemester Three ME113Cheng XinvennBelum ada peringkat
- CA STD Clinician Guide Sexual History TakingDokumen5 halamanCA STD Clinician Guide Sexual History TakingCheng XinvennBelum ada peringkat
- AtaxiaDokumen1 halamanAtaxiaCheng XinvennBelum ada peringkat
- Module 4 - History Taking and Risk AssessmentDokumen22 halamanModule 4 - History Taking and Risk AssessmentCheng XinvennBelum ada peringkat
- 03 (Selective BLS)Dokumen1 halaman03 (Selective BLS)Cheng XinvennBelum ada peringkat
- Stem CellDokumen17 halamanStem Cellsonal agarwalBelum ada peringkat
- Baromtric (89 Pages)Dokumen89 halamanBaromtric (89 Pages)jahangirealamBelum ada peringkat
- Benign Prostat HiperplasiaDokumen17 halamanBenign Prostat HiperplasiahawhawnurBelum ada peringkat
- 1978 Caramazza Berndt PBDokumen21 halaman1978 Caramazza Berndt PBEddiesvoiceBelum ada peringkat
- Carrie Frechette, LVN: ContactDokumen2 halamanCarrie Frechette, LVN: ContactCARRIE FRECHETTEBelum ada peringkat
- SMA Advanced Sports Taping Presentation DSR Handouts PDFDokumen4 halamanSMA Advanced Sports Taping Presentation DSR Handouts PDFHamada Said AliBelum ada peringkat
- Chapter 3 PsychDokumen12 halamanChapter 3 Psychred_gyrl9282Belum ada peringkat
- Bionic Eye: Department of Electronics and Communication Engineering Kle Institute of Technology HubliDokumen25 halamanBionic Eye: Department of Electronics and Communication Engineering Kle Institute of Technology HubliAbhishek MatBelum ada peringkat
- Medsurg (112) Rle: Care of Patients With Problems of The Hematologic SystemDokumen5 halamanMedsurg (112) Rle: Care of Patients With Problems of The Hematologic SystemChelsea Faith SarandiBelum ada peringkat
- Valve Repair & ReplacementDokumen27 halamanValve Repair & ReplacementmaibejoseBelum ada peringkat
- CTNeoBC Pooled AnalysisDokumen9 halamanCTNeoBC Pooled AnalysistabaresgonzaloBelum ada peringkat
- Magnet Therapyncreasingly Gaining in Popularity The World OverDokumen3 halamanMagnet Therapyncreasingly Gaining in Popularity The World OverPradeep PatilBelum ada peringkat
- Total Standards: - Total Sub-Standards: - Total ESR StandardsDokumen8 halamanTotal Standards: - Total Sub-Standards: - Total ESR StandardsHCX dghhqBelum ada peringkat
- Epidural Hematom Merck ManualDokumen4 halamanEpidural Hematom Merck ManualA Novita Dewi AryantiBelum ada peringkat
- A&E BlepharitisDokumen3 halamanA&E BlepharitisNadine Bär-SchmitzBelum ada peringkat
- Lewis COPD Case StudyDokumen2 halamanLewis COPD Case Studyatarisgurl08Belum ada peringkat
- In-Patient Claim Form - ConventionalDokumen2 halamanIn-Patient Claim Form - ConventionalAbdul Qayyum Sipra Madduki50% (2)
- Good Posture... Just How Important Is It?: Source: Kansas Chiropractic Foundation WebsiteDokumen4 halamanGood Posture... Just How Important Is It?: Source: Kansas Chiropractic Foundation WebsiteJacklynlim LkcBelum ada peringkat