United States Patent Office: Patented Jan. I9, 1954
Diunggah oleh
Javier0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
16 tayangan2 halamanThis 3-sentence summary provides the high-level information from the document:
The document describes a patented method for making stable cupric hydroxide and sodium sulfate through a controlled reaction between copper sulfate and sodium hydroxide in alternating steps. The method involves dissolving trisodium phosphate and copper sulfate, then adding sodium hydroxide, and repeating the additions of copper sulfate and sodium hydroxide until cupric hydroxide accumulates, which is then filtered out while the sodium sulfate remains in solution. This cyclic process allows for continuous production of cupric hydroxide and sodium sulfate through repeated chemical reactions between the compounds.
Deskripsi Asli:
Patente: METHOD OF MAKING STABLE CUPRIC
HYDROXIDE
Judul Asli
Us 2666688
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniThis 3-sentence summary provides the high-level information from the document:
The document describes a patented method for making stable cupric hydroxide and sodium sulfate through a controlled reaction between copper sulfate and sodium hydroxide in alternating steps. The method involves dissolving trisodium phosphate and copper sulfate, then adding sodium hydroxide, and repeating the additions of copper sulfate and sodium hydroxide until cupric hydroxide accumulates, which is then filtered out while the sodium sulfate remains in solution. This cyclic process allows for continuous production of cupric hydroxide and sodium sulfate through repeated chemical reactions between the compounds.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
16 tayangan2 halamanUnited States Patent Office: Patented Jan. I9, 1954
Diunggah oleh
JavierThis 3-sentence summary provides the high-level information from the document:
The document describes a patented method for making stable cupric hydroxide and sodium sulfate through a controlled reaction between copper sulfate and sodium hydroxide in alternating steps. The method involves dissolving trisodium phosphate and copper sulfate, then adding sodium hydroxide, and repeating the additions of copper sulfate and sodium hydroxide until cupric hydroxide accumulates, which is then filtered out while the sodium sulfate remains in solution. This cyclic process allows for continuous production of cupric hydroxide and sodium sulfate through repeated chemical reactions between the compounds.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 2
2,6'66,688
Patented Jan. I9, 1954
UNITED STATES PATENT OFFICE
2,666,688
METHOD OF MAKING STABLE CUPRIC
HYDROXIDE
William H. Furness, Haddonfield, N. J., assignm
to Copper Research, a corporation of New
Jersey
No Drawing. Application May 5, 1951,
Serial No. 224,840
3 Claims. (Cl. 23-147)
1
The object of this invention is to provide a
simple, inexpensive method for making stable
cupric hydroxide, with a by-product of sodium
sulphate, by means of a controlled, indirect re
action between copper sulphate and sodium hy
droxide.
It is known that stable, separable cupric hy
droxide cannot be made by direct reaction be
tween copper sulphate and sodium hydroxide.
The combination of these two chemicals results
in the formation of a gelatinous, inseparable
sludge of blue cupric hydroxide which rapidly
changes to a useless sludge of black cupric oxide.
Because of the lack of a simple, inexpensive
As the volume remaining in the tank is greatly
reduced by removal of the supernatant sodium
sulphate solution the step procedure may be re
sumed by adding caustic soda, as in step 4, fol
lowed by copper sulphate as in step 3, repeatedly
until the tank is again nearly ?lled, when settling
and decantation are again carried out.
The step process is continued until the in
soluble cupric hydroxide, resulting from the in
termittent alternate additions and reactions of
copper sulphate and sodium hydroxide, nearly
?lls the tank after settling. At this stage the
process is stopped at a point corresponding to
step 4, that is, with a ?nal addition of the quan
method of manufacture, cupric hydroxide has
tity of sodium hydroxide speci?ed in that step.
not heretofore been generally available for com
merce in the large quantities required for use as
The entire contents of the tank is then fed to a
?lter on which the cupric hydroxide is collected;
the ?ltrate, containing the original quantity of
trisodium phosphate, in solution, is returned to
the mixing tank, where the process of adding
alternate quantities of copper sulphate and so
dium hydroxide may be repeated indefinitely as
described.
The following chemical formula is offered as
a fungicide in agriculture, for antifouling ship
bottom paints, and other similar purposes.
In my process, pure, stable cupric hydroxide is
made from copper sulphate and sodium hydrox
ide in the following manner:
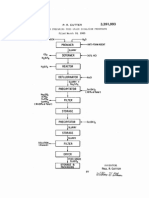
Step 1.-Dissolve 380 parts trisodium phos
phate (NaaPOdZI-IzO) using only enough Water
to afford complete solution at room temperature.
Step 2.-Place the trisodium phosphate solu
tion of step 1 in a tank of large size compared
to the volume of the solution; for example, a 100
gallon tank if the volume of solution prepared
under step 1 is one gallon.
an indication of probable reactions which occur
repeatedly during the process as new additions of
copper sulphate and sodium hydroxide are made:
CuSO4 +NaaPO4=CuNaPO4+Na2SO4
2NaOH+CuNaPO4=NaaPOr + Cu (OH) 2
As the time element of the chemical reactions
Step 3.To the trisodium phosphate solution
involved in the process is very small, and no ap
in the tank add 250 parts copper sulphate
preciable increase of temperature occurs, the
(CuSO45H2O) dissolved in the minimum quantity
alternate additions of copper sulphate and so
of water required for solution at room tempera
ture. Mix bystirring.
do dium hydroxide may be made at brief intervals,
and large quantities of cupric hydroxide and so
Step 4.-Add to the contents of the tank a
solution containing 80 parts sodium hydroxide
(NaOH), in a concentration of about 80 gm. per
liter at room temperature. Mix by stirring.
Step 5.Add a second quantity of copper sul
phate solution as in step 3.
Step 6.-Add a second quantity of sodium hy
droxide as in step 4.
Continue the process by adding successive
alternate quantities of copper sulphate and so
dium hydroxide until the tank is nearly full. No
trisodium phosphate is employed other than the
original quantity speci?ed in step 1.
When the tank is nearly full the process is
stopped at a point corresponding with step 3,
that is, after the addition of copper sulphate, and
the precipitate is permitted-to settle. The super
giium sulphate are thus accumulated in a short
me.
While my method may be used to make single
batches of cupric hydroxide, I prefer to employ
the step method described, in which only a rela
tively small and ?xed quantity of trisodlum phos
phate or copper sodium phosphate alternatively
are present. Copper sodium phosphate is dliii
cult to remove by settling or ?ltration on a large
scale, while the small quantities present at inter
vals in the step process are carried down with the
rapidly settling accumulation of cupric hydrox
ide, and do not interfere with the decantation of
the supernatant liquid containing the sodium
sulphate in solution.
The action of trisodium phosphate on copper
sulphate in aqueous solution is such that all of
natant liquid is then drawn off for recovery of
sodium sulphate, which at this point is the only
the copper contained in a molecular equivalent
55 of copper sulphate is precipitated by the addition
chemica1 in solution.
2,666,688
of less than a molecular equivalent or trisodium
copper sodium phosphate, and to react with all
phosphate. For example, if 250 gms. of trisodium
of the sulphate radical of the copper sulphate to
phosphate (NaJPOlIZHzO) are added to 250 gms
form sodium sulphate in solution, and then add
copper sulphate (CuSO45HzO) in aqueous solu
ing to the mixture a sufficient quantity of caus
tion, all of the copper will be precipitated and
tic soda to convert the insoluble copper sodium
the water will show a neutral reaction with phe~
phosphate to insoluble cupric hydroxide and tri
nolphthalein. Any additional amount of tri
sodium phosphate and removing the cupric hy
sodium phosphate iNaaPo-llzllzo) up to a total
droxide by filtration; thereafter adding to the ?l
of 380 gms. will react with the precipitated cop
trate suilicient copper sulphate to react with the
per, the water remaining neutral. The nature ll trisodium phosphate present therein to form cop
of the copper precipitate varies with the amount
per sodium phosphate and sodium sulphate and
of trisodium phosphate added but all such pre
removing the insoluble copper sodium phosphate
cipitates are converted to cupric hydroxide by the
by ?ltration and recovering the sodium sulphate
addition of a molecular proportion of sodium
from the ?ltrate by crystallization or evaporation.
hydroxide, the trisodium phosphate being regen
3. The cyclic process of making cupric hydrox
erated in the amount originally used in each
ide from copper sulphate and caustic soda in
instance. While I prefer to use trisodium phos
which molecular-1y equivalent and equal unit
phate in the proportion of 380 gms.
masses of copper sulphate and caustic soda are
added repeatedly and alternately to a volume of
20 water containing a quantity of trisodium phos
to 250 gms. copper sulphate (CIISOq?HzOl because
phate su?icient to convert all of the copper con
tained in the unit of copper sulphate ?rst added
a better grade of cuprlc hydroxide results from
to insoluble copper sodium phosphate and sodium
that formula, it is to be understood that the scope
sulphate, and in which each addition of copper
of my invention includes the use of any smaller
proportion of trisodium phosphate which will " sulphate is followed by a molecularly equivalent
entirely precipitate copper sulphate from solution.
quantity of caustic soda which reacts with the
copper sodium phosphate resulting from the pre~
I claim:
ceding step to form cupric hydroxide and tri
1. The method of making cupric hydroxide and
sodium phosphate, the repeated alternate addi
sodium sulphate from copper sulphate and sodium
hydroxide which consists of mixing 250 parts of
tions of the unit masses of copper sulphate and
copper sulphate (CUSO45H2O) with 380 parts of
caustic soda resulting in an accumulation of
cupric hydroxide and sodium sulphate in the mix~
trisodium phosphate (NaaPOdZHzO) in aqueous
solution and subsequently adding thereto an
ture, from which part of the cupric hydroxide is
aqueous solution containing 80 parts of sodium
removed from time to time by ?ltration at any
hydroxide (NaOH), to form approximately 9;?
interval following an addition of caustic soda
parts cupric hydroxide Cu(0H)2, 142 parts of
when the phosphate radical is in solution, and
sodium sulphate (NazSOq), and 194 parts of tri
part of the accumulated sodium sulphate is
sodium phosphate (Na:PO4) , removing the cupric
removed from time to time by ?ltration or decan
hydroxide by ?ltration and repeating the process
tation during an interval following an addition
by adding250 gms. copper sulphate
of copper sulphate when the phosphate radical
is present in the mixture in insoluble form.
(CuSOalOHzO)
WILLIAM H. FURNESS.
to the ?ltrate, followed by 80 gms. caustic soda
(Na-OH) to produce a second 98 gms. cupric
References Cited in the ?le of this patent
hydroxide.
UNITED STATES PATENTS
2. The method of making cupric hydroxide and
sodium sulphate from copper sulphate and caus
Number
Name
Date
tic soda by adding trisodium phosphate to a cop
1,800,828
Furness __________ __ Apr. 14, 1931
per sulphate solution in suf?cient quantity to pre
2,168,985
Gulbrandsen ______ __ Aug. 8, 1939
cipitate all of the copper contained therein as 50 2,532,308
Hoffman __________ __ Dec. 5, 1950
Anda mungkin juga menyukai
- This Invention Relates To A Process For Making A Stable CopperDokumen3 halamanThis Invention Relates To A Process For Making A Stable CopperAngel BuenoBelum ada peringkat
- Determination of Gamma No and TSDokumen3 halamanDetermination of Gamma No and TSAditya ShrivastavaBelum ada peringkat
- Mm'mon FOR MAKIN: Filed Aug. 8, 1925Dokumen4 halamanMm'mon FOR MAKIN: Filed Aug. 8, 1925arufatoBelum ada peringkat
- United States Patent Office: Production of Disopum PhosphateDokumen2 halamanUnited States Patent Office: Production of Disopum PhosphatefredyBelum ada peringkat
- US2321218Dokumen3 halamanUS2321218shirazizadehsinaBelum ada peringkat
- Translate Paten US5976485Dokumen24 halamanTranslate Paten US5976485Lenywulandari AyundaBelum ada peringkat
- A Safe Method For Preparation of Uncontaminated Hydrazoic AcidDokumen1 halamanA Safe Method For Preparation of Uncontaminated Hydrazoic Acidgeovani2Belum ada peringkat
- KOH From K2SO4 and NaOHDokumen3 halamanKOH From K2SO4 and NaOHamirBelum ada peringkat
- Production of Sodium DithioniteDokumen10 halamanProduction of Sodium DithioniteDhaval PadaliaBelum ada peringkat
- Arsenal Philadelphia, Pa. 19137: FrankfordDokumen23 halamanArsenal Philadelphia, Pa. 19137: FrankfordPutri PramodyaBelum ada peringkat
- Filed June l5, 1935Dokumen6 halamanFiled June l5, 1935Yustinus Selis ToronBelum ada peringkat
- Unite Sites Fret (19) : DahlinDokumen3 halamanUnite Sites Fret (19) : Dahlintrinh xuan hiepBelum ada peringkat
- US3347627Dokumen3 halamanUS3347627Nuttapong JongjitsatitmunBelum ada peringkat
- Patent Office: 5 Claims. (CL 260-69)Dokumen2 halamanPatent Office: 5 Claims. (CL 260-69)Teleson MarquesBelum ada peringkat
- US1960211 (Sudah)Dokumen3 halamanUS1960211 (Sudah)aris_nurhidayatBelum ada peringkat
- Sodium Hydroxide Production With A Calcium CarbonaDokumen7 halamanSodium Hydroxide Production With A Calcium CarbonaFebri SandiBelum ada peringkat
- United States Patent PO: Patented Nov. 20, 1956Dokumen2 halamanUnited States Patent PO: Patented Nov. 20, 1956shenn0Belum ada peringkat
- American Government 13th Edition Volkomer Test Bank Full Chapter PDFDokumen22 halamanAmerican Government 13th Edition Volkomer Test Bank Full Chapter PDFnicholassmithyrmkajxiet100% (14)
- United States Patent 0: '3, l50, l74 ICCDokumen2 halamanUnited States Patent 0: '3, l50, l74 ICCMuhammadAmdadulHoqueBelum ada peringkat
- Effect of Sodium Salts On Demercaptanization ProcessDokumen4 halamanEffect of Sodium Salts On Demercaptanization ProcessInternational Journal of Science and Engineering InvestigationsBelum ada peringkat
- UNITED Starts: Patented Apr. 16, 1935Dokumen2 halamanUNITED Starts: Patented Apr. 16, 1935shalsinia chantalBelum ada peringkat
- Patente 2Dokumen3 halamanPatente 2Saul MamaniBelum ada peringkat
- United States: Patent OfficeDokumen3 halamanUnited States: Patent OfficefredyBelum ada peringkat
- Storage: o - InzoDokumen4 halamanStorage: o - InzoOscar SobradosBelum ada peringkat
- US3036885Dokumen2 halamanUS3036885pradipBelum ada peringkat
- US3303001Dokumen3 halamanUS3303001Lokesh RavichandranBelum ada peringkat
- AzufreDokumen8 halamanAzufreKike KikinBelum ada peringkat
- US3689216Dokumen5 halamanUS3689216PABLO URIZ CEREZOBelum ada peringkat
- Us 744128Dokumen2 halamanUs 744128haviedBelum ada peringkat
- J. N. CarothersDokumen4 halamanJ. N. CarothershaviedBelum ada peringkat
- Indigo Prodn. From Phenyl-Glycine Carboxylic Acid Salt - by Fusion in Mixed Potassium Hydroxide and Sodium Hydroxide Melt, Then OxidnDokumen4 halamanIndigo Prodn. From Phenyl-Glycine Carboxylic Acid Salt - by Fusion in Mixed Potassium Hydroxide and Sodium Hydroxide Melt, Then OxidnCillian CreedonBelum ada peringkat
- Process For Preparing Both Barium Sulfate and Calcium Chloride From Waste Ardealite DregsDokumen6 halamanProcess For Preparing Both Barium Sulfate and Calcium Chloride From Waste Ardealite DregsAgam WirasaniBelum ada peringkat
- The Reaction Between Sulfur and Calcium Hydroxide PDFDokumen4 halamanThe Reaction Between Sulfur and Calcium Hydroxide PDFJavier Aviles100% (1)
- LD2668T41956F45Dokumen49 halamanLD2668T41956F45bitisa5368Belum ada peringkat
- Hydrogen Peroxide PropertiesDokumen22 halamanHydrogen Peroxide PropertiesValentin HueBelum ada peringkat
- United States Patent (19) : Diercks Et Al. (11) Patent NumberDokumen5 halamanUnited States Patent (19) : Diercks Et Al. (11) Patent NumberVirginia Rosales OlmosBelum ada peringkat
- Boiler Water Treatment FundamentalsDokumen8 halamanBoiler Water Treatment FundamentalssauravsinhaaBelum ada peringkat
- Us 2375054Dokumen3 halamanUs 2375054haviedBelum ada peringkat
- US2364993Dokumen2 halamanUS2364993aditya_lomteBelum ada peringkat
- EPA Method 9030B - Acid Soluble and Acid Insoluble Sulfides DistillationDokumen15 halamanEPA Method 9030B - Acid Soluble and Acid Insoluble Sulfides DistillationArmando Fuentes BenitesBelum ada peringkat
- IntroductionDokumen10 halamanIntroductionAmith Singh J100% (1)
- Quantitative Analysis of Potassium and Sodium in The UringDokumen2 halamanQuantitative Analysis of Potassium and Sodium in The UringidownloadbooksforstuBelum ada peringkat
- A Novel Process To Recover Sulfur in Aqueous Phase Under Ambient Condition - SpringerLinkDokumen16 halamanA Novel Process To Recover Sulfur in Aqueous Phase Under Ambient Condition - SpringerLinkmodikiritBelum ada peringkat
- Sulfur and Sulfuric AcidDokumen24 halamanSulfur and Sulfuric AciddhavalBelum ada peringkat
- Process For The Manufacturing of Copper SulphateDokumen3 halamanProcess For The Manufacturing of Copper Sulphaterajesh80% (5)
- Merox Catalyst ImpregnationDokumen8 halamanMerox Catalyst ImpregnationLijo ThomasBelum ada peringkat
- US3416887Dokumen6 halamanUS3416887khairulBelum ada peringkat
- Patent US2446233Dokumen3 halamanPatent US2446233Alan ConnorBelum ada peringkat
- Production of Sulfuric AcidDokumen29 halamanProduction of Sulfuric Aciddeshaka11Belum ada peringkat
- Us 2021699Dokumen5 halamanUs 2021699haviedBelum ada peringkat
- Unied States W 0: Patented Nov. 11, 1969Dokumen2 halamanUnied States W 0: Patented Nov. 11, 1969Yeisson MoraBelum ada peringkat
- United States Patent 0: Patented Jan. 26, 1965 2Dokumen5 halamanUnited States Patent 0: Patented Jan. 26, 1965 2Waheed ZebBelum ada peringkat
- Chlorine Dioxide ProcessesDokumen4 halamanChlorine Dioxide ProcessesKani Kanii50% (2)
- The Determination of Sulfate and Sulfide Sulfur in Rocks or MineralsDokumen9 halamanThe Determination of Sulfate and Sulfide Sulfur in Rocks or Mineralssupendra phuyalBelum ada peringkat
- United States Patent Office: Patented Sept. 5, 1950Dokumen2 halamanUnited States Patent Office: Patented Sept. 5, 1950ari factoryBelum ada peringkat
- Benzoic Acid To Benzaldehyde, P-Nitrobenzoic Acid To Nitrobenzene and More.Dokumen3 halamanBenzoic Acid To Benzaldehyde, P-Nitrobenzoic Acid To Nitrobenzene and More.banjo010% (1)
- Hummer's MethodDokumen1 halamanHummer's MethodPrajwal Bikram Thapa0% (1)
- Legal Chemistry: A Guide to the Detection of Poisons, Examination of Tea, Stains, Etc., as Applied to Chemical JurisprudenceDari EverandLegal Chemistry: A Guide to the Detection of Poisons, Examination of Tea, Stains, Etc., as Applied to Chemical JurisprudenceBelum ada peringkat
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresDari EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresPenilaian: 5 dari 5 bintang5/5 (1)
- Dehydration AssessmentDokumen2 halamanDehydration AssessmentzaheerbdsBelum ada peringkat
- The Story of An Hour QuestionpoolDokumen5 halamanThe Story of An Hour QuestionpoolAKM pro player 2019Belum ada peringkat
- STAB 2009 s03-p1Dokumen16 halamanSTAB 2009 s03-p1Petre TofanBelum ada peringkat
- EffectivenessDokumen13 halamanEffectivenessPhillip MendozaBelum ada peringkat
- ACTIVITY Design - Nutrition MonthDokumen7 halamanACTIVITY Design - Nutrition MonthMaria Danica89% (9)
- Aryan Civilization and Invasion TheoryDokumen60 halamanAryan Civilization and Invasion TheorySaleh Mohammad Tarif 1912343630Belum ada peringkat
- Actara (5 24 01) PDFDokumen12 halamanActara (5 24 01) PDFBand Dvesto Plus CrepajaBelum ada peringkat
- Class 12 Unit-2 2022Dokumen4 halamanClass 12 Unit-2 2022Shreya mauryaBelum ada peringkat
- Service M5X0G SMDokumen98 halamanService M5X0G SMbiancocfBelum ada peringkat
- Suggestions On How To Prepare The PortfolioDokumen2 halamanSuggestions On How To Prepare The PortfolioPeter Pitas DalocdocBelum ada peringkat
- Reviewer CSCDokumen22 halamanReviewer CSCChristopher CocalBelum ada peringkat
- Technical Data - Tad1342veDokumen9 halamanTechnical Data - Tad1342veRachid SmailiBelum ada peringkat
- Cognitive Coaching AdelaideDokumen3 halamanCognitive Coaching AdelaideBusiness-Edu100% (2)
- Service Manual: NISSAN Automobile Genuine AM/FM Radio 6-Disc CD Changer/ Cassette DeckDokumen26 halamanService Manual: NISSAN Automobile Genuine AM/FM Radio 6-Disc CD Changer/ Cassette DeckEduardo Reis100% (1)
- ETSI EG 202 057-4 Speech Processing - Transmission and Quality Aspects (STQ) - Umbrales de CalidaDokumen34 halamanETSI EG 202 057-4 Speech Processing - Transmission and Quality Aspects (STQ) - Umbrales de Calidat3rdacBelum ada peringkat
- Dekker V Weida Amicus Brief by 17 AGsDokumen35 halamanDekker V Weida Amicus Brief by 17 AGsSarah WeaverBelum ada peringkat
- Industry GeneralDokumen24 halamanIndustry GeneralilieoniciucBelum ada peringkat
- Agreement - IDP - Transformation Institute - 2023Dokumen16 halamanAgreement - IDP - Transformation Institute - 2023Elite ProgramBelum ada peringkat
- Promotion of Coconut in The Production of YoghurtDokumen4 halamanPromotion of Coconut in The Production of YoghurtԱբրենիկա ՖերլինBelum ada peringkat
- Catalog de Aparatura Si Instrumentar Veterinar Eikemeyer-GermaniaDokumen336 halamanCatalog de Aparatura Si Instrumentar Veterinar Eikemeyer-GermaniaDr. Dragos CobzariuBelum ada peringkat
- Supreme Court Case Analysis-Team ProjectDokumen5 halamanSupreme Court Case Analysis-Team ProjectJasmineA.RomeroBelum ada peringkat
- HSG Vs SonohysterographyDokumen4 halamanHSG Vs Sonohysterography#15Belum ada peringkat
- MultiZone Limitations and HintsDokumen2 halamanMultiZone Limitations and HintsRubén Darío Becerra GalindoBelum ada peringkat
- Top Survival Tips - Kevin Reeve - OnPoint Tactical PDFDokumen8 halamanTop Survival Tips - Kevin Reeve - OnPoint Tactical PDFBillLudley5100% (1)
- Skills Check Extra 2ADokumen1 halamanSkills Check Extra 2AVishmi JayawardeneBelum ada peringkat
- Mastering American EnglishDokumen120 halamanMastering American Englishmarharnwe80% (10)
- Case Studies InterviewDokumen7 halamanCase Studies Interviewxuyq_richard8867100% (2)
- Fabric DefectsDokumen30 halamanFabric Defectsaparna_ftBelum ada peringkat
- Test 1801 New Holland TS100 DieselDokumen5 halamanTest 1801 New Holland TS100 DieselAPENTOMOTIKI WEST GREECEBelum ada peringkat