Assignment 5 - Solution
Diunggah oleh
mlhy26800 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
82 tayangan17 halamanCHEE3005
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniCHEE3005
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
82 tayangan17 halamanAssignment 5 - Solution
Diunggah oleh
mlhy2680CHEE3005
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF atau baca online dari Scribd
Anda di halaman 1dari 17
Chee3005, assignment 5
Due time: 12:30 pm 21 Oct 2013
Problem set H 8.2,
Fogler P11-5 (a) and (b), question please see tute week 11
Problem set H8.4, H8.8
Fogler 12-3 (a) -(g), question attached
Fogler P12-6 (a) and (b), question attached
82
84
88
Suppose an enzyme catalyzed reaction has the following mechanism
A+E@AE (a)
A+ AE © AE Q)
AXE > P+ QB)
in which A is substrate, P is product, E is the enzyme and AB, AoE are enzyme-
substrate complexes. The third reaction is rate controlling and is irreversible and of
first order. Derive an overall rate expression for the formation of P in terms of the
concentration of A and total concentration of enzyme
The following rates were observed for a first order irreversible reaction carried out on
a spherical catalyst:
for dp= 0.6cm; ross = 0.09 mol/gcat-hr
for dy = 0.3cm; raps = 0.162 mol/gcat.hr
Significant diffusional limitations were observed in both cases. Determine the true
rate of reaction. Is diffusional resistance still important with d, = 0.lem?
‘The first order reaction A —> B is conducted in a laboratory fixed bed reactor. The
reactor is isothermal, and the following data are provided for the gas-phase reaction,
the catalyst particles and reactor:
Peclet no. = 5
Reactor length 750m
Reactor diameter 7 linch
Bed porosity 0.35
Rate Law = kCy, moles/em?.cat-sec
Inlet flow rate = 0.5em’/see
k 10° cm/sec
Surface area = 60mg
Pellet density = 1.5 g/cm?
Pellet diameter = 3mm (spheres)
D, of A in pellet = 10%cm*/see
Estimate the conversion of A in the react
transfer resistance
Question 8.2
A,E—*->P +E rate controlling step
“We want tp = f(Ca, Cua)
“K. K. Ca
PO Cn Clue
kCa
KC yCye
1, = KK CiCe
but Cy = Ce + Cap + Cae
Cy = Cp + Ky Cle + KC Car
Cy = Cp + Ky Cale + K aK aCiCy
—_Su_
“Cp = ;
T#K,C,+ Kak Ch
=K,K,C}| ———*"__
14 KyC,+KKyCy
Question 8.4
Actual Rate
3
Actual Rate __ 3-4 eotnyg)—1
Rare at surface condition = g@ ON) -D
KC us Eyp,S
Mast PSs Ey _ 0.09 _ 9 556
rasa RCycEyp,S, Ey 0.162
= 0.556 ----(1)
& Sinceg = R,
= 28,
From equation (1):
jiecothin)—D
a = 0.556
S ,coth(d,) -D
&
3
grin oa) —1) = 0 sd 7
(0.59,)"
(0.54, coth(0.54,) |
9, coth(g,)-1= oss (0.5¢, coth(0.5¢,) = »
G, coth(g, )—1 = 2.222(0.5¢, coth(0.5¢,) - 1)
0=1.1114, coth(0.5¢,) ~¢, coth(g,) ~ 1.222
«Using excel, 4, =11
oh =55
E, = 0.248
For case 1:
Tray 0.09 _ mol
E, 0.248 geat —hr
True rate of equation =
For dy =0.lem,
Therefore, diffusion is still important.
Question 8.8
on} >,
0.3 flO 1.5 60x10"
10°
.987
rite = kp,S,E,C,(—6,)
ky = kppS,E,(\-#,)
kyr = kppS,E,(~e,)e
4p
DP penn?
= 0.948
Eternal fusion telson Heterogeneous Rascton
‘ooh Lea Hain 20 atm
ro nw
1 ao
oer he slave importance of tauipase don ud spe
eee ee ie oft Teactln? (Caliornla Profesional
sa)
Pa mcunaber extinct «humans tal tue op,
J Ne edit sal arg of is lin were expove 10 mvOH68 gt. Ag
sees espn sre becue eh, with sal ster ety
Akin, Morte! the skin as consisting of to adjacent layers, one OF thiknes 9,
saa verity of Be If souveritfiskon o He ot th essen
Ft a iy acs tt the sin a ha ptt he by
Tre ea ote rl pesires maxi? he saturn pan Nese
Mth gres 101 Pac ea he ser esl of ese
Aree well prosunes exceeding te satura pal eu ae py
‘coming out of the solution (Le, the skin)?
re aoewerng any of these Gusti
les oe Ny a ee an ayes, He
1 durve the concentration po
jee Side Note.
Diftusivity of He and Na i the inn skin layer
= 5% 10 YemYs and 1.5 % 10-? ems, respects
Ditfusviy of He and Ny inthe outer skin
10-3 ems and 3.3 X 10-4
Ta ka D
nypisition of eyefohesane to benzene sd yogis ss
ied at high temperatures, The reaction is cari out ia a Som 1D WR
The dee
fort
in fei The pellet
‘ao costed with the eatalyst only om the aatside
tring volumetsc
Mow eat is 60 min
tyelchexane from an entering gas stream of 5% eye!
at 2 wim ane 500°C
(0) Plot conversion as # fanction of length
ue number of pipes necessary to achieve 99.9% conve
I
hap. 14
Puts,
Puite
PIS,
Questions and Problems 805:
(©) How much would your answer change ifthe pellet diarneter and length
were each eut in half?
{@) How would your answer to part (a) change if the feed were pure cyclo-
hexane?
(e) What do you belive is the point of this problem?
‘Assume the raiuimam respiration rate of a chipmunk is 1.5 micsomoles of
(Ogimin, The corresponding volumetric rate of gas lake is 0.05 dmn¥inin at
str,
(@) What is the deepest a chipmunk can burow @ 3m diameter tole
beneath the surface in Ann Arbor, Michigan? Dag = 18 x 10 mis
(&) In Boulder, Colorado?
(©) How would your answers to (a) and (b) change in the dead of winter
when = 0°F?
(@) Critique and extend this problem (e ., CO poisoning).
Cachon disultide (A) is evaporating ito air (B) at 25°C (Pygg = 510 mm Hg)
tnd 1 atm fom the bottom of a 1.0 em diameter vertical tube. The distance
frow the CS; surface to the open end is 20.0 om, and this ie held constant by
continuous adition of iguid CS, from below. The experiment is aranged so
that the vapor concentration of CS, at the open end is zero.
(a) Calculate the molecular difusivity of CS, i air (Dy) and ts vapor pres
sure at 35°C, (ans. Dyy = 0.12 cris)
(b) Find the moiae and mass Buxes (My, me of CS,) in the tube
(©) Caleulae the folowing properties st 0.0, 3.0, 100, 15.0, 18.0, and 20.0
cm (roma the CS; surface. Areange columns in the foliwing onder on one
Sheet of paper. (Additional columas may be inchaded for compatational
purposes if desired) On a epacete sheet give the relations used Wo obtain
Each quanti. Try 40 put cach relation into a form involving the mini
mun computation and the highest accuracy
(1) yn andy Cole fractions), Cy
@) Va. Va, V8, V mass velocity)
Q) Inde
(2) Blow etch of the groups of quantities in (@(1), (2), and (3) on separate
sgapbs, Nanve all vaiables and show units, Do'nor plot those parameters
in parentheses
{@) What isthe cate of evaporation of CS, in erway?
{Discuss the physical meaning of the value of VY, and Jy at the open end
of the tube
(@) Is malecutar difsion of aie taking place?
AA device for meusuting the diffusion cuefReient of « gas mixtre (Figure
PLI-R,) comsiats of two chambers coneetel by a small tube, Initially the
chambers contain different proportions of two gases, A and B. The tote ps
is the same in each chamber
Fogler Problem: P11-5
Renetion is CgHiz > Colle + 3H
A > B +3C
Assume: = Isothermal Ideal Gas - Perfect plug flow - Constant P
a) Mol Balance:
Mole Balance
Inital Change Final
A Fw “FaX4 — Feo(l-X,)
Bo Fy FX, Foot FX,
BF eX, 3a,
BFoX, Fro (143% 0X4)
Start with the mass balance for @ PER:
dk,
a”
Need to get r, (rate per unit volume) in terms of mass transfer
14 = kat (Car~Cur)
4, is the area of catalyst per volume of bed
‘mass transfer limited, so C,, = 0.
aX, RIX)
dV” v(1¥0.15X,)
a, RIX)
dz U,(1+0.15%,)
X,)
Use Thoenes & Kramers correlation (or similar) for km: Sh’ = (Re’) *(Se') *
kad, (
oC Late Loss
Caloulate the effective diameter ofthe particle:
10 aos?
4, ($) [ena 203) -osv
Caloulate the diffusivity of cyclohexane in hydrogen:
oor (& as
M,
De = +
PUPP)
Fuller, Schettler, Gi
(Perry’s Handbook)
ings for binary mixtures, low pressure, non polar
‘My, My = molecular masses = 84 and 2 g/mol, respectively
V4, Va = diffusion volumes = 122 and 7.07cm*/mol, respectively
o.oox(773.13)( 42)
EY, 0.857 mts
2{(122)" +(7.07)" |
Duy
porosity, = 0.4,
1.146
Initial velocity:
% 60000
A adi
60
=50.9 em? /s
Expression for velocity:
i (1+ 3¥40%,)=50.9(1+0.15%,)
thet
vy w(+0.15X,) (1+0.1S%,)
jal density:
(840.05 + 20,95) 202.65
y= Mada? , Marr? _ ( +2%0.95)202-65 «919 kein? 0.00019 ple?
RP ORT 83145x73.15
p= Oper p/om.s (Hz , 500 K, 2 atm)
9.8001}
te _509%(14. 0:15, )x0.572%0.00019 _ yo 5
0.00017 (1+ 0.15, )x(1— OA) xt. 145 —
0.00017%(1+0.15%,
=1.044(1+0.15%,)
0,000190.857
a (=5)7 patiggn
4,6,
17.98(1+0.15¥,)”
(note the typing error, ke should be kz)
577x6.88%1.014x(1+0.15%,)”
‘Need to calculate a, for a non-porous clyndrical catalyst:
ws) (1-8 )(2a7,b4 207)
ab
29 om’
aK, X,) _17.98x(1-X,)!" x6.29%(1-X,)
de O,(1+0.15X,) 50.9% (IF 0.15X,)
a, -x,)
4-22 _ “5
eG osK,)
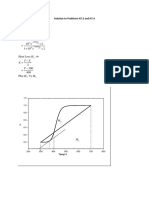
Plotting in excel. Only 3.3em required to reach 99.9% conversion. Much less than one tube,
(b) Plot
(©) Can use the same mass balance from before:
dx, R(I-X,)
de U,(140.15X,)
Need to find new effective radius and shape factor:
6v,)” _(6xx0.125 0.25)"
4(%) (2 0.125 2) = 0.286
= x
a 2
228 snd 27225 5 90.25%0.25
p= 4 its
ad 10.286"
Assume porosity remains unchanged, find new Re (Sc unchanged):
__S09(14 045%, )x0.2864 0.00019 5g
00017 (140.15. Jx(I Oat 4S
Dy (I=: p
Pall) nese as 195% 4.866x1.014x(1+0.15%,)"
k, =25.43(1+0.15%,)
(note the typing error, ke should be ky)
Calculate new a:
3
1-6,)(2mr,L+2r2
(eal “ ) 21259 em
aL
aX, ka,(I-X,) _ 2843x(1-X,)" «12.59«(1-X,)
“dz U,(1+0.15X,) ——-50.9x(1+0.15X,)
ws (4)
dz (140.15x,)"
Integrating the equation again in excel shows that only 1.16 cm of reactor is required. About
123 of the previous length required.
wns cower eal
P1239
Hatt Fame
the temperate were ines iil (3) 1 Bn vel
iipled? (2) He peti wore dee Dy 8 fh 9 Ny
‘Would the rector Heng ehange in each exse? (5) What ley 2” Pe,
{quire to achieve DDG conversion oF the pollutant NOP” MH
What it.
(e) yo applied the Meaes and Weisz-Prter criteria to Examples 1.4
12-4? What would you fwd? What would you Team if yg, Md
kcal/mol, f= 100 BeufheM%E and £ = 20 keal/mo!? 2s
would your effectiveness factor change and the rate of reaction a iow
powitive or negative effects that oeeur? t
CO+3H, > CH HO
H,0+C0 --—> CO, +H,
hae
‘emporature in CDROM Example 812.2 were inreased” Yow woul
felative resistances in the sly reactor change
(4) you were asked fr ll che things that cauld go wrong inthe operation uf
slung reaeing, what would you say?
Te eataytieresetion
AB
cakes place within # xed bed containing spherical porous eatlyst X22. Fi
te P12-3y shows the averll rates of reaction at a point in the reser a5
function of temperature for various entering total molar flow rates, Fre
(a), Is the renetion limited by external diffusion?
{b) if your answer to part (a) was “yes,” under what conditions [of tse
shown (ie, J Fy] is the reaction litnited by external dlfusion?
(0) Is the seaetion “feation-te-limited"?
{@) If your answer w pat (c) was "yes," unr what conditions [oF those shaw
(ie. T, Felli the reaction limited by the sate of the surface reaction”
(e) Ts she egation Hinited by internal ditfusion?
(I your answer to past (6) wax “yess” under what conltions (of Hes
‘07, Fr) isthe reaetion nied by the rate of internal Cision
(2) For a flow rate oF 10 g mul, determine (Hf possi) the overall
iveness factor, £2, at 360 K.
(h) Estimate (if possible) the intemal effectiveness fret, at 367 K
G@_ Ir the concentration atthe extenal eatalyst surface is 0.01 mot" ¢
‘late (GF possible) the concentration at y = A/2 inside the porous
Iyst at 367 K. (Assume a fistrder rection)
(chap. 12 Questo
na Problems as7
29
Fg = 1000 g mam
0360 a¢0 3700 “00
TH)
Figure P1233, Reueton ste in extalyst bod
Addlvional informatio:
Gas properties: Bod properties:
Diffosivty: 0.1 ean's Tortwosty of pellet: 1414
Density: 0.001 fem? Bed permeability: 1 millidarey
Viscosity: 0.0001 glem's Porosity = 03
PI24,. The reaction
es) Ae
Ii is carried out in a differentia) pucked-bed reactor at different temperatures, flow
HH ii ané price son. To ela shows In Figen nes nd
Figure P24 Resotion rte a etalys ed
(a) What regions (ie, conditions d,. 7 Py) ave externa mas eansfer Hite
(b) What regions are teaction-ate limited
(6) What region is imteral-diffusion-contrlled?
(4) What i the intra effectiveness factor at 7 = 490 K and dy = 08 en!
bm
Fogler Problem: P12-3 (15 mark
(a) Yes (im)
(b) All temperatures for Fro = LOmol/hr and for T > 360 K for F7o = 100mol/hr. That the
reaction is mass transfer limited is shown by the fact it increases with flow rate and increases
linearly (as opposed to exponentially) with temperature. (im)
(© Yes (im)
(@) At low temperatures and at high flow rates:
T'< 363 K, Fro = 1000 mol/hr, 5000 molar
1360 K, Fro= 100 mol/hr
‘as shown by independence to flow and exponential increase with temperature of the rate at
these conditions. (im)
(Yes (un)
(O At high temperatures and high flow rates
T > 363K, Fro = 1000 mov/hr, 5000 mol¢hr
tas shown by independence to low and non-exponential (surface reaction control) and non-
linear (mass transfer control) increase with temperature of the rate at these conditions.
(im)
(g) As stated above for Fro = LOmol/hr, the reaction is mass transfer limited. So its overall
effectiveness can be obtained by comparing the rate against those at high flow conditions:
actual rate of reaction =r, (T =360K, Fy =10)
rate of reaction without mass transfer limitations —r3 (T"
= 8, =975 036
07
2m)
(hy As stated above for Fro = 5000mol/hr, the reaction is diffusion limited above 363 K. So
its overall effectiveness can be obtained by comparing the actual rate at 367 K against a rate
extrapolated from the region where there is negligible diffusional resistance:
actual rate of reaction =r, (T = 367K, Fy = $000)
7, extrapolated from 360K ro 367 K
'r, without diffusion limitations
12 0.86
(2m)
(i The internal effectiveness factor can be used to determine the Thiele modulus of this
system at 367 K, We can use the effectiveness factor from part (h) since it is the internal
effectiveness factor (i.e. no external limitations). Using the equation for E;:
86 = (Bes) -1) solving numerically
$=16
‘Now use the equation for the concentration profile of a spherical pellet:
Cx(a) _ sind)
Cy nsinh(g)
:
=Ze05
TR
Nd
—SinhO.8) 9.75,
C, _ OSsinh(1.6)
=> C,(r = R/2)=0.0075 mol/ém?
(3m)
(2m)
PID
Agno
nde
or Probl
i
Curves A. Band C in Figuce 12-5, show the vatatins in
ae ee on cea lols pela.
Figure PI2-5, Tenpeine dependence of thes teatons,
A fintorder inteiogoneons itevnible rastion WY ling place wy
gap wich ped wt pati hago ge
spre «
{Gee Figure 12-0, The reaciant concentration halfway betvcen th
Sune and the eentce ofthe pelle (Le, 7 = KF2) is equal tw ny
* plat’ exter yurfoes. "The concentration ti
voter (2R) is 2% 10" em
coneetttation oft
Surface is 0.001 gama? the
sion coefficient i 0.1 em2/s
A—>B
(@) What is the concentntion of reactant ata distance of 3%
from the exteral pellet surface? (Ans. Cy = 2.36 % 10 + m
() ‘To what diameter should the pelle be reduced i te effective
is to be O.8? Uns. dy = 48 10° em, Critique this answe
atalyst auppart were not ye plated ith platinuin, how
st thatthe catalyst support be pel afte i had been
ate ofa sonll gain 1, Theoret, Mis 264 1
the energy released y the hydrolysis of adenosine i
telat
ATP) 49 adevosinediphowphate (ADP). The nite of hyucoysis
tae of effusion of ATP feom the mice to thecal (ase Pagan
ieient of ATP in the midpiece a tail 3.61
ierted ATP an the eit, where its
Tine ofusysectonal ae OF Ue Nal
Figure P12
7
Swimming ofan organs
(a) Derive equation for difuston and reaction in the til
(b) rive ais equation For the effectiveness tactoc in the tl
{e) “Taking the ration in the tll (o be of zer order, eakeate
the tail, The rate of reaction bythe tall fs 23. 10. mols
(a) Compare your answer with the average tall Iength of 41
possible sources of error?
8 yon
he en
Wet
the extern
whe dt,
10 em in
wld)
‘weal ym
(19703 4
phase
th
yh The
su, ADE
iw 10
ny, Wha
Fogler Problem: P12-6
Assume: = Isothermal
a) For a first order reaction in a spherical pellet, we can use the equation:
“Ca sinh(9)
From the data we are given:
n=Z=05
09) 91
O=6
sinh(0.56)
Seinnggy *0ig nunecoally
At the new radius:
= C,(r = 7x10" cm) =2.36x10 movdm?
'b) Use the equation for internal effectiveness factor:
A $= (GeomK@)-") solving numerially
o=2
‘Now the Thiele modulus is proportional to the radius (and hence diameter) of the particle.
Since it is 1/3 of the ‘Thiele modulus above, then:
This is a very small catalyst particle!
Anda mungkin juga menyukai
- CHEE 3006 - 2008 Exam and SolutionsDokumen15 halamanCHEE 3006 - 2008 Exam and Solutionsmlhy2680Belum ada peringkat
- CHEE 3006 - 2011 Exam and Solutions PDFDokumen19 halamanCHEE 3006 - 2011 Exam and Solutions PDFmlhy2680Belum ada peringkat
- CHEE 3006 - 2009 Exam and Solutions PDFDokumen20 halamanCHEE 3006 - 2009 Exam and Solutions PDFmlhy2680Belum ada peringkat
- CHEM1090 Week 7 PASS Worksheet - AnswersDokumen9 halamanCHEM1090 Week 7 PASS Worksheet - Answersmlhy2680Belum ada peringkat
- Solution To Problems H7.2 and H7.4Dokumen4 halamanSolution To Problems H7.2 and H7.4mlhy2680Belum ada peringkat
- Solution To H7 3Dokumen2 halamanSolution To H7 3mlhy2680Belum ada peringkat
- 2007 Quiz 2 AnwersDokumen5 halaman2007 Quiz 2 Anwersmlhy2680Belum ada peringkat
- Tutorial Sheet 7 SolutionDokumen6 halamanTutorial Sheet 7 Solutionmlhy2680Belum ada peringkat
- CHEM1090 Week 12 PASS Worksheet+AnswersDokumen10 halamanCHEM1090 Week 12 PASS Worksheet+Answersmlhy2680Belum ada peringkat
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (120)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)