02 Nuclear Properties
Diunggah oleh
Muhammad Sohil0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
75 tayangan15 halamanThe document provides an overview of elementary nuclear physics concepts. It discusses that protons and neutrons have both wave and particle properties, and that protons and neutrons are made of quarks. It defines nuclear notation using atomic mass (A), atomic number (Z), and number of neutrons (N). It also discusses that the mass of a nuclide is less than the sum of its constituent parts, with the difference appearing as binding energy. Nuclear sizes are small and energies are high, requiring different units of measurement than typical atomic processes. The document outlines several factors that contribute to nuclear binding energy, and introduces the semi-empirical mass formula. It presents evidence for the nuclear shell model from nuclear decay and reaction data.
Deskripsi Asli:
slides on ENP
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniThe document provides an overview of elementary nuclear physics concepts. It discusses that protons and neutrons have both wave and particle properties, and that protons and neutrons are made of quarks. It defines nuclear notation using atomic mass (A), atomic number (Z), and number of neutrons (N). It also discusses that the mass of a nuclide is less than the sum of its constituent parts, with the difference appearing as binding energy. Nuclear sizes are small and energies are high, requiring different units of measurement than typical atomic processes. The document outlines several factors that contribute to nuclear binding energy, and introduces the semi-empirical mass formula. It presents evidence for the nuclear shell model from nuclear decay and reaction data.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
75 tayangan15 halaman02 Nuclear Properties
Diunggah oleh
Muhammad SohilThe document provides an overview of elementary nuclear physics concepts. It discusses that protons and neutrons have both wave and particle properties, and that protons and neutrons are made of quarks. It defines nuclear notation using atomic mass (A), atomic number (Z), and number of neutrons (N). It also discusses that the mass of a nuclide is less than the sum of its constituent parts, with the difference appearing as binding energy. Nuclear sizes are small and energies are high, requiring different units of measurement than typical atomic processes. The document outlines several factors that contribute to nuclear binding energy, and introduces the semi-empirical mass formula. It presents evidence for the nuclear shell model from nuclear decay and reaction data.
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 15
Nuclear Properties
Elementary Nuclear Physics PG-111
Lecture by: Dr. Zahra Ali
Atomic Nuclei
The protons and neutrons have both wave and particle properties just like
electrons and the interactions are very complicated
Later we will find that both protons and neutrons are NOT elementary
particles like electrons but have an internal structure and are made of
quarks
Nuclear Notation
atomic mass = A
atomic number = Z = number of protons (+) = number of
electrons ()
AZ = number of neutrons (no charge) = N
e.g. 238U.
A = 238 and U has Z = 92 protons. Therefore, 146 neutrons.
Nuclear Mass & Binding Energy
Nuclear Units
Nuclear energies are very high compared to atomic
processes, and need larger units
Nuclear sizes are quite small and need smaller units
Nuclear masses are measured in terms of atomic mass
units (amu, u) with the carbon-12 nucleus defined as
having a mass of exactly 12 amu.
It is also common practice to quote the rest mass energy
E = mc2 as if it were the mass. The conversion to amu is:
1amu = 1.6605 x 10-27 kg = 931.5 MeV/c2
Binding Energy
The mass of any nuclide is less than the mass
of its constituent parts!!!
Where did the mass go?
Appears as the binding energy (energy of the
bonds between the nucleons)
B.E.(A,Z) = [ Zmp + Nmn (M-Zme) ] c2
B.E.(A,Z) = [ ZmH + Nmn - M(A,Z) ] c2
The binding energy is just missing mass times
c2
m
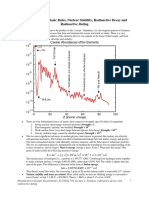
Binding Energy per Nucleon Curve
If these atoms are split
into two nearly equal
parts energy is
released and again the
mass defect increases
Any alteration of the
nuclear structure which
causes a movement
towards this point
results in released
energy. In fact, there is
so much of this
element in the universe
because of its highest
mass defect
If these atoms can be
joined together to
form heavier atoms,
the mass defect
increases, so energy
has been
released
8
Contributions to Binding Energy
EB = strong nuclear force binding -surface tension binding-Coulomb repulsion
+Asymmetry Term + spin pairing+ shell binding
1) strong nuclear force -- the more nucleons the better
2) surface tension -- the less surface/volume the better (U better than He)
3) Coulomb repulsion -- packing more protons into nucleus comes at a cost (although
neutron addition will stabilize high Z nuclei)
4) Asymmetry Term -- Deviation from this symmetry
5) spin pairing -- neutrons and protons have + and - spins, paired spins better
6)shell binding -- nucleus has quantized shells which prefer to be filled (magic
numbers)
Semi Empirical Mass Formula
10
Shell Model - data
T show sharp discontinuities near N,Z of
28, 50, 82, 126
BE for last n added: sharp discontinuities
near, 50, 82, 126
e.g., (d,p), (n,), ( ,n), (d,t) reactions
And, the observation of discrete photon
energies E emitted from nuclear deexcitation
11
Conclusion so far
Nuclear structure BEHAVES alike
electron structure
Magic number a Closed Shell
12
Conclusion so far
Nuclear structure BEHAVES alike
electron structure
Magic number a Closed Shell
Properties:
1.
Spherical symmetric
2.
Total angular momentum = 0
3.
Specially stable
13
Nuclear Forces
protons have a positive electric charge and neutrons
have no electric charge at all, there must be some sort
of extra force a force even stronger than the
electromagnetic force to hold these nuclei together.
15
Strong Nuclear Forces
force between nucleons has following properties:
These are very strong
short-range in order of 10-15 m force between nucleons
Strongly repulsive at very short separations
Strong force is charge symmetric
16
Questions
29
Anda mungkin juga menyukai
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3Dari EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 3: Gravitational and Inertial Control, #3Belum ada peringkat
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2Dari EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2Belum ada peringkat
- Nuclie PDFDokumen6 halamanNuclie PDFSamBelum ada peringkat
- 12 Nuclear Physics and IsotopesDokumen12 halaman12 Nuclear Physics and IsotopesProf.Dr.Mohamed Fahmy Mohamed HusseinBelum ada peringkat
- CBSE TERM2 - NucleiDokumen50 halamanCBSE TERM2 - Nucleivenom eBelum ada peringkat
- Chapter 13Dokumen13 halamanChapter 13Shyam 07Belum ada peringkat
- Transmutation and Nuclear Fission - NotesDokumen6 halamanTransmutation and Nuclear Fission - Notesapi-327272079Belum ada peringkat
- Nuclear Physics and Examples-2022Dokumen14 halamanNuclear Physics and Examples-2022OSCARBelum ada peringkat
- Nuclear Physics Notes For A-Level by HubbakDokumen33 halamanNuclear Physics Notes For A-Level by HubbakHubbak Khan100% (1)
- 7.2 Nuclear ReactionsDokumen2 halaman7.2 Nuclear ReactionsEjaz AhmadBelum ada peringkat
- Nuclei 22912Dokumen7 halamanNuclei 22912user 003Belum ada peringkat
- Course No. Phy1121, Course Title: Physics: Professor Dr. Md. NuruzzamanDokumen21 halamanCourse No. Phy1121, Course Title: Physics: Professor Dr. Md. Nuruzzamanabsar jahanBelum ada peringkat
- Nuclear PHYSICSDokumen195 halamanNuclear PHYSICSAugustte StravinskaiteBelum ada peringkat
- Nuclear Physics 1Dokumen7 halamanNuclear Physics 1dramita15Belum ada peringkat
- Ib HL Study Guide Organizer-Nuclear PhysicsDokumen20 halamanIb HL Study Guide Organizer-Nuclear PhysicsCarlos Andres Vásquez100% (1)
- Chapter Four (Nuclear Power)Dokumen121 halamanChapter Four (Nuclear Power)luter alexBelum ada peringkat
- 23 Nuclear PhysicsDokumen52 halaman23 Nuclear Physicsdayana hanis hassanBelum ada peringkat
- PC Chapter 44Dokumen85 halamanPC Chapter 44ultimuBelum ada peringkat
- Nucli Note ADokumen5 halamanNucli Note AMani MajhiBelum ada peringkat
- Nuclear Power Plant. EEE-481, CUETDokumen96 halamanNuclear Power Plant. EEE-481, CUETbaruaeee100% (1)
- NucleiDokumen8 halamanNucleiZahaan SajidBelum ada peringkat
- Nuclear Physics PrimerDokumen10 halamanNuclear Physics PrimerAnthony AffulBelum ada peringkat
- Module Nuc - Phys I (Phys382) New1Dokumen26 halamanModule Nuc - Phys I (Phys382) New1davididosa40Belum ada peringkat
- Atoms and Nuclei2012-Notes UnlockedDokumen29 halamanAtoms and Nuclei2012-Notes Unlockedapi-250079701Belum ada peringkat
- Chapitre 1 1Dokumen15 halamanChapitre 1 1Ri HabBelum ada peringkat
- Atomic Structure: Lauren Saunders 12 September 2014Dokumen3 halamanAtomic Structure: Lauren Saunders 12 September 2014LaurenBelum ada peringkat
- Chapter I Cours 1 23 24Dokumen4 halamanChapter I Cours 1 23 24ma3kdelacruzzzBelum ada peringkat
- Chapter IvDokumen15 halamanChapter Ivdedy krisnayanaBelum ada peringkat
- g485 5 3 4 Fission and FusionDokumen14 halamang485 5 3 4 Fission and Fusionapi-236179294Belum ada peringkat
- Atomic NucleusDokumen24 halamanAtomic Nucleussudha24_7Belum ada peringkat
- Nuclear PhysicsDokumen20 halamanNuclear PhysicsShubh GuptaBelum ada peringkat
- 12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive DatingDokumen8 halaman12.744/12.754 The Basic Rules, Nuclear Stability, Radioactive Decay and Radioactive Datingshawnah jamesBelum ada peringkat
- Nuclei NewDokumen15 halamanNuclei NewB 06 Tenzin StopdenBelum ada peringkat
- Intro Nuclear PhysicsDokumen32 halamanIntro Nuclear PhysicsMichael VernonBelum ada peringkat
- NucleiDokumen28 halamanNucleisomarshidubeyBelum ada peringkat
- 9-Lecture 9Dokumen29 halaman9-Lecture 9george.1023077Belum ada peringkat
- Nuclear Binding EnergyDokumen7 halamanNuclear Binding EnergyWhite HeartBelum ada peringkat
- Review of Rad PhysicsDokumen268 halamanReview of Rad PhysicsZuhra Jabeen100% (3)
- Mass Defect and Binding EnergyDokumen3 halamanMass Defect and Binding EnergyMark ProchaskaBelum ada peringkat
- Ch.7 Topic7.2 (With Solution)Dokumen46 halamanCh.7 Topic7.2 (With Solution)Amr El SharkawyBelum ada peringkat
- 'NUCLEIDokumen39 halaman'NUCLEIRITIKABelum ada peringkat
- ATOMS NUCLEI (Cbseworldonline - Blogspot.com)Dokumen24 halamanATOMS NUCLEI (Cbseworldonline - Blogspot.com)Sagar VermaBelum ada peringkat
- Nuclei (6 Lecture)Dokumen197 halamanNuclei (6 Lecture)kumarchandanyadav276Belum ada peringkat
- Nuclear Tutorial SolutionDokumen19 halamanNuclear Tutorial SolutionGordon GohBelum ada peringkat
- Nuclei: Atomic Mass Unit (U)Dokumen10 halamanNuclei: Atomic Mass Unit (U)Jatin PanjwaniBelum ada peringkat
- Atoms and NucleiDokumen12 halamanAtoms and NucleiBablu ChaudharyBelum ada peringkat
- Nuclear Models Liquid Drop Model of The Nucleus: Bethe-Weizacker Semi-Empirical Mass Formula (SEMF)Dokumen4 halamanNuclear Models Liquid Drop Model of The Nucleus: Bethe-Weizacker Semi-Empirical Mass Formula (SEMF)Ainebyoona ChriscentBelum ada peringkat
- Nuclear Binding Energy: For The "Special" AP Physics Students Montwood High School R. CasaoDokumen22 halamanNuclear Binding Energy: For The "Special" AP Physics Students Montwood High School R. CasaoAris JuliantoBelum ada peringkat
- Nuclear Physics (Lecture 22-24) - Compatibility ModeDokumen41 halamanNuclear Physics (Lecture 22-24) - Compatibility ModeBibhu Prasad SahooBelum ada peringkat
- A Level Physics NotesDokumen80 halamanA Level Physics NotesAsghar Abbas100% (2)
- Nucleus - 2021Dokumen5 halamanNucleus - 2021Aswin P TBelum ada peringkat
- Nuclei 2022-23Dokumen3 halamanNuclei 2022-23GayatriBelum ada peringkat
- Unit I Nuclear Structure & RadioactivityDokumen66 halamanUnit I Nuclear Structure & RadioactivityAnupriya AnuBelum ada peringkat
- Atomic Mass UnitDokumen6 halamanAtomic Mass UnitVansh SinghaiBelum ada peringkat
- NucleiDokumen24 halamanNucleihembrampriyanka07Belum ada peringkat
- Nuclear Physics NotesDokumen8 halamanNuclear Physics NotesCai Peng Fei100% (1)
- 3 Nuclear Reactions, Fission, Fusion - PresentationDokumen105 halaman3 Nuclear Reactions, Fission, Fusion - PresentationVorizionBelum ada peringkat
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 4: Gravitational and Inertial Control, #4Dari EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 4: Gravitational and Inertial Control, #4Belum ada peringkat
- What is Charge? – The Redefinition of Atom - Energy to Matter ConversionDari EverandWhat is Charge? – The Redefinition of Atom - Energy to Matter ConversionBelum ada peringkat
- CVTemplateDokumen2 halamanCVTemplateMuhammad SohilBelum ada peringkat
- Radiation Chemistry: 22.55 "Principles of Radiation Interactions"Dokumen17 halamanRadiation Chemistry: 22.55 "Principles of Radiation Interactions"Muhammad SohilBelum ada peringkat
- IAEA Pub-Accident Analysis For PWR Pub1162 - Web PDFDokumen77 halamanIAEA Pub-Accident Analysis For PWR Pub1162 - Web PDFMuhammad SohilBelum ada peringkat
- CeramicsDokumen11 halamanCeramicsMuhammad SohilBelum ada peringkat
- Composites: Group#04 Roll#25-OnwardsDokumen5 halamanComposites: Group#04 Roll#25-OnwardsMuhammad SohilBelum ada peringkat
- NPE-632 Nuclear Reactor MaterialsDokumen18 halamanNPE-632 Nuclear Reactor MaterialsMuhammad SohilBelum ada peringkat
- Polymers: Group#03 Roll # 918 - 927-OnwardsDokumen8 halamanPolymers: Group#03 Roll # 918 - 927-OnwardsMuhammad SohilBelum ada peringkat
- Presentation Title: Your Company InformationDokumen3 halamanPresentation Title: Your Company InformationMuhammad SohilBelum ada peringkat
- Char & Paralel DC GenDokumen22 halamanChar & Paralel DC GenMuhammad SohilBelum ada peringkat
- Cooling Towers: Pgtp-9, KinpoeDokumen40 halamanCooling Towers: Pgtp-9, KinpoeMuhammad Sohil100% (3)
- PB - 1Dokumen22 halamanPB - 1Muhammad SohilBelum ada peringkat
- Linear Algebra A Gentle IntroductionDokumen23 halamanLinear Algebra A Gentle IntroductionMuhammad SohilBelum ada peringkat
- Gaussian Elimination MatlabDokumen3 halamanGaussian Elimination MatlabMuhammad SohilBelum ada peringkat
- Nuclear Energy A Viable Option For PakistanDokumen3 halamanNuclear Energy A Viable Option For PakistanMuhammad SohilBelum ada peringkat
- Introduction To Nuclear Engineering by Lamarsh Exercise Questions & SolutionsDokumen2 halamanIntroduction To Nuclear Engineering by Lamarsh Exercise Questions & SolutionsMuhammad Sohil67% (6)
- Abdus Salam - Astro-Particle-PhysicsDokumen6 halamanAbdus Salam - Astro-Particle-PhysicsVasmazxBelum ada peringkat
- High Compressed Solar Light: (Free, Clean and Infinite Energy)Dokumen1 halamanHigh Compressed Solar Light: (Free, Clean and Infinite Energy)Anonymous SV2eVOigBelum ada peringkat
- Dual Nature of Radiation and Matter PDFDokumen11 halamanDual Nature of Radiation and Matter PDFW.R DavidBelum ada peringkat
- CHAPTER 4-Energy Analysis of Closed SystemDokumen26 halamanCHAPTER 4-Energy Analysis of Closed SystemChelsie Patricia Demonteverde MirandaBelum ada peringkat
- PGC 2016 Edition - 20160527-2Dokumen176 halamanPGC 2016 Edition - 20160527-2Glenn TanBelum ada peringkat
- Guidebook For Solar Photo Voltaic ProjectsDokumen87 halamanGuidebook For Solar Photo Voltaic ProjectsnashamikiBelum ada peringkat
- Challenges Facing The Electricity Sector Industry in UgandaDokumen11 halamanChallenges Facing The Electricity Sector Industry in UgandaLucie Bulyaba100% (1)
- RAEC Capability Final PDFDokumen0 halamanRAEC Capability Final PDFkggganiBelum ada peringkat
- Lista Standarde IEC PVDokumen10 halamanLista Standarde IEC PVMariana PanaitBelum ada peringkat
- Atomic StructureDokumen28 halamanAtomic StructureRirin AlchinBelum ada peringkat
- Active Management of Distribtion Networks PDFDokumen95 halamanActive Management of Distribtion Networks PDFmuhannad11061975Belum ada peringkat
- Arsalis 2011Dokumen10 halamanArsalis 2011KESAVARAPU UMA SAI MAHESHBelum ada peringkat
- Slides Cp3Dokumen41 halamanSlides Cp3gunarmotaBelum ada peringkat
- Coal-Water Slurry Presentation2Dokumen19 halamanCoal-Water Slurry Presentation2cumpio425428Belum ada peringkat
- Equipment Capital Budget JustificationDokumen4 halamanEquipment Capital Budget Justificationapi-249635202Belum ada peringkat
- Ship's Hull Theory II, Stability (Module 1)Dokumen17 halamanShip's Hull Theory II, Stability (Module 1)ScarletRahadyanBelum ada peringkat
- 5 Chapter Atomic Structure McqsDokumen11 halaman5 Chapter Atomic Structure McqsKamran Khan100% (1)
- Andersson - E-A - 2009 - Efficient Waste Heat Usage W Seasonal (BTES) at ITT W&W - (At Effstock)Dokumen8 halamanAndersson - E-A - 2009 - Efficient Waste Heat Usage W Seasonal (BTES) at ITT W&W - (At Effstock)northernerBelum ada peringkat
- Atomic Structure (Grade 8) - Free Printable Tests and Worksheets - HelpTeaching PDFDokumen1 halamanAtomic Structure (Grade 8) - Free Printable Tests and Worksheets - HelpTeaching PDFnick2107067% (3)
- Nelson Ch1 - What The Ancients KnewDokumen34 halamanNelson Ch1 - What The Ancients KnewElizabeth LeeBelum ada peringkat
- Mercado SDokumen26 halamanMercado SarvindBelum ada peringkat
- 01-Masterplan E-HotCat Prefinal 09-12-1Dokumen163 halaman01-Masterplan E-HotCat Prefinal 09-12-1Gaurav DhallBelum ada peringkat
- Lecture 1Dokumen41 halamanLecture 1KiKon KwakBelum ada peringkat
- Ministry of Energy and Mineral Resources Agency of R&D For Energy and Mineral ResourcesDokumen27 halamanMinistry of Energy and Mineral Resources Agency of R&D For Energy and Mineral ResourcesRdy SimangunsongBelum ada peringkat
- CRET Class 01 - 10 - 2013Dokumen15 halamanCRET Class 01 - 10 - 2013Erj DaniyaroffBelum ada peringkat
- Si-Con 4X Low Concentration PV: Technology of The FutureDokumen2 halamanSi-Con 4X Low Concentration PV: Technology of The FutureRoberto Ignacio DíazBelum ada peringkat
- Formula Sheet For PhysicsDokumen6 halamanFormula Sheet For PhysicsAsim ButtBelum ada peringkat
- A2 Particle Physics Worksheet PDF MS - Part 2Dokumen18 halamanA2 Particle Physics Worksheet PDF MS - Part 2TahirBelum ada peringkat
- Minimization of Blast Furnace Fuel Rate by Optimizing Burden and Gas DistributionsDokumen1 halamanMinimization of Blast Furnace Fuel Rate by Optimizing Burden and Gas DistributionsOsama AlwakkafBelum ada peringkat
- Momentum: 1 - Praveen AlwisDokumen7 halamanMomentum: 1 - Praveen Alwispraveen alwisBelum ada peringkat