Achondroplasia Online
Diunggah oleh
api-3404211950 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
49 tayangan12 halamanJudul Asli
achondroplasia online
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
49 tayangan12 halamanAchondroplasia Online
Diunggah oleh
api-340421195Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 12
By: Melisa Robinson
The most common form of human short limbed dwarfism
Literally means without cartilage formation
Defective endochondral ossification
Symptoms
Average sized trunk with rhizomelic shortening of the
arms and legs
Macrocephaly (enlarged head) and midface hypoplasia
Trident configuration of hands
Normal intelligence
Resulting conditions
sleep apnea
Genu varum (bowed legs)
lordosis or kyphosis of the spine
spinal stenosis
Occurs in 1/25,000 births
Dominant Gain-of-function mutation
Heterozygous mutation causes ACH
Homozygous mutation is lethal
80% of cases are de novo mutations
linked to paternal germline
one of the most mutable nucleotides in the genome
Majority of other cases are inherited from an affected parent
50% chance that a living child will be affected
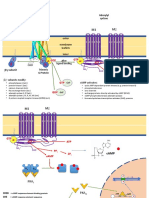
Mutation in the FGFR3 gene
Located at chromosome 4p16.3
Codes for the FGFR3 protein- a transmembrane tyrosine kinase
receptor
3 domains- an extracellular ligand-binding domain, a transmembrane
domain and an intracellular split tyrosine kinase domain
Mutation occurs within the transmembrane domain
GA or GC point mutation at nucleotide 1138
Causes a p.G380R missense mutation
G380R substitution causes ligand independent
stabilization of FGFR3 dimers
Due to hydrogen bonds formed between the 2 side chains of
the arginine residues
Fibroblast growth factor receptor 3
FGFR3 is activated by FGF ligands
Normal physiological function is the regulation of cartilage growth- ERK
and MAPK pathway
A negative regulator of skeletal growth
restricts the length of the long bones via inhibition of chondrocyte
proliferation
With an ACH mutation, the FGFR3 receptor no longer requires a
ligand to activate.
Constitutive activation- receptor is constantly signaling for ERK activation
and activation of p38 arms of the MAPK pathway
Sustained ERK activiation is associated with decreased
chondrocyte proliferation and extracellular matrix production,
increased matrix degradation, and altered cell shape and
differentiation
MAPK signals not only negatively influence proliferation but
also disrupt terminal differentiation and post-mitotic matrix
synthesis
Multiple Disorders from various mutations within the FGFR3 gene
Five different forms of skeletal dysplasia
Hypochondroplasia, Achondroplasia, SADDAN dysplasia, Thanatophoric dysplasia, &
Platyspondylic Lethal skeletal dysplasia
Also linked to epidermal nevi, seborrhoeic keratosis, acanthosis
nigricans caused by keratinocyte overgrowth, and various forms of
cancer
A genetic paradox
Why do mutations cause inhibition of proliferation in chondrocytes,
but have a mitogenic effect on many other types of cells?
Fibroblasts, keratinocytes, melanocytes, epithelial cells, lymphocytes,
and spermatocytes all increase in proliferation in response to a mutation
Currently, the only treatments available are designed to
manage symptoms after they manifest
Bilateral limb lengthening
Neurosurgery to correct spinal malformations
In the early 2000s, treatments using Human Growth
Hormone (HGH) injections to counteract the downregulation
of ossification began
Treatments saw early success, then failed to cause a
significant increase in bone length
Developing Treatments
Targeting the FGFR3 protein
interfere with FGFR3 synthesis, block its activation,
inhibit its tyrosine kinase activity, promote its
degradation, and antagonize its downstream signals
CNP antagonizes FGFR3 downstream signaling by inhibiting the
ERK/MAPK pathway
A paracrine/autocrine factor that signals through natriuretic
peptide receptor B and modulates the activity of FGFR3
Enhances and restores chondrocyte proliferation and
differentiation which results in bone growth
Studied in mice and cynomolgus monkeys
Many CNP variants limited as a treatment due to rapid clearance
rates by natriuretic clearance receptors (NCR) and neutral
endopeptidase (NEP)
BMN-111 has extended serum half-life due to resistance to NEP
39 amino acid CNP variant
small enough to penetrate successfully to target location
In mouse models, treatment resulted in increased axial and
appendicular skeletal lengths, improvement in ACH-related
clinical features, and correction of growth plate defects
BMN-111 recently completed a successful Phase II clinical trial
26 children in 3 cohorts tested the drug for 6 months
50% increase in growth rate
No serious adverse affects
Anda mungkin juga menyukai
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Mechanism of Hormone ActionDokumen54 halamanMechanism of Hormone ActionDr. Tapan Kr. Dutta100% (1)
- War Dogs 1Dokumen26 halamanWar Dogs 1api-340421195Belum ada peringkat
- British Journal of Nutrition (2008), 99, E-Suppl.Dokumen50 halamanBritish Journal of Nutrition (2008), 99, E-Suppl.nihadtoussounBelum ada peringkat
- Resume Online Edit 2Dokumen1 halamanResume Online Edit 2api-340421195Belum ada peringkat
- Plant Diversity EcoDokumen18 halamanPlant Diversity Ecoapi-340421195Belum ada peringkat
- GM Mosquitos UpdatedDokumen8 halamanGM Mosquitos Updatedapi-340421195Belum ada peringkat
- 2010 A Level H2 Biology P2 AnsDokumen8 halaman2010 A Level H2 Biology P2 AnsImagreenbucklegirl SGBelum ada peringkat
- Leucocyte Circulation and Migration Into The TissuesDokumen21 halamanLeucocyte Circulation and Migration Into The TissuesNalumenya MathewBelum ada peringkat
- Harald H. H. W. Schmidt - Franz B. Hofmann - Johannes-Peter Stasch CGMP Generators - Effectors and Therapeutic Implications 2008Dokumen578 halamanHarald H. H. W. Schmidt - Franz B. Hofmann - Johannes-Peter Stasch CGMP Generators - Effectors and Therapeutic Implications 2008Rose DNBelum ada peringkat
- Perceptual ProcessDokumen4 halamanPerceptual ProcessIndrajith KrBelum ada peringkat
- L RhamnosusDokumen16 halamanL RhamnosusGonzalo Báez VBelum ada peringkat
- G Protein Coupled Receptor (GPCR) Adenylyl Cyclase: Ligand BindingDokumen3 halamanG Protein Coupled Receptor (GPCR) Adenylyl Cyclase: Ligand BindingPanda DaoBelum ada peringkat
- Swiss Target PredictionDokumen5 halamanSwiss Target PredictionDishank PBelum ada peringkat
- UMR cDNA Resource Center Clone CollectionDokumen1 halamanUMR cDNA Resource Center Clone Collectiongheorghe2Belum ada peringkat
- Chapter 25: Strees PhysiologiDokumen44 halamanChapter 25: Strees PhysiologiMuhammad AbduhBelum ada peringkat
- Adrenergic ReceptorsDokumen1 halamanAdrenergic ReceptorsYavuz Burak KÜÇÜKGÜLBelum ada peringkat
- Th17 Cells InflamationDokumen4 halamanTh17 Cells InflamationJayanth BalakuntlaBelum ada peringkat
- Hnrs 199 Senior Capstone Project SQDokumen24 halamanHnrs 199 Senior Capstone Project SQapi-618702342Belum ada peringkat
- Epidermal Growth FactorDokumen11 halamanEpidermal Growth FactorDanièle FeudjioBelum ada peringkat
- Immune Regulation by GlucocorticoidsDokumen35 halamanImmune Regulation by GlucocorticoidsSyifa SariBelum ada peringkat
- Imunologi PersalinanDokumen16 halamanImunologi PersalinanRidanna HartateanaBelum ada peringkat
- Targeting Tumor Associated Macrophages (TAMs) Via NanocarriersDokumen15 halamanTargeting Tumor Associated Macrophages (TAMs) Via Nanocarriersyuvrajsingh3Belum ada peringkat
- CBCS Syllabus For Post-Graduate Courses: Subject: ZoologyDokumen35 halamanCBCS Syllabus For Post-Graduate Courses: Subject: Zoologyanurag kumarBelum ada peringkat
- Chitosan Nanoparticles Preparation and ApplicationsDokumen15 halamanChitosan Nanoparticles Preparation and ApplicationsAnonymous ahNUZsrQpSBelum ada peringkat
- Testosterone and Its Metabolites - Modulators of Brain FunctionsDokumen22 halamanTestosterone and Its Metabolites - Modulators of Brain FunctionsPablo MiraBelum ada peringkat
- A Biomolecular Computing Method Based On Rho Family Gtpases: Jian-Qin Liu and Katsunori Shimohara, Member, IeeeDokumen5 halamanA Biomolecular Computing Method Based On Rho Family Gtpases: Jian-Qin Liu and Katsunori Shimohara, Member, IeeeNathan McCorkleBelum ada peringkat
- Introduction To Biochemistry: by Zaheer Uddin (Pharm-D, M Phil (Pharmacy Practice) MBA (MARKETING)Dokumen63 halamanIntroduction To Biochemistry: by Zaheer Uddin (Pharm-D, M Phil (Pharmacy Practice) MBA (MARKETING)Izat KhanBelum ada peringkat
- Chapter PP.5 (Signals)Dokumen68 halamanChapter PP.5 (Signals)NaHuynJungBelum ada peringkat
- Her-2 +ve Ca BreastDokumen3 halamanHer-2 +ve Ca BreastwillyoueverlovemenkBelum ada peringkat
- SignallingDokumen22 halamanSignallingpalakBelum ada peringkat
- Apoptosis: Dr. Prabesh K Choudhary Final Year Resident MD PathologyDokumen47 halamanApoptosis: Dr. Prabesh K Choudhary Final Year Resident MD PathologyAhmed BioBelum ada peringkat
- M - 114 Second Messenger in The Hormone ActionDokumen3 halamanM - 114 Second Messenger in The Hormone ActionDr. Tapan Kr. DuttaBelum ada peringkat
- Stress Responses in PlantsDokumen293 halamanStress Responses in PlantsIonela RădulescuBelum ada peringkat
- MCQ of Neurochemistry and Signal Transduction-2022-FinalDokumen6 halamanMCQ of Neurochemistry and Signal Transduction-2022-FinalKaif AliBelum ada peringkat