17 JChromatPAPER PDF
Diunggah oleh
Shelley HernandezJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
17 JChromatPAPER PDF
Diunggah oleh
Shelley HernandezHak Cipta:
Format Tersedia
JOURNAL OF
CHROMATOGRAPHY A
ELSEVIER
Journal
of Chromatography
A, 688 (lYY4) 363-367
Short communication
Selectivity
Pilar Fernandez,
Department
effects in semi-polar
Rosa Vilanova,
26 May lYY4; revised
II
Lourdes Berdie, Joan 0. Grimalt
of Environmental Chemistry (CID-CSIC).
First received
columns.
Jordi Girona 18, 08034-Barcelona,
manuscript
received
13 September
Catalonia, Spain
1994
Abstract
Significant selectivity differences between semi-polar capillary gas chromatographic
columns involving the elution
order of acyclic and polycyclic lipid molecules have been observed. These differences
occur despite these columns
being reported to be equivalent
in catalogue specifications.
In Part I, the changes in retention indices between
columns manufactured
by different companies
were described.
In this paper, it is shown that these variations
in
selectivity may even be observed among semi-polar
columns originating
from the same manufacturer.
1. Introduction
In Part I [l], we showed that the use of
different
capillary columns in the trace organic
analysis of environmental
samples may give rise
to significant
changes
in the relative retentions
of
several
major
compounds.
These
selectivity
changes essentially
concerned
the elution order
of linear
vs. polycyclic
molecules
such as
squalene
and benzopyrenes
and alkan-1-01s and
sterols,
and were observed
among semi-polar
stationary
phase
columns
(5%
phenyl-95 %
methyl type) which are reported to be equivalent
in commercial
catalogues.
The study involved a
comparison
of semi-polar
columns from J & W
Scientific
(DB-5),
Carlo Erba (SE-52 and SE54), Chrompack
(CP-Sil 8 CB) and HewlettPackard (HP-5).
In the context of the chromatographic
work on
environmental
problems
regularly performed
in
our Department,
we have found
that these
previously
described
differences
[l] may even
* Corresponding
author.
@
0021~Y673/941$07.00
SSDI
0021.9673(93)00898-
1Y94 Elsevier
Science
B.V. All rights
involve columns manufactured
by the same company. In this paper we present
one of these
cases, which extends the previous description
of
selectivity
differences
between
semi-polar
columns.
2. Experimental
2. I. Materials
Chromatography
quality n-hexane,
methanol,
dichloromethane,
isooctane and neutral silica gel
(Kieselgel 40, 70-230 mesh) were obtained from
Merck (Darmstadt,
Germany).
The silica gel was
extracted
with dichloromethane-methanol
(2:1,
v/v) in a Soxhlet apparatus
for 24 h. After
solvent evaporation,
the silica was heated for 12
h at 120C. A total of 5% (w/w) of water
obtained
with a Milli-Q system (Millipore)
was
then added to the chromatographic
adsorbents
for deactivation,
The purity of the solvents was
checked by concentrating
under vacuum 100 ml
of solvent
to 10 ~1 for GC analysis.
Blank
reserved
364
P. Femandea
et al. ! J. Chromarogr.
A 688 (1994) 363-367
requirements
were as follows: splitless injection
of 2 ~1 should result in chromatograms
with no
unresolved
GC envelope
and only a few peak
representing
up to 1 ng in terms of their flame
ionization
detector response.
2.2.
Sampling,
DBS-MS
2/
extraction and fractionation
Samples were obtained
by sediment coring in
high-altitude
lakes. The cores were divided into
sections at the sampling site and frozen at -20C
until analysis
in the laboratory.
Lipids were
extracted
by sonication
after
freeze-drying.
About
1 g of sediment
was extracted
with dichloromethane-methanol
(2: 1, v/v) (3 x 20 ml;
20 min), the extract was vacuum and nitrogen
evaporated
almost to dryness and diluted to 0.5
ml with n-hexane,
then fractionated
by column
chromatography
according
to previously
established methods [2]. A column filled with 2 g of
5% water-deactivated
silica was used. The hydrocarbon
and alcohol fractions
were obtained
by successive
elution
with 8 ml n-hexane-dichloromethane
(1 :l) and 12 ml of dichloromethan+methanol
(18:2), respectively.
These fractions were vacuum
and nitrogen
concentrated
almost to dryness and silylated with isooctanebis( trimethylsilyl)trifluoroacetamide
(1: 1) (60
min, 70C).
-1
L.3.
The samples were analysed
by GC and GCMS. These analyses were performed
with a Carlo
Erba HRGC Mega 2 Series gas chromatograph
Table 1
Semi-polar
Column
capillary
columns
~
time
r
I I
InstrumeniaI analysts
compared
time
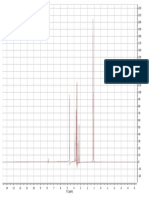
Fig. 1 CC profiles showing the elution order of the TMS
ether derivatives
of alkan-1-01s and A5-sterols with the DB-5
and DBS-MS semi-polar
columns.
Peaks: 1 = chotest-5en3p-01; 2 = octacosan-l-01;
3 = p-sitosterol;
4 = triacontan-l-
01.
in this study
Stationary
phase
Dimensions
Film
thickness
Manufacturer
(cl.m)
DB-5
5% phenyl-Y5l:
methyl
30 m x 0.25 mm I.D.
0.2
J & W Scientific
DB5-MS
SC; phenyl-YS?
methyl
30 m X (1.25 mm I.D.
0.2
J & W Scientific
(Folsom, CA, USA)
a As defined
by the manufacturer
P. Fernander
et al. i J. Chomaragr.
equipped with a flame ionization detector and
with a Carlo Erba GC8000 Series gas chromatograph cuupled to a Fisons MD 800 mass spectrumeter, respectively. The GC analyses were
performed with the capillary columns described
in Table 1. The oven temperature
was programmed from 90 to 300C at fYC/min, and the
injector and detector temperatures were set at
280 and 330X, respectively. Hydrogen was used
as the carrier gas at a flow-rate of 50 cm/s. The
oven temperature programme for the GC-MS
analyses was from 90 to 300C at 4C/ min, the
injector and transfer line temperatures were 280
and 3OOC, respectively, and helium was used as
the carrier gas at a flow-rate of 50 cm/s. Data
were acquired in the electron impact mode (70
eV), scanning from 50 ta 550 mass units at 1 s
per decade. In both instances the injector was in
the splitless mode (1 ~1; hot needle technique),
the split valve being closed for 35 s.
3. Results and discussion
C,,,-C3, alkan-1-01s constitute the dominant
compounds in the polar fractions of the sediments considered in this study. In addition to
these, sterols are also major compounds, encompassing a mixture af C,,-CZo homologues
dominated by cholest-5-en-3P-ol
and /3-sitosterol. The gas chromatograms corresponding to
the analysis af the alcohol-sterol
mixtures with
365
A 688 (1994) 363-367
the two columns indicated in Table 1 are shown
in Fig. 1. These chromatograms illustrate that
important changes in relative retention are observed when using the different columns. Thus,
with the DB-5 column, the trimethylsilyl (TMS)
ethers of octacosan-l-01 and triacontan-l-01 elute
before than the TMS ethers of cholest-5-en-3P-ol
and p-sitosterol, respectively, whereas with the
DB-5 column the elution urder is the reverse.
Repeated analyses with several coIumns and
comparison of the resulting relative retention
indices (I) of the sterols (Table 2) showed that
the discrepancy is maintained despite using columns from different manufacturing batches. The
f dispersion is low (relative standard deviation
0.17-0.26%)
in comparison with the DB-5DBS-MS I discrepancies (1.4-1.8%),
which results in very significant mean I differences between the two column types (confidence level in
Students t-test >> 0.9995).
These selec! ivity effects parallel the elution
order changes t jbserved in our previous work [ 11
and show that this new J & W Scientific column,
DBS-MS, behaves similarly to CP-Sil 8 CB, SE54 and HP-5, with which the elution of the linear
compounds foflows that of the polycyciic malecules occurring at close retention times (Table
3). In particular, the retention index of /?-sitosterol with this column is coincident with that
with CP-Sil 8 CB.
The difference in selectivity is independent of
the specific functionality of the analyte mole-
Table 2
Relative retention indices (I) of alkan-1-01s and sterols with the DB-S and DBS-MS columns
Column
Octacosan-l-o1
I values relative to alkan-I-ols.
Cholest-S-en-@-&
Triacontan-P-o1
j?-Sitosterot
2831.1
2825.6
X827.(1
2Sl9.7
3OOO.O
3ocN).o
3m1.0
3000.0
3037.7
3035.2
3035.8
3020.3
2781.h
2791.7
2787.5
2781.4
3000.0
3000.0
3000.0
3000.0
2973-2
2983.5
2979.2
2974.0
366
P. Fernandez
Table 3
Average relative retention
in a previous study [l]
Column
indices (I) of alkan-1-01s
DB-5
SE-52
CP-SiI 8 CB
SE-54
HP-5
DB5-MS
and sterols with the semi-polar
1-01
columns
listed in Table
1 and those considered
28W.O
2800.0
2800.0
2800.0
2800.0
2800.1)
relative
Cholest-5-en-3P-ol
Triacontan-l-01
&Sitosterol
2826. I
2803.6
2776.1)
2782.5
2774.4
2785.6
3000.0
3000.0
3000.0
3000.0
3000.0
3000.0
3032.2
2998.2
2974.1
2985.3
2969.1
2977.5
to alkan-I-ols
cules. Thus. the comparison
of the elution order
of C,,-C,Z
n-alkanes
and several triterpenes
and
triterpanes
[taraxer-16ene,
olean-12-ene,
hop17(21)-ene,
glutinane,
heterolupene.
diploptene
and (22R)-17a(H),21p(
H)-homophopane]
in the
31
32.00
33 .EE
34.88
two columns
shows the same displacement
towards higher I for the polycyclic
molecules
eluting in the DB-5 column (Fig. 2 and Table 4).
With these hydrocarbons,
the general displacement effect gives rise to elution order inversions
between
hop-17(21)-ene
and n-hentriacontane
and between heterolupene
and n-dotriacontane.
The same type of I differences
between
the
DB-5 and the CP-Sil 8 CB, SE-54 and HP-5
columns was observed
in our previous study [l]
when comparing
the elution
time of squalene
Hence the selectivity
effect
and benzopyrenes.
Relative
carbons
DBS-MS
Bl.Bfl
A 6$$ (1994) 363-367
I
Octacosan-
I values
et al. i J. Chromatogr.
35 .ea
36 .ee
time
Fig. 2. GC profiles showing the elutmn order of n-alkanes
and triterpanes
with the DB-5 and DBS-MS
semipolar
columns.
Numbers
on peaks refer to n-alkane
chain length.
T = taraxer-14-ene;
0 = olean-l?-ene;
H = hop-lJ(Zl)-ene:
G = glutinane:
He = heterolupene;
D = diploptene;
H,, =
(22R)-17a(H),21@(
HI-homohopane.
Time scale m mm.
retention
indices (I) of linear and polycyclic
with the DB-5 and DBS-MS columns
,I-Triacontane
Taraxcr-I-I-ene
Olcan- I?-enc
n-Henrriacontane
Hop-17(;!1)-ene
Glutinane
n-Dotriacontane
Heterolupenc
Dipioptene
(22R)-17a(
H),21P(H)-Homohopane
~Tritriacontane
hydro-
DB-5
DBS-MS
3000.0
3059.0
3077.0
3100.0
3109.8
3161.0
3200.0
3210.8
3271.4
3278.4
33t10.0
3000.0
3020.7
3040.7
3100.0
3070.0
3124.1
3200.0
3170.4
3231.2
3240.4
3300.0
I values relative to n-alkanes.
a Compounds
showing reversal of elution order
to the n-alkane with the nearest retention.
with respect
P. Fernandez
et al. / J. Chromatogr.
which differentiates the DES-5 column essentialty
concerns the elution time of cyclic vs. polycyclic
molecules.
A 6M (1994) 363-367
367
tant peak assignment errors may be produced
from the straightforward comparison of equivalent semi-polar capillary columns such as those
described here.
4, Conclusions
The important changes in relative retention on
analysis of organic compounds with different
semi-polar columns described in Part I [l] are
also observed even with columns produced by
the same manufacturer. Catalogue spe~i~~atio~s
do not allow differentiation between semi-polar
columns corresponding to one or another group.
Hence, the use of a standard mixture containing
linear and polycyclic molecules with similar retention times is recommended as a guideline for
environmental applications where both types of
molecules are easily encountered.
This is particularly relevant when comparing GC trace.s
obtained with different detectors, such as flame
ionization and mass spectrometric types. Impor-
Financial support from ALPE-II Project (EEC
Project EVS-CT92-0205)
is acknowledged. P.F.
thanks CIRIT (Generalitat de Catalunya) for a
LB,
thanks the Spanish
research contract.
Ministry of Education for a Ph.D. fellowship.
References
[ lj M. Aceves and J.O. Grimalt, f. ~~r~~at~~~.,
607 (1992)
261.
121 M. Aceves,
J.O. Grimalt, J. Albaiges, F. Broto, L.
Cornellas and M. Gassiot, 1. Chromatogr.,
436 (1988)
503.
Anda mungkin juga menyukai
- Alkene WorksheetDokumen4 halamanAlkene WorksheetShelley HernandezBelum ada peringkat
- Ras Time ResolvedDokumen8 halamanRas Time ResolvedShelley HernandezBelum ada peringkat
- Chapter 1wDokumen26 halamanChapter 1wShelley HernandezBelum ada peringkat
- 2015-16 Salary Schedule A PDFDokumen2 halaman2015-16 Salary Schedule A PDFShelley HernandezBelum ada peringkat
- XfelDokumen12 halamanXfelShelley HernandezBelum ada peringkat
- Mass Cytometry: An ICP-MS Approach For Single-Cell AnalysisDokumen68 halamanMass Cytometry: An ICP-MS Approach For Single-Cell AnalysisShelley HernandezBelum ada peringkat
- 2015-16 Salary Schedule ADokumen2 halaman2015-16 Salary Schedule AShelley HernandezBelum ada peringkat
- Efficient Synthesis of AnastrephinDokumen5 halamanEfficient Synthesis of AnastrephinShelley HernandezBelum ada peringkat
- 1d Spectrum g1Dokumen1 halaman1d Spectrum g1Shelley HernandezBelum ada peringkat
- IugfiougfiDokumen3 halamanIugfiougfiShelley HernandezBelum ada peringkat
- CalibrationVDS 031813Dokumen7 halamanCalibrationVDS 031813Shelley HernandezBelum ada peringkat
- WefewfDokumen11 halamanWefewfShelley HernandezBelum ada peringkat
- SpillDokumen1 halamanSpillShelley HernandezBelum ada peringkat
- EthicsDokumen1 halamanEthicsShelley HernandezBelum ada peringkat
- (+) - 5fluorodeoxyuridine MSDS: Section 1: Chemical Product and Company IdentificationDokumen5 halaman(+) - 5fluorodeoxyuridine MSDS: Section 1: Chemical Product and Company IdentificationShelley HernandezBelum ada peringkat
- WdqwdqwedDokumen2 halamanWdqwdqwedShelley HernandezBelum ada peringkat
- Bomb Cal LabDokumen4 halamanBomb Cal LabShelley HernandezBelum ada peringkat
- WdqwdqwedDokumen2 halamanWdqwdqwedShelley HernandezBelum ada peringkat
- Supplemental Material Synthesis and Analyzation of ManganeseDokumen3 halamanSupplemental Material Synthesis and Analyzation of ManganeseShelley HernandezBelum ada peringkat
- WdqwdqwedDokumen2 halamanWdqwdqwedShelley HernandezBelum ada peringkat
- WdqwdqwedDokumen2 halamanWdqwdqwedShelley HernandezBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (120)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Use of GC-MS Instruments On The DeterminationDokumen10 halamanThe Use of GC-MS Instruments On The DeterminationAryani AtmajaBelum ada peringkat
- MediHerb Catalog PDFDokumen153 halamanMediHerb Catalog PDFSaaol VizagBelum ada peringkat
- FundamentalsofHPLCWebinar TRS 102012 PDFDokumen103 halamanFundamentalsofHPLCWebinar TRS 102012 PDFsalmanBelum ada peringkat
- Calculation of System Suitability ParametersDokumen10 halamanCalculation of System Suitability ParametersMubarak Patel100% (1)
- INK ChromatographyDokumen10 halamanINK ChromatographyRaymond Godfrey Dagwasi100% (1)
- Iso 11024 1 1998Dokumen9 halamanIso 11024 1 1998Lauture SeideBelum ada peringkat
- Determination of Caffeine Theobromine and Theophylline in Standard Reference Material 2384 Baking Chocolate Using Reversed Phase Liquid ChromatographyDokumen5 halamanDetermination of Caffeine Theobromine and Theophylline in Standard Reference Material 2384 Baking Chocolate Using Reversed Phase Liquid ChromatographyAdab GlezBelum ada peringkat
- 1st Year Chemistry Chapter 2Dokumen3 halaman1st Year Chemistry Chapter 2Zeeshan ahmedBelum ada peringkat
- ADC MethodDokumen16 halamanADC MethodPhilip K MathewBelum ada peringkat
- Chem 26.1 FR E12Dokumen6 halamanChem 26.1 FR E12JR CastorBelum ada peringkat
- 7E Mixtures and SeparationDokumen2 halaman7E Mixtures and Separationakshyta gantanBelum ada peringkat
- Tutorial 6Dokumen22 halamanTutorial 6Merna El SayeghBelum ada peringkat
- Standard Operating Procedure To Learn How To Behave in Quality Control Laboratory in PharmaceuticalsDokumen38 halamanStandard Operating Procedure To Learn How To Behave in Quality Control Laboratory in PharmaceuticalsYulis AdrianaBelum ada peringkat
- Rapid Identification of Pork For Halal Authentication Using The Electronic Nose and Gas Chromatography Mass Spectrometer With Headspace AnalyzerDokumen7 halamanRapid Identification of Pork For Halal Authentication Using The Electronic Nose and Gas Chromatography Mass Spectrometer With Headspace AnalyzerekosaputrobbppbatuBelum ada peringkat
- Canarium L.: A Phytochemical and Pharmacological ReviewDokumen8 halamanCanarium L.: A Phytochemical and Pharmacological ReviewMogana RajagopalBelum ada peringkat
- Astm D4059Dokumen2 halamanAstm D4059septhiadi100% (1)
- Comparison Bet. US FDA, USP & ICH GuidelinesDokumen10 halamanComparison Bet. US FDA, USP & ICH GuidelinesRavi KantBelum ada peringkat
- Iron Sucrose InjectionDokumen4 halamanIron Sucrose Injectionngoc tan tranBelum ada peringkat
- (PEJT5025-02) Reporting Particle Count by ISO CodeDokumen12 halaman(PEJT5025-02) Reporting Particle Count by ISO Codevictor.ciprianiBelum ada peringkat
- M.Prasad Naidu MSC Medical Biochemistry, PH.D Research ScholarDokumen31 halamanM.Prasad Naidu MSC Medical Biochemistry, PH.D Research ScholarDr. M. Prasad NaiduBelum ada peringkat
- En 14372 Child Use and Care Articles - Cutlery and Feeding UtensilsDokumen25 halamanEn 14372 Child Use and Care Articles - Cutlery and Feeding UtensilsLaura Marcela100% (1)
- Principle and Applications of Gel Fitration ChromatographyDokumen18 halamanPrinciple and Applications of Gel Fitration ChromatographyGanesh V GaonkarBelum ada peringkat
- CAPE Chemistry U2 - P1Dokumen10 halamanCAPE Chemistry U2 - P1Moya-Dean WalcottBelum ada peringkat
- Capstone Project Chapter 1 4Dokumen11 halamanCapstone Project Chapter 1 4jaterBelum ada peringkat
- 2012 Catalogo GraceDokumen84 halaman2012 Catalogo GraceValery FujitaBelum ada peringkat
- Ficoll Paque PDFDokumen20 halamanFicoll Paque PDFBabbooBelum ada peringkat
- Chem Xi FB Mcqs & Short QDokumen95 halamanChem Xi FB Mcqs & Short QficpeshawarBelum ada peringkat
- Paper Chromatography of Gel Ink PensDokumen4 halamanPaper Chromatography of Gel Ink PensdaveBelum ada peringkat
- UltiMate 3000 Nano and Cap System Installation and ApplicationDokumen73 halamanUltiMate 3000 Nano and Cap System Installation and ApplicationValery MurilloBelum ada peringkat