Hyperbilirubinemia Guidelines in Newborn
Diunggah oleh
Nisa UcilHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Hyperbilirubinemia Guidelines in Newborn
Diunggah oleh
Nisa UcilHak Cipta:
Format Tersedia
that in 19% of the sudden, unexpected infant death cases, knowledge about the history was essential for
the final diagnosis.
Siri Hauge Opdal, PhD
Torleiv Ole Rognum, MD
Institute of Forensic Medicine
Rikshospitalet
0027 Oslo, Norway
REFERENCES
1. Gregersen MRJ, Laursen H, Baandrup U, et al. Pathologic criteria for the
Nordic Study of SIDS. In: Rognum TO, ed. Sudden Infant Death Syndrome, New Trends in the Nineties. Oslo, Norway: Scandinavian University Press; 1995:50 58
2. Vege A, Rognum TO. Use of new Nordic criteria for classification of
SIDS to re-evaluate diagnoses of sudden unexpected infant death in the
Nordic countries. Acta Paediatr. 1997;86:391396
3. Opdal SH, Rognum TO. The sudden infant death syndrome gene: does
it exist? Pediatrics. 2004;114(4). Available at: www.pediatrics.org/cgi/
content/full/114/4/e506
4. Rognum TO, Arnestad M, Bajanowski T, et al. Consensus on diagnostic
criteria for the exclusion of SIDS. Nord Rettsmedisin. 2003;9:6273
5. Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome
and unclassified sudden infant deaths: a definitional and diagnostic
approach. Pediatrics. 2004;114:234 238
6. Stray-Pedersen A, Arnestad M, Opdal SH, Vege A, Rognum TO, Byard
RW. Globally accepted definition and diagnostic criteria crucial for
solving the SIDS enigma. Scand J Forensic Sci. 2004;10:70 71
7. Fleming PJ, Blair PS, Sidebotham PD, Hayler T. Investigating sudden
unexpected deaths in infancy and childhood and caring for bereaved
families: an integrated multiagency approach. BMJ. 2004;328:331334
8. Krous HF. The international standardized autopsy protocol for sudden
unexpected infant death. In: Rognum TO, ed. Sudden Infant Death Syndrome, New Trends in the Nineties. Oslo, Norway: Scandinavian University Press; 1995:8195
9. Hunt CE. Genes and sudden infant death syndrome. Pediatr Res. 2004;
56:321322
10. Arnestad M, Vege A, Rognum TO. Evaluation of diagnostic tools applied in the examination of sudden unexpected deaths in infancy and
early childhood. Forensic Sci Int. 2002;125:262268
doi:10.1542/peds.2004-2743
Hyperbilirubinemia Guidelines in Newborn
Infants
To the Editor.
We read with interest the recent hyperbilirubinemia guidelines
by the American Academy of Pediatrics1 (AAP). We found them
to be very informative, but we believe these guidelines can be
adapted further to become more user friendly. To follow the
guidelines, the practicing pediatrician needs to use information
from 1 algorithm, 3 nomograms, and 4 tables that are spread over
several pages. In our experience, using a 1-page practice guideline
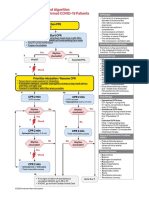
is a feasible solution to this problem (see Fig 1).
The AAP allows 2 options for predischarge assessment of newborns: (1) predischarge bilirubin measurement, which is a universal screening, and/or (2) assessment of clinical risk factors without
a universal screening. Either of these 2 options can be used with
appropriate postdischarge follow-up. The AAP also states, however, that [t]he best documented method for assessing the risk of
TABLE 1.
subsequent hyperbilirubinemia is to measure the TSB [total serum
bilirubin] or TcB [transcutaneous bilirubin] level and plot the
results on a nomogram [by Bhutani et al2].
Between 1992 and 2002, bilirubin encephalopathy was voluntarily reported in 125 healthy term and near-term infants to the
Kernicterus Pilot Registry.3 Kernicterus and severe hyperbilirubinemia are not reportable conditions. These cases probably underestimate the true incidence of kernicterus in the United States
because of the lack of a universal registry, missed diagnosis, and
underreporting. In addition, there is an inability to assess adequately neonatal jaundice by clinical judgment alone.4 For these 2
reasons, the AAP identified universal screening of all term or
near-term infants before their discharge as the best documented
method for hyperbilirubinemia screening.
In July 2003, we were able to create and implement 1-page
hyperbilirubinemia prevention and management guidelines for
term and near-term infants in the Cedars-Sinai Medical center
Well Infant Nursery (Fig 1). These guidelines were based on the
previous AAP guidelines,5 the Bhutani et al nomogram,2 and the
neonatal jaundice task force of NICU-Cedars-Sinai Medical Center. It is notable that our guidelines are similar to the current AAP
guidelines.2
One-year experience showed that since the implementation of
our guidelines in July 2003, the hyperbilirubinemia readmission
rate to the pediatric ward and NICU combined decreased by 38%
(P .001). Between July 2002 and June 2003, we had a total of 66
hyperbilirubinemia readmissions versus 41 between July 2003 and
June 2004. The ratio of readmitted newborns to the pediatric ward
versus the NICU went up from 1.2 to 4.85. Of note, the number of
newborns born in academic years 2002 and 2003 were similar
(Table 1). Per our policy, newborns admitted to the pediatric ward
have lower serum bilirubin levels on admission in comparison to
those admitted to the NICU (20 vs 20 mg/dL, respectively).
Thus, a reduction in hyperbilirubinemia frequency and severity
was observed.
In the future, a prospective, large, multicenter, long-term follow-up study is necessary to evaluate the impact of these guidelines on readmission rate, severity of hyperbilirubinemia at the
time of admission, and the rate of kernicterus. Such a study will
become increasingly challenging in our profession equipoise on
this key aspect of neonatal clinical management.
Arie L. Alkalay, MD
Charles F. Simmons, MD
Department of Pediatrics, Division of Neonatology
Ahmanson Pediatric Center
Cedars-Sinai Medical Center
David Geffen School of Medicine
University of California Los Angeles
Los Angeles, CA 90048
REFERENCES
1. American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia.
Management of hyperbilirubinemia in the newborn infant 35 or more
weeks of gestation. Pediatrics. 2004;114:297316
2. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge
hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:
6 14
3. Bhutani VK, Johnson LH, Maisles MJ, et al. Kernicterus: epidemiological
strategies for its prevention through systems-based approaches. J Perinatol. 2004;24:650 662
Readmissions Due to Neonatal Hyperbilirubinemia
Academic
Year
Ward Readmissions
(n Newborns)
NICU Readmissions
(n Newborns)
Ward NICU
Readmissions Total
(per 1000 Deliveries)
Ward NICU
Readmission Ratio
Annual Deliveries
(n Newborns)
2002*
2003
36
34
30
7
66 (9.4)
41 (5.9)
1.20
4.85
6981
6890
P .001 (2).
* Before implementation of hyperbilirubinemia guidelines.
After implementation of hyperbilirubinemia guidelines.
824
LETTERS TO THE EDITOR
Downloaded from by guest on January 21, 2017
Hyperbilirubinemia Guidelines for Infants 34 Weeks of Gestation
Newborn infant in the WBN
Pre-discharge Bilia+EM
Nomogram*
428
25
342
95th % ile
High Risk Zone
15
In
High
Lo
10
ter
te
w In
iat
med
rme
diat
eR
eR
isk Z
one
75th % ile
40th % ile
on
isk Z
257
mol/L
Serum Bilirubin (mg/dl)
20
171
Low Risk Zone
85
0
0
12
24
36
48
60
72
84
96
108
120
132
144
Postnatal Age (hours)
*PEDIATRICS, 1999; 103:6-14 (modified)
Risk factors
no
yes
Low Zone: Regular follow-up by PMD*
Low-inter Zone: Bili in 48 4hrs
High-inter Zone: Bili in 24 4hrs
High Zone: Bili in 12 4hrs, consider Photox
*If discharge at <24, 24-47, 48-72hrs, to be
seen by PMD at 72, 96, and 144hrs of age,
respectively.
Table* Hyperbilirubinemia Management Guidelines
Photox
Exchange
Risk Factors
1. Family history of jaundice or hemolysis
2. Near term infants (34-38 wks)
3. Polycythemia
4. Internal or external bleeding
5. Postnatal hemolysis
6. Increase Bili rise (>0.5mg/dl/hr)
7. Increased Bili production (high ETCOc)
8. Hypoxemia, acidosis, sepsis, hypoalbuminemia
Abbreviations
Bili=Total bilirubin
EM=Educational material
ETCOc=End tidal volume corrected CO
Photox=Phototherapy
PMD=Attending pediatrician
WBN=Well Baby Nursery
Low Zone: Follow-up by PMD in 48 4hrs
Low-inter Zone: Bili in 36 4hrs
High-inter Zone: Bili in 18 4hrs
High Zone: Bili in 6 2hrs, Photox
24hrs
10-12 (7-10)
20 (18)
25-48hrs 12-15 (10-12)
20-25 (20)
49-72hrs 15-18 (12-15)
25-30 (>20)
>72hrs
18-20 (12-15)
25-30 (>20)
Bilirubin levels expressed in mg/dl
In brackets are Bili levels for infants with risk factors
*PEDIATRICS, 1994; 94:558-565 (modified)
a). Bilirubin can be obtained either by blood sample or by transcutaneous measurement
This algorithm is a suggested practice guidelines
and do not intend to replace clinical judgement.
Neonatology Division, Ahmanson Pediatric Center,
Cedars--Sinai Medical Center, Los Angeles, California; July, 2003
Arie L. Alkalay, MD; Charles F. Simmons, MD
Fig 1. Hyperbilirubinemia guidelines for infants 34 weeks gestation.
4. Moyer VA, Ahn C, Sneed S. Accuracy of clinical judgement in neonatal
jaundice. Arch Pediatr Adolesc Med. 200;154:391394
5. American Academy of Pediatrics, Provisional Committee for Quality
Improvement and Subcommittee on Hyperbilirubinemia. Practice
parameter: management of hyperbilirubinemia in the healthy term newborn. Pediatrics 1994;94:558 562
doi:10.1542/peds.2004-2442
Downloaded from by guest on January 21, 2017
LETTERS TO THE EDITOR
825
Hyperbilirubinemia Guidelines in Newborn Infants
Arie L. Alkalay and Charles F. Simmons
Pediatrics 2005;115;824
DOI: 10.1542/peds.2004-2442
Updated Information &
Services
including high resolution figures, can be found at:
/content/115/3/824.full.html
References
This article cites 4 articles, 3 of which can be accessed free
at:
/content/115/3/824.full.html#ref-list-1
Citations
This article has been cited by 6 HighWire-hosted articles:
/content/115/3/824.full.html#related-urls
Subspecialty Collections
This article, along with others on similar topics, appears in
the following collection(s):
Fetus/Newborn Infant
/cgi/collection/fetus:newborn_infant_sub
Hyperbilirubinemia
/cgi/collection/hyperbilirubinemia_sub
Permissions & Licensing
Information about reproducing this article in parts (figures,
tables) or in its entirety can be found online at:
/site/misc/Permissions.xhtml
Reprints
Information about ordering reprints can be found online:
/site/misc/reprints.xhtml
PEDIATRICS is the official journal of the American Academy of Pediatrics. A monthly
publication, it has been published continuously since 1948. PEDIATRICS is owned, published,
and trademarked by the American Academy of Pediatrics, 141 Northwest Point Boulevard, Elk
Grove Village, Illinois, 60007. Copyright 2005 by the American Academy of Pediatrics. All
rights reserved. Print ISSN: 0031-4005. Online ISSN: 1098-4275.
Downloaded from by guest on January 21, 2017
Hyperbilirubinemia Guidelines in Newborn Infants
Arie L. Alkalay and Charles F. Simmons
Pediatrics 2005;115;824
DOI: 10.1542/peds.2004-2442
The online version of this article, along with updated information and services, is
located on the World Wide Web at:
/content/115/3/824.full.html
PEDIATRICS is the official journal of the American Academy of Pediatrics. A monthly
publication, it has been published continuously since 1948. PEDIATRICS is owned,
published, and trademarked by the American Academy of Pediatrics, 141 Northwest Point
Boulevard, Elk Grove Village, Illinois, 60007. Copyright 2005 by the American Academy
of Pediatrics. All rights reserved. Print ISSN: 0031-4005. Online ISSN: 1098-4275.
Downloaded from by guest on January 21, 2017
Anda mungkin juga menyukai
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Maintain CVP ≥8 mm Hg in Septic ShockDokumen6 halamanMaintain CVP ≥8 mm Hg in Septic ShockIbrahim DharmawanBelum ada peringkat
- The Prevalence of Interatrial Septal Openings in Newborns and Predictive Factors For Spontaneous ClosureDokumen5 halamanThe Prevalence of Interatrial Septal Openings in Newborns and Predictive Factors For Spontaneous ClosureNisa UcilBelum ada peringkat
- Perinatology Journal Reading by AhimsaDokumen31 halamanPerinatology Journal Reading by AhimsaNisa UcilBelum ada peringkat
- Abstrak Anu Lampung Revisi FinalDokumen1 halamanAbstrak Anu Lampung Revisi FinalNisa UcilBelum ada peringkat
- Algo Pals Pediatric Cardiac ArrestDokumen1 halamanAlgo Pals Pediatric Cardiac ArrestDevi ChrestellaBelum ada peringkat
- Carrot, Angel and Oreo cake recipesDokumen4 halamanCarrot, Angel and Oreo cake recipesNisa UcilBelum ada peringkat
- Airway Management Complications in Children With Difficult Tracheal Intubation From The Pediatric Difficult Intubation PeDI Registry A Prospective Cohort AnalysisDokumen10 halamanAirway Management Complications in Children With Difficult Tracheal Intubation From The Pediatric Difficult Intubation PeDI Registry A Prospective Cohort AnalysisNisa UcilBelum ada peringkat
- HSR - USCOM FinalDokumen49 halamanHSR - USCOM FinalNisa UcilBelum ada peringkat
- NeurologicDokumen7 halamanNeurologicFarrah ErmanBelum ada peringkat
- AlgorithmPALS CACOVID 200406 PDFDokumen1 halamanAlgorithmPALS CACOVID 200406 PDFEka RahmanizarBelum ada peringkat
- Airway Management Complications in Children With Difficult Tracheal Intubation From The Pediatric Difficult Intubation PeDI Registry A Prospective Cohort AnalysisDokumen10 halamanAirway Management Complications in Children With Difficult Tracheal Intubation From The Pediatric Difficult Intubation PeDI Registry A Prospective Cohort AnalysisNisa UcilBelum ada peringkat
- Nihms 701642Dokumen10 halamanNihms 701642Nisa UcilBelum ada peringkat
- Algo Pals BLS Pediatric Cardiac ArrestDokumen1 halamanAlgo Pals BLS Pediatric Cardiac ArrestSiti NabilaBelum ada peringkat
- Algo Pals Pediatric BradycardiaDokumen1 halamanAlgo Pals Pediatric BradycardiaNisa UcilBelum ada peringkat
- Exercise During Pregnancy A Practical ApproachDokumen7 halamanExercise During Pregnancy A Practical ApproachNisa UcilBelum ada peringkat
- Infantile Pityriasis Alba and Comorbid DisordersDokumen5 halamanInfantile Pityriasis Alba and Comorbid DisordersNisa UcilBelum ada peringkat
- Early detection of autism with CHAT checklistDokumen44 halamanEarly detection of autism with CHAT checklistNisa UcilBelum ada peringkat
- Exercise During Pregnancy A Practical ApproachDokumen7 halamanExercise During Pregnancy A Practical ApproachNisa UcilBelum ada peringkat
- Weight-For-Length GIRLS: Birth To 2 Years (Z-Scores)Dokumen1 halamanWeight-For-Length GIRLS: Birth To 2 Years (Z-Scores)Malisa LukmanBelum ada peringkat
- Karagol 2010Dokumen4 halamanKaragol 2010Nisa UcilBelum ada peringkat
- Adverson, J. 2013. Feeding Children With CP Swallowing Difficulties PDFDokumen4 halamanAdverson, J. 2013. Feeding Children With CP Swallowing Difficulties PDFNicoleOrtegaAguileraBelum ada peringkat
- Maternal and Infant Risk Factors Associated with Neonatal Asphyxia in BaliDokumen6 halamanMaternal and Infant Risk Factors Associated with Neonatal Asphyxia in BaliNisa UcilBelum ada peringkat
- 66b3 PDFDokumen6 halaman66b3 PDFNisa UcilBelum ada peringkat
- Isk AafpDokumen7 halamanIsk Aafpbebekdd22Belum ada peringkat
- Review Article: Autoimmune/Inflammatory Arthritis Associated Lymphomas: Who Is at Risk?Dokumen12 halamanReview Article: Autoimmune/Inflammatory Arthritis Associated Lymphomas: Who Is at Risk?Nisa UcilBelum ada peringkat
- Isk AafpDokumen7 halamanIsk Aafpbebekdd22Belum ada peringkat
- Cerebrospinal Fluid Lactate and PyruvateDokumen8 halamanCerebrospinal Fluid Lactate and PyruvateNisa UcilBelum ada peringkat
- Validity and ReliabilityDokumen6 halamanValidity and ReliabilityfarlynzBelum ada peringkat
- Correlation of Procalcitonin Level and Neutrophil-Lymphocyte Ratio in SIRS PatientsDokumen1 halamanCorrelation of Procalcitonin Level and Neutrophil-Lymphocyte Ratio in SIRS PatientsNisa UcilBelum ada peringkat
- PCH 32 s1 039 PDFDokumen4 halamanPCH 32 s1 039 PDF79lalalaBelum ada peringkat
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Sensing System Assisted Novel PID Controller For Efficient Speed Control of DC MDokumen4 halamanSensing System Assisted Novel PID Controller For Efficient Speed Control of DC Mu2005044Belum ada peringkat
- Improving Students' Science Process SkillsDokumen9 halamanImproving Students' Science Process SkillsNovia RahmawatiBelum ada peringkat
- STC Ratings PDFDokumen3 halamanSTC Ratings PDFDiseño SonidoBelum ada peringkat
- SPECIFIC GRAVITY - DENSITY OF HYDRAULIC CEMENT (IS - 4031-Part 11-1988)Dokumen6 halamanSPECIFIC GRAVITY - DENSITY OF HYDRAULIC CEMENT (IS - 4031-Part 11-1988)Pritha DasBelum ada peringkat
- Rooftop Solar PV Opportunity in Tempe, Arizona: A Consultancy PlanDokumen4 halamanRooftop Solar PV Opportunity in Tempe, Arizona: A Consultancy PlanAli KhanBelum ada peringkat
- Macroeconomics II: Search and Matching: Luiz BrotherhoodDokumen18 halamanMacroeconomics II: Search and Matching: Luiz BrotherhoodMartin GutovskieBelum ada peringkat
- Structural Notes SampleDokumen14 halamanStructural Notes SampleNicole FrancisBelum ada peringkat
- Canadian Wood Council Publications and Tools For Wood Design Robertson RocchiDokumen62 halamanCanadian Wood Council Publications and Tools For Wood Design Robertson RocchiDj MacBelum ada peringkat
- Surface Roughness Measurement - MitutoyoDokumen2 halamanSurface Roughness Measurement - MitutoyoSelvaraj BalasundramBelum ada peringkat
- Ego7 Manual enDokumen76 halamanEgo7 Manual ensullivanj69Belum ada peringkat
- Error Correction - Test 1Dokumen4 halamanError Correction - Test 1phucnguyen0429Belum ada peringkat
- Smart Asthma ConsoleDokumen35 halamanSmart Asthma ConsoleMohamad Mosallam AyoubBelum ada peringkat
- Eb4069135 F enDokumen13 halamanEb4069135 F enkalvino314Belum ada peringkat
- BS 7941-1-2006Dokumen20 halamanBS 7941-1-2006Willy AryansahBelum ada peringkat
- Pharmaceutics | Water Solubility and Dissolution RateDokumen11 halamanPharmaceutics | Water Solubility and Dissolution RateAnnisa AgustinaBelum ada peringkat
- Brosur Sy135cDokumen9 halamanBrosur Sy135cDenny KurniawanBelum ada peringkat
- Omcmle Physiology Workbook Part 5 PDFDokumen63 halamanOmcmle Physiology Workbook Part 5 PDFloiuse shepiralBelum ada peringkat
- Steel 17-4PH MmpdsDokumen18 halamanSteel 17-4PH MmpdsManoj ManoharanBelum ada peringkat
- Ichroma™ COVID-19 Ab (With Ichroma™ II Reader) Test SystemDokumen6 halamanIchroma™ COVID-19 Ab (With Ichroma™ II Reader) Test SystemGopinath AgnihotramBelum ada peringkat
- Christmas Around the WorldDokumen16 halamanChristmas Around the WorldVioleta Veljanovska100% (1)
- HTTP Verbs GET POST PUT PATCH DELETE (39Dokumen12 halamanHTTP Verbs GET POST PUT PATCH DELETE (39Jefferson EducacionBelum ada peringkat
- Sepuran® N Module 4": in NM /H at 7 Barg 25°CDokumen2 halamanSepuran® N Module 4": in NM /H at 7 Barg 25°CsanjaigBelum ada peringkat
- Research Article (Lavandula Angustifolia) Essential Oil On: Effect of Lavender Acute Inflammatory ResponseDokumen10 halamanResearch Article (Lavandula Angustifolia) Essential Oil On: Effect of Lavender Acute Inflammatory ResponseAndreeaBelum ada peringkat
- 43-101 Technical Report Quimsacocha, February 2009Dokumen187 halaman43-101 Technical Report Quimsacocha, February 2009Marco Vinicio SotoBelum ada peringkat
- Steam Turbine Unloading and Shut-Down of Operation Turbine/Generator Shut-Down Diagram (General)Dokumen1 halamanSteam Turbine Unloading and Shut-Down of Operation Turbine/Generator Shut-Down Diagram (General)parthibanemails5779Belum ada peringkat
- Manual de Uso Ecografo GE Logiq e PDFDokumen192 halamanManual de Uso Ecografo GE Logiq e PDFDaniel CortesBelum ada peringkat
- داينمك الملزمة كاملةDokumen79 halamanداينمك الملزمة كاملةarno assassin33% (3)
- Project Information for 2x660 MW Lube Oil PumpsDokumen93 halamanProject Information for 2x660 MW Lube Oil PumpsghostamirBelum ada peringkat
- TacoDokumen12 halamanTaconguyennhan2190Belum ada peringkat
- Traffic Sign Detection and Recognition Using Image ProcessingDokumen7 halamanTraffic Sign Detection and Recognition Using Image ProcessingIJRASETPublicationsBelum ada peringkat