Natsci 02
Diunggah oleh
Sofia Coleen Naluaran TemplonuevoJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Natsci 02
Diunggah oleh
Sofia Coleen Naluaran TemplonuevoHak Cipta:
Format Tersedia
Templonuevo, Sharmaine N.
NAT-SCI
NB703 M-TH 10:30-12:00
1) Definition of the following:
a) Atom
The smallest component of an element having the chemical properties of the element, consisting
of a nucleus containing combinations of neutrons and protons and one or more electrons bound
to the nucleus by electrical attraction; the number of protons determines the identity of the
element.
b) Molecule
Is the smallest particle in a chemical element or compound that has the chemical properties of
that element or compound.
c) Compound
Is a substance formed when two or more chemical elements are chemically bonded together.
d) Inorganic Compound
Is any compound that lacks a carbon atom, for lack of a more in-depth definition.
e) Organic Compound
Those compounds with a carbon atom.
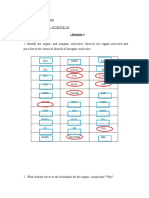
2) Enumerate the inorganic compound . Give the function and examples.
Function of inorganic compound:
1. Many of our enzymes serve as chelators for inorganic elements (usually ions). It's a bit
complicated, but what that basically means is that enzymes will fold around an inorganic
element, and the resulting conformation will help it carry out its function. Hemoglobin, which is of
course a vital protein that carries oxygen throughout our bodies, wouldn't work at all if it were
not for the iron ion it's functional units are wrapped around. The iron 'heme' group of hemoglobin
is what actually binds oxygen. Some structural proteins will chelate minerals as well; the calcium
in our bones for example.
2. Secondly, inorganic substances are crucial for transporting substances across membranes. The
different gradients of inorganic elements (like sodium, potassium, calcium and chlorine ions) in
and around cells allows membrane bound transport proteins to function correctly. A variety of
critically important functions of our cells depend on these gradients; from nerve impulse
transmission to the transport of proteins in and out of cells.
3. Oxygen and water are both inorganic compounds as well (with respect to chemistry, organic
means containing carbon). Oxygen of course has a integral role in aerobic metabolism; water has
so many functions and roles in our bodies.
Examples of Inorganic Compounds:
1. H2O - Water is a simple inorganic compound, even though it contains hydrogen, a key
atom (along with carbon) in many organic compounds. The atoms in a molecule of water have
formed very simple bonds due to this lack of carbon.
2. HCl - Hydrochloride, also known as hydrochloric acid when it is dissolved in water, is a
colorless, corrosive acid with a fairly strong pH. It is found in the gastric juices of many
animals, helping in digestion by breaking down food.
3. CO2 - Carbon dioxide, despite the presence of a carbon atom in the formula, is classified
as an inorganic compound. This has caused a dispute within the scientific community, with
questions being raised as to the validity of our current methods of classifying compounds.
Currently, organic compounds contain a carbon or a hydrocarbon, which forms a stronger
bond. The bond formed by carbon in CO2 is not a strong bond.
4. NO2 - Nitrogen dioxide gas presents a variety of colors at different temperatures. It is
often produced in atmospheric nuclear tests, and is responsible for the tell-tale reddish color
displayed in mushroom clouds. It is highly toxic, and forms fairly weak bonds between the
nitrogen and oxygen atoms.
5. Fe2O3 - Iron (III) oxide is one of the three main oxides of iron, and is an inorganic
compound due to the lack of a carbon atom or a hydrocarbon. Iron (III) oxide occurs naturally
as hematite, and is the source of most iron for the steel production industry. It is commonly
known as rust, and shares a number of characteristics with its naturally occurring
counterpart.
3) Enumerate the organic compound . Give the function and examples.
Function of inorganic compound:
All these components provide the energy and the means to maintain and create life.
Examples of Inorganic Compounds:
Carbohydrates- provide energy for all living things and form the basis for some of
their structures. Simple carbohydrates, or sugars, are monosaccharides that are
directly absorbed. Glucose is a monosaccharide that is the basic fuel for life. Cellular
respiration begins with glucose, and is the main product of photosynthesis.
Monosaccharides & Polyssacharides- Linked monosaccharides, or polysaccharides,
are complex carbohydrates that are broken down by enzymes into simple sugars so
they can be absorbed by the body. Most important are the starches, which are a
storage form for carbohydrates. Glycogen is stored in the liver as glucose. Cellulose,
such as cotton fabric and paper, is primarily a structural carbohydrate.
Lipids- make up waxes, fats and the steroids that compose many hormones. When
oxidized, fat provides almost twice the energy of carbohydrates. Fats are often stored
in plant seeds and in the adipose tissue of animals for reserve energy.
Protein- All life consists of proteins, which build and repair tissues and make up the
enzymes that catalyze chemical reactions inside cells. Proteins also store a cells
reserve energy
Anda mungkin juga menyukai
- Chemistry of Cell Review: Quick Review Notes Chapter 2Dari EverandChemistry of Cell Review: Quick Review Notes Chapter 2Belum ada peringkat
- AP BioDokumen15 halamanAP BioFatma AyadBelum ada peringkat
- Atoms and MoleculesDokumen5 halamanAtoms and MoleculesRelaisa CimafrancaBelum ada peringkat
- Anatomy Physiology and Disease For The Health Professions 3rd Edition Booth Solutions ManualDokumen25 halamanAnatomy Physiology and Disease For The Health Professions 3rd Edition Booth Solutions ManualCathyHowardokqm100% (64)
- MolBiol HL (2.1, 2.2,2.3.2.4,7.3) BookletDokumen37 halamanMolBiol HL (2.1, 2.2,2.3.2.4,7.3) BookletSeo Young YOONBelum ada peringkat
- Topic 1 Chemistry of LifeDokumen31 halamanTopic 1 Chemistry of LifeHapsah Muhammad100% (1)
- Bio Study SheetDokumen9 halamanBio Study SheetJennyBelum ada peringkat
- Chemical Basis Assignment-ANSDokumen2 halamanChemical Basis Assignment-ANSAj MirandaBelum ada peringkat
- BIOL 307 Biochemistr Y: by Çağdaş D. SonDokumen101 halamanBIOL 307 Biochemistr Y: by Çağdaş D. SonMetehan KaraBelum ada peringkat
- Introduction of BiochemistryDokumen38 halamanIntroduction of Biochemistrygghalia033Belum ada peringkat
- Bio 101 Notes Chemistry of Living ThingsDokumen8 halamanBio 101 Notes Chemistry of Living ThingsIbraheem AribidesiBelum ada peringkat
- NOTES Unit 2 - Chemistry, Matter and LifeDokumen5 halamanNOTES Unit 2 - Chemistry, Matter and LifeCarina LattoBelum ada peringkat
- Anatomy and Physiology for Students: A College Level Study Guide for Life Science and Allied Health MajorsDari EverandAnatomy and Physiology for Students: A College Level Study Guide for Life Science and Allied Health MajorsBelum ada peringkat
- Organic Versus Inorganic CompoundsDokumen3 halamanOrganic Versus Inorganic CompoundsSophia Margareth PulgadoBelum ada peringkat
- Introdn and CHOs E.CDokumen39 halamanIntrodn and CHOs E.Cfentaw melkieBelum ada peringkat
- Chapter 1 Intro To BiochemistryDokumen28 halamanChapter 1 Intro To BiochemistryddaylenroseBelum ada peringkat
- BiochimieDokumen11 halamanBiochimieecosysBelum ada peringkat
- Lesson 1 Gen BiologyDokumen14 halamanLesson 1 Gen BiologyPrincess Kyl JaymeBelum ada peringkat
- AP Biology Notebook PDFDokumen105 halamanAP Biology Notebook PDFعمر gamerBelum ada peringkat
- B. Chapter 3 Lesson 2-Organic CompoundsDokumen20 halamanB. Chapter 3 Lesson 2-Organic CompoundsShelrenBelum ada peringkat
- Anatomy Physiology and Disease For The Health Professions 3rd Edition Booth Solutions ManualDokumen31 halamanAnatomy Physiology and Disease For The Health Professions 3rd Edition Booth Solutions Manualbiolysis.roomthyzp2y100% (21)
- 1.2 BioDokumen5 halaman1.2 Bioaelinsmy93Belum ada peringkat
- Science 9 Q2module 4Dokumen8 halamanScience 9 Q2module 4alexablisssBelum ada peringkat
- What Is Organic Compound?Dokumen3 halamanWhat Is Organic Compound?Mark Joseph LatadeBelum ada peringkat
- Chapter 1 Outline: The Human Organism: I. DefinitionsDokumen13 halamanChapter 1 Outline: The Human Organism: I. DefinitionsSteffi MurielBelum ada peringkat
- Identify The Organic and Inorganic Molecules. Encircle The Organic Molecules and Put A Box To The Chemical Formula of Inorganic MoleculesDokumen9 halamanIdentify The Organic and Inorganic Molecules. Encircle The Organic Molecules and Put A Box To The Chemical Formula of Inorganic MoleculesMark Brian FloresBelum ada peringkat
- 1 - Chapter NotesDokumen40 halaman1 - Chapter NotesMuhammad MuneebBelum ada peringkat
- Topic 2 Complete Study GuideDokumen38 halamanTopic 2 Complete Study GuidechalihflBelum ada peringkat
- 1-Chapter 1 Introduction To BiochemistryDokumen53 halaman1-Chapter 1 Introduction To BiochemistryFarhana Mohd Hatta100% (1)
- Chapter - 2 ShortDokumen16 halamanChapter - 2 ShortNadeem ArainBelum ada peringkat
- Introduction of BiochemistryDokumen34 halamanIntroduction of Biochemistrygghalia033Belum ada peringkat
- Carbon and The Molecular Diversity of LifeDokumen4 halamanCarbon and The Molecular Diversity of LifesamBelum ada peringkat
- Chapter 7Dokumen10 halamanChapter 7AlexandraBelum ada peringkat
- Document From UsmanDokumen37 halamanDocument From UsmanzzzBelum ada peringkat
- FAQ MetalsDokumen6 halamanFAQ Metals1126playpubgBelum ada peringkat
- Module 4-Biomolecules: Chemical Composition of Living FormsDokumen6 halamanModule 4-Biomolecules: Chemical Composition of Living Formsmpstme placementBelum ada peringkat
- Ilovepdf Merged PDFDokumen12 halamanIlovepdf Merged PDFharshBelum ada peringkat
- General BiologyDokumen82 halamanGeneral BiologyNanashiBelum ada peringkat
- The Chemical Basis of Life II:: Organic MoleculesDokumen16 halamanThe Chemical Basis of Life II:: Organic MoleculesdontcareBelum ada peringkat
- ReviewerDokumen13 halamanReviewerSi sielBelum ada peringkat
- Week 3: September 27 - October 2: MC 2: BiochemistryDokumen6 halamanWeek 3: September 27 - October 2: MC 2: BiochemistryMary Rose CuentasBelum ada peringkat
- Biology Fall Final ReviewDokumen5 halamanBiology Fall Final ReviewtaemintBelum ada peringkat
- Science 9 - Q2 - Mod4 - CARBON ATOM A UNIQUE ONE - VerFinalDokumen8 halamanScience 9 - Q2 - Mod4 - CARBON ATOM A UNIQUE ONE - VerFinalHerdie Anne LedesmaBelum ada peringkat
- BiochemistryDokumen68 halamanBiochemistryAbby FranciscoBelum ada peringkat
- Anatomy and Physiology Chapter 2Dokumen28 halamanAnatomy and Physiology Chapter 2Marshalee FrancisBelum ada peringkat
- Introduction To Biochemistry - Course Notes 1Dokumen8 halamanIntroduction To Biochemistry - Course Notes 1jefov39379Belum ada peringkat
- ES202L-Part 1 - (GPRoy)Dokumen60 halamanES202L-Part 1 - (GPRoy)Adnan RizviBelum ada peringkat
- Chemistry in BiologyDokumen15 halamanChemistry in BiologyJohn OsborneBelum ada peringkat
- TOPIC 2 - Chemical Basis of LifeDokumen22 halamanTOPIC 2 - Chemical Basis of LifeKezziah KarylleBelum ada peringkat
- Lec Chapter 2 Enviro Systems Matter Energy LifeDokumen61 halamanLec Chapter 2 Enviro Systems Matter Energy LifeAdrian GomezBelum ada peringkat
- Activity 2 Carbohydrates Preiy Julian M de GuiaDokumen3 halamanActivity 2 Carbohydrates Preiy Julian M de GuiaPreiy Julian De GuiaBelum ada peringkat
- Unit 1 Chemistry of LifeDokumen9 halamanUnit 1 Chemistry of Liferaghad mohammedBelum ada peringkat
- Biomolecules (Carbohydrates, Fats, Proteins and Nucleic Acids)Dokumen4 halamanBiomolecules (Carbohydrates, Fats, Proteins and Nucleic Acids)rommel benamirBelum ada peringkat
- Biochemistry Lecture 2Dokumen14 halamanBiochemistry Lecture 2Izhan AhmedBelum ada peringkat
- Functional GroupsDokumen11 halamanFunctional GroupsAvinejBelum ada peringkat
- Unit 2 - Envi Sci HandoutsDokumen2 halamanUnit 2 - Envi Sci HandoutsRuby SamarBelum ada peringkat
- Molecules of Biology BiochemistryDokumen5 halamanMolecules of Biology BiochemistryAbdalrahman AldsokyBelum ada peringkat
- Module 2: The Chemical Level of Organization: Topic: Basic Chemistry & Biochemistry Learning TargetsDokumen15 halamanModule 2: The Chemical Level of Organization: Topic: Basic Chemistry & Biochemistry Learning Targetsalmira garciaBelum ada peringkat
- Chapter Six, GeneticsDokumen10 halamanChapter Six, GeneticsHashim GhazoBelum ada peringkat
- 06.disorder of Carbohydrate MetabolismDokumen47 halaman06.disorder of Carbohydrate MetabolismRizka NizarBelum ada peringkat
- Caramellization A Review 2018Dokumen13 halamanCaramellization A Review 2018eleazarBelum ada peringkat
- Cambridge IGCSE: Biology 0610/22Dokumen16 halamanCambridge IGCSE: Biology 0610/22SAFFANAH NURBelum ada peringkat
- Burke 2011 - Carbohydrates For Training and CompetitionDokumen12 halamanBurke 2011 - Carbohydrates For Training and Competitionacolpo100% (1)
- My TestDokumen9 halamanMy Test1005012 Kary Luo HouBelum ada peringkat
- Dr. Jérôme Le Nours Group Leader: Jerome - Lenours@monash - EduDokumen33 halamanDr. Jérôme Le Nours Group Leader: Jerome - Lenours@monash - EduSophia BeamBelum ada peringkat
- Blood Glucose HomeostasisDokumen21 halamanBlood Glucose HomeostasisAastha SinhaBelum ada peringkat
- Dismantling The Ketogenic Diet With DR - NicholasgonzalezDokumen49 halamanDismantling The Ketogenic Diet With DR - NicholasgonzalezCristina100% (3)
- Pathophysiology of DMDokumen45 halamanPathophysiology of DMAmanuel MaruBelum ada peringkat
- ResultDokumen15 halamanResultWei Loon100% (1)
- MC3 - MIDTERM NotesDokumen18 halamanMC3 - MIDTERM NotesJustineBelum ada peringkat
- Siridhanyallu Updated Book English - BasavarajuDokumen25 halamanSiridhanyallu Updated Book English - BasavarajuDesi DallasBelum ada peringkat
- Iodometric Determination of GlucoseDokumen2 halamanIodometric Determination of GlucoseAnuj jainBelum ada peringkat
- Unit 1 Molecules Their Interaction Relevant To Biology CSIR UGC NET Life SciencesDokumen5 halamanUnit 1 Molecules Their Interaction Relevant To Biology CSIR UGC NET Life SciencesKishan KoyaniBelum ada peringkat
- Kertas 3Dokumen19 halamanKertas 3Ct CalifaBelum ada peringkat
- Bioplastic Corn StarchDokumen16 halamanBioplastic Corn StarchVIPratik KOLIBelum ada peringkat
- BIOMOLECULES Ncert Class 12 Most Important QuestionsDokumen6 halamanBIOMOLECULES Ncert Class 12 Most Important QuestionsRamBelum ada peringkat
- Salacia Reticulata (Kothala Himbutu) Revisited A Missed Opportunity To Treat Diabetes and Obesity?Dokumen8 halamanSalacia Reticulata (Kothala Himbutu) Revisited A Missed Opportunity To Treat Diabetes and Obesity?DEEPAK KUMARBelum ada peringkat
- Glucose Dinitrosalicylic Acid MethodDokumen3 halamanGlucose Dinitrosalicylic Acid Methodarcheologists100% (1)
- 566 Enzymatic Conversion of Biomass For Fuels Production: ACS Symp0Sium SeriesDokumen20 halaman566 Enzymatic Conversion of Biomass For Fuels Production: ACS Symp0Sium SeriesMARCOSBelum ada peringkat
- Nutrition Study GuideDokumen3 halamanNutrition Study GuidedianaAZPBelum ada peringkat
- BIOMOLECULESDokumen41 halamanBIOMOLECULESbam yeontanieBelum ada peringkat
- Carbohydrates and NutrigeneticsDokumen7 halamanCarbohydrates and NutrigeneticsMada madalinaBelum ada peringkat
- General Biology (Biol. 1012) : Course Instructor: Kabeta LegeseDokumen48 halamanGeneral Biology (Biol. 1012) : Course Instructor: Kabeta LegeseKena MegersaBelum ada peringkat
- Four Essential MacromoleculesDokumen34 halamanFour Essential MacromoleculesCosetteBelum ada peringkat
- Biology Teachers' Manual PDFDokumen72 halamanBiology Teachers' Manual PDFAndrewBelum ada peringkat
- Carbohydrates OverviewDokumen144 halamanCarbohydrates OverviewButz AnthonyBelum ada peringkat
- TB ch18Dokumen12 halamanTB ch18Lolei Garnace100% (1)
- To Bee or Not To Bee Varianta 2Dokumen31 halamanTo Bee or Not To Bee Varianta 2Remus GuiuBelum ada peringkat
- पञ्चकर्म Part ADokumen100 halamanपञ्चकर्म Part AAnil DasBelum ada peringkat