Date
Diunggah oleh
gianganteng0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
8 tayangan3 halamanfdgr
Hak Cipta
© © All Rights Reserved
Format Tersedia
DOCX, PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen Inifdgr
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai DOCX, PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
8 tayangan3 halamanDate
Diunggah oleh
giangantengfdgr
Hak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai DOCX, PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 3
Nama : Elvina Dheborah Silalahi
NIM : 161424008
Kelas : 1A- Teknik Kimia Produksi Bersih
1. Date : 14 March 2017
Source : http://www.instructables.com/
Title : Separate Hydrogen and Oxygen From Water Through Electrolysis
Topic :
Electrolysis a method of separating elements by pushing an electric current through a
compound. It is used in various industrial applications such as removing copper from
its ore. It is also used to separate hydrogen and oxygen from water. Electrolysis isn't
the most efficient way to obtain hydrogen, but it is one of the easiest and cheapest
ways to "homebrew" hydrogen.Water molecule is formed by two elements: two
positive Hydrogen ions and one negative Oxygen ion. The water molecule is held
together by the electromagnetic attraction between these ions.
2. Date : 15 March 2017
Source : http://www.finishing.com
Title : How to Get Copper from Copper Sulphate
Topic :
Here CuSO4 (solution= CuSO4 + water) plus add iron foam; here CuSO4 reacts with
iron to become iron sulphate and the copper becomes as residue or precipitate.
Method: take a plastic jug, put in CuSO4, add water, stir well, then put iron foam into
this solution. We get the copper residue immediately. It's a 10 minute reaction. This
looks like conversion from iron into copper. This is one method of low energy nuclear
reaction.
3. Date : 16 March 2017
Source : http://www.omega.co.uk/
Title : pH Meter
Topic :
A pH meter is an instrument used to measure acidity or alkalinity of a solution - also
know as pH. pH is the unit of measure that describes the degree of acidity or
alkalinity. It is measured on a scale of 0 to 14.The pH value of a substance is directly
related to the ratio of the hydrogen ion [H+] and the hydroxyl ion [OH-]
concentrations. If the H+ concentration is greater than OH-, the material is acidic; i.e.,
the pH measurement is less than 7. If the OH- concentration is greater than H+, the
material is basic, with a pH value greater than 7. If equal amounts of H+ and OH- ions
are present, the material is neutral, with a pH of 7.
4. Date : 17 March 2017
Source : http://www.chem.latech.edu/
Title : Standardization of Acid and Base Solutions
Topic :
Standardization is the process of determining the exact concentration (molarity) of a
solution. Titration is one type of analytical procedure often used in standardization. In
a titration, an exact volume of one substance is reacted with a known amount of
another substance.
The point at which the reaction is complete in a titration is referred to as the endpoint.
A chemical substance known as an indicator is used to indicate (signal) the endpoint.
The indicator used in this experiment is phenolphthalein. Phenolphthalein, an organic
compound, is colorless in acidic solution and pink in basic solution.
5. Date : 18 March 2017
Source : http://chemed.chem.purdue.edu
Title : What is a Titration
Topic :
A titration is a technique where a solution of known concentration is used to
determine the concentration of an unknown solution. Typically, the titrant (the know
solution) is added from a buret to a known quantity of the analyte (the unknown
solution) until the reaction is complete. Knowing the volume of titrant added allows
the determination of the concentration of the unknown. Often, an indicator is used to
usually signal the end of the reaction, the endpoint.
6. Date : 19 March 2017
Source : http://www.californiaavocado.com
Title : How Avocado Oil Is Made
Topic :
There are many different ways to produce avocado oil. We believe that the purest way
to make avocado oil starts from California Avocados. That is key when making raw
avocado oil, as with life itself, what you put into something is what you get out of it.
We naturally ripen California grown avocados once picked from our avocado grove in
San Diego. When the fruit is perfectly ripe the avocados are pushed through the press.
We ensure that only quality fruit is used and we do this by inspecting each piece of
fruit by hand before its processed and after its washed.
7. Date : 20 March 2017
Source : http://www.chemicool.com
Title : Extraction
Topic :
Extractions are a way to separate a desired substance when it is mixed with others.
The mixture is brought into contact with a solvent in which the substance of interest is
soluble, but the other substances present are insoluble.
Extractions use two immiscible phases to separate the substance from one phase into
the other.
Typical lab extractions are of organic compounds out of an aqueous phase and into an
organic phase. The distribution of a solute between two phases is an equilibrium
condition described by partition theory.
Anda mungkin juga menyukai
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Mercedes DifferentialsDokumen8 halamanMercedes DifferentialsAntonio Sanchez SanchezBelum ada peringkat
- Installation Manual - GRE PIPE - For Marine - Rev.2Dokumen37 halamanInstallation Manual - GRE PIPE - For Marine - Rev.2HuongtrinhAkay100% (1)
- Cover Conductor ReportDokumen73 halamanCover Conductor ReportCarlosBelum ada peringkat
- Bolts (Al Rashed Fastners) PDFDokumen71 halamanBolts (Al Rashed Fastners) PDFAbu100% (1)
- Zinc Flake Technology-Technical PDFDokumen1 halamanZinc Flake Technology-Technical PDFKalyan DhakaneBelum ada peringkat
- Typical Exchanger & Reboiler Piping-BNDokumen12 halamanTypical Exchanger & Reboiler Piping-BNManan100% (2)
- Weld Repair For Pressure Vessels Made From Cr-Mo SteelsDokumen8 halamanWeld Repair For Pressure Vessels Made From Cr-Mo SteelsVedad ColakBelum ada peringkat
- 6 - Baker Hughes - Glen BengeDokumen21 halaman6 - Baker Hughes - Glen BengegeosedBelum ada peringkat
- ZCS300 ManualDokumen24 halamanZCS300 ManualFabricio BorgattaBelum ada peringkat
- Service Manual: CB 18S CB 27S CB 37SDokumen85 halamanService Manual: CB 18S CB 27S CB 37SMack DieselBelum ada peringkat
- (161424011) ToluenDokumen1 halaman(161424011) ToluengiangantengBelum ada peringkat
- (161424011) CombustionDokumen1 halaman(161424011) CombustiongiangantengBelum ada peringkat
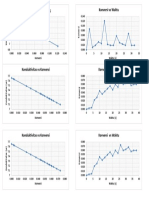
- Konduktivitas Vs Konversi Konversi Vs WaktuDokumen1 halamanKonduktivitas Vs Konversi Konversi Vs WaktugiangantengBelum ada peringkat
- Types of Infrared ThermometersDokumen2 halamanTypes of Infrared ThermometersgiangantengBelum ada peringkat
- PrintDokumen2 halamanPrintgiangantengBelum ada peringkat
- Natal Pemuda Gkps Bandung 2 DESEMBER 2017 Rundown Kebaktian Hari / Tanggal Waktu Durasi Kegiatan Penanggung JawabDokumen2 halamanNatal Pemuda Gkps Bandung 2 DESEMBER 2017 Rundown Kebaktian Hari / Tanggal Waktu Durasi Kegiatan Penanggung JawabgiangantengBelum ada peringkat
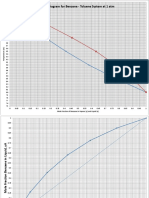
- T-X-Y Diagram For Benzene - Toluene System at 1 AtmDokumen3 halamanT-X-Y Diagram For Benzene - Toluene System at 1 AtmgiangantengBelum ada peringkat
- WWW - Sciencedirect: The Control of Pore Size in Alumina Catalyst Supports: A ReviewDokumen3 halamanWWW - Sciencedirect: The Control of Pore Size in Alumina Catalyst Supports: A ReviewgiangantengBelum ada peringkat
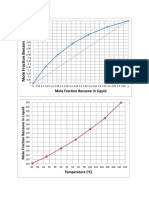
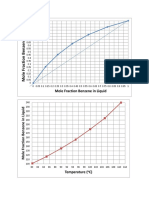
- Mole Fraction Benzene in LiquidDokumen2 halamanMole Fraction Benzene in LiquidgiangantengBelum ada peringkat
- (161424011) BFD AlkoholDokumen1 halaman(161424011) BFD AlkoholgiangantengBelum ada peringkat
- Advances, 4 (52), 27184-27200Dokumen2 halamanAdvances, 4 (52), 27184-27200giangantengBelum ada peringkat
- Indeks Bias Campuran Indeks Bias CampuranDokumen1 halamanIndeks Bias Campuran Indeks Bias CampurangiangantengBelum ada peringkat
- Conceptual Process Design and Techno-Economic Assessment of Ex Situ Catalytic Fast Pyrolysis of BiomassDokumen2 halamanConceptual Process Design and Techno-Economic Assessment of Ex Situ Catalytic Fast Pyrolysis of BiomassgiangantengBelum ada peringkat
- Memprihatinkan: Materials Chemistry B, 2 (42), 7459-7463Dokumen1 halamanMemprihatinkan: Materials Chemistry B, 2 (42), 7459-7463giangantengBelum ada peringkat
- Pengolahan DataDokumen11 halamanPengolahan DatagiangantengBelum ada peringkat
- Bin Er RRRRRRRRDokumen2 halamanBin Er RRRRRRRRgiangantengBelum ada peringkat
- Sampel1 23,3mL Sampel 2 7,8 Sampel 3 4,2 Sampel 4 2,5 Sampel 5 0,9 Sampel 6 0,6 AquadesnyaDokumen1 halamanSampel1 23,3mL Sampel 2 7,8 Sampel 3 4,2 Sampel 4 2,5 Sampel 5 0,9 Sampel 6 0,6 AquadesnyagiangantengBelum ada peringkat
- Laporan Praktikum Kimia Fisika Adsorpsi Pada Larutan: Oleh Gian Habli Maulana 161424011Dokumen10 halamanLaporan Praktikum Kimia Fisika Adsorpsi Pada Larutan: Oleh Gian Habli Maulana 161424011giangantengBelum ada peringkat
- I Data Percobaan A Metode Kenaikan Titik DidihDokumen3 halamanI Data Percobaan A Metode Kenaikan Titik DidihgiangantengBelum ada peringkat
- Comparison of Costs - Chlorine Gas Versus Hypochlorites: Application 1Dokumen4 halamanComparison of Costs - Chlorine Gas Versus Hypochlorites: Application 1fatsoe1Belum ada peringkat
- HD465.605-7 Field AssemblyDokumen72 halamanHD465.605-7 Field Assemblysoelist teoBelum ada peringkat
- Sc. Cl. - 001 - SAR0214HFESL-F3 (IE) CDokumen1 halamanSc. Cl. - 001 - SAR0214HFESL-F3 (IE) CSudarshika SumathipalaBelum ada peringkat
- Embuild BrochureDokumen38 halamanEmbuild BrochureKawish TamourBelum ada peringkat
- Quality Requirements New Format-06.04.13Dokumen1 halamanQuality Requirements New Format-06.04.13beeyesyemBelum ada peringkat
- Project: 4 Doors Duplex Type Boarding House: Item Materials Qty. Unit U/P AmountDokumen4 halamanProject: 4 Doors Duplex Type Boarding House: Item Materials Qty. Unit U/P AmountReynaldo PesqueraBelum ada peringkat
- Mil DTL 17849FDokumen23 halamanMil DTL 17849FOm Parkash SharmaBelum ada peringkat
- Polymer Processing Design Week 1Dokumen53 halamanPolymer Processing Design Week 1Abdul RahmanBelum ada peringkat
- Gambar ValveDokumen28 halamanGambar ValveIrfan FahmiBelum ada peringkat
- Fabricated Bridge Products: CatalogDokumen24 halamanFabricated Bridge Products: CatalogMuhammad TobiBelum ada peringkat
- ISO 1459 Hots Dip GalvanizingDokumen4 halamanISO 1459 Hots Dip GalvanizingLe Van TamBelum ada peringkat
- Mepoxe ANewDokumen4 halamanMepoxe ANewHasbi Ashidiqi PBelum ada peringkat
- Epoxy CuringDokumen8 halamanEpoxy CuringUrban Renewal Development100% (1)
- A Presentation On PumpsDokumen34 halamanA Presentation On PumpsSajjad Rasool ChaudhryBelum ada peringkat
- MSDS EonDokumen5 halamanMSDS EonIndieBelum ada peringkat
- Advanced Polymer Panel TDS PDFDokumen2 halamanAdvanced Polymer Panel TDS PDFsaint222Belum ada peringkat
- Lecture 02 01 Process DevelopmentDokumen20 halamanLecture 02 01 Process DevelopmentDon ReloBelum ada peringkat
- UENR58410001Dokumen12 halamanUENR58410001Jari GoethuysBelum ada peringkat
- 6-Absorption Stripping Pt1Dokumen18 halaman6-Absorption Stripping Pt1Naufal FasaBelum ada peringkat
- Cabin Pressure:: ExampleDokumen47 halamanCabin Pressure:: ExamplePrasanth ViratBelum ada peringkat