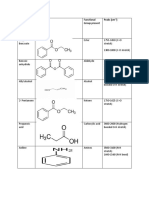
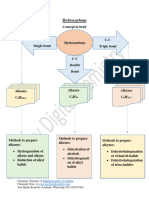
Chemistry Data
Diunggah oleh
J LevinsHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Chemistry Data
Diunggah oleh
J LevinsHak Cipta:
Format Tersedia
Appendix 8: Data Booklet
This appendix shows the data included in a Data Booklet that will be available on
our website. Centres will be sent copies of the Data Booklet for the first
examination series.
Centres can make additional fresh copies by printing the Data Booklet from our
website. Candidates must use an unmarked copy of the Data Booklet in
examinations.
Acknowledgement of source
The data used in the Data Booklet is derived from the Nuffield Advanced Science,
Revised Book of Data (ISBN 058235448X), Nuffield Foundation.
Pearson Edexcel Level 3 Advanced GCE in Chemistry 93
Specification Issue 2 March 2016 Pearson Education Limited 2016
Physical constants
Avogadro constant (L) 6.02 x 1023 mol-1
Elementary charge (e) 1.60 x 10-19 C
Gas constant (R) 8.31 J mol-1 K-1
Molar volume of ideal gas:
at r.t.p. 24 dm3 mol-1
Specific heat capacity of water 4.18 J g-1 K-1
Ionic product of water (Kw) 1.00 x 10-14 mol2 dm-6
1 dm3 = 1 000 cm3 = 0.001 m3
94 Pearson Edexcel Level 3 Advanced GCE in Chemistry
Specification Issue 2 March 2016 Pearson Education Limited 2016
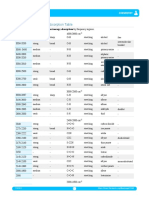
Infrared spectroscopy
Correlation of infrared absorption wavenumbers with molecular structure
Group Wavenumber range/cm-1
C-H stretching vibrations
Alkane 2962-2853
Alkene 3095-3010
Alkyne 3300
Arene 3030
Aldehyde 2900-2820 and 2775-2700
C-H bending variations
Alkane 1485-1365
Arene 5 adjacent hydrogen atoms 750 and 700
4 adjacent hydrogen atoms 750
3 adjacent hydrogen atoms 780
2 adjacent hydrogen atoms 830
1 adjacent hydrogen atom 880
N-H stretching vibrations
Amine 3500-3300
Amide 3500-3140
O-H stretching vibrations
Alcohols and phenols 3750-3200
Carboxylic acids 3300-2500
C=C stretching vibrations
Isolated alkene 1669-1645
Arene 1600, 1580, 1500, 1450
C=O stretching vibrations
Aldehydes, saturated alkyl 1740-1720
Ketones, alkyl 1720-1700
Ketones, aryl 1700-1680
Carboxylic acids, alkyl 1725-1700
Carboxylic acids, aryl 1700-1680
Carboxylic acid, anhydrides 1850-1800 and 1790-1740
Acyl halides, chlorides 1795
Acyl halides, bromides 1810
Esters, saturated 1750-1735
Amides 1700-1630
Triple bond stretching vibrations
CN 2260-2215
CC 2260-2100
Pearson Edexcel Level 3 Advanced GCE in Chemistry 95
Specification Issue 2 March 2016 Pearson Education Limited 2016
1
H nuclear magnetic resonance chemical shifts relative to
tetramethylsilane (TMS)
CH3OH
3.39 HCC=C
alkenes
arenes
benzene ethene propanone
7.27 5.28 2.10
HCN
amine
amide
CONH ArNH CNH
amide phenylamines amine
HCC=O
aldehyde
ketone
ester
COOH ArOH amide
carboxylic acid phenol acid
HCO
alcohol TMS
ether
ester
OH
alcohol
O ArH HC=C HChalogen HCC
arene ring alkene halogenoalkane alkane
CH R2CHF>R2CHCl> R3CH>R2CH2>
aldehyde
R2CHBr>R2CHI RCH3
12.0 11.0 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0
/ppm for TMS
13
C nuclear magnetic resonance chemical shifts relative to
tetramethylsilane (TMS)
C-N
O
CO CC
O
O C=C
TMS
CCC C-OH
CN
O CC CCl
Arene
CCH CBr CH2O
220 200 180 160 140 120 100 80 60 40 20 0 -20 -40
96 Pearson Edexcel Level 3 Advanced GCE in Chemistry
Specification Issue 2 March 2016 Pearson Education Limited 2016
Pauling electronegativities
Pauling electronegativity index
H He
21
Li Be B C N O F Ne
10 15 20 25 30 35 40
Na Mg Al Si P S CI Ar
09 12 15 19 21 25 30
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
08 10 13 15 16 16 15 18 18 18 19 16 16 20 20 24 28
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
08 10 12 13 16 21 19 22 22 22 19 16 17 19 19 21 25
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn
07 09 11 13 15 23 19 22 22 22 25 20 16 18 19 20 22
Relation in electronegativity difference, Ne and ionic character P/%
Electronegativity
difference Ne 01 03 05 07 10 13 15 17 20 25 30
Percentage
ionic character 05 2 6 12 22 34 43 51 63 79 89
P/%
Indicators
pKin acid pH range alkaline
(at 298 K)
1 Thymol blue (acid) 1.7 red 1.22.8 yellow
2 Screened methyl orange 3.7 purple 3.24.2 green
3 Methyl orange 3.7 red 3.24.4 yellow
4 Bromophenol blue 4.0 yellow 2.84.6 blue
5 Bromocresol green 4.7 yellow 3.85.4 blue
6 Methyl red 5.1 red 4.26.3 yellow
7 Litmus red 5.08.0 blue
8 Bromothymol blue 7.0 yellow 6.07.6 blue
9 Phenol red 7.9 yellow 6.88.4 red
10 Phenolphthalein (in 9.3 colourless 8.210.0 red
ethanol)
Pearson Edexcel Level 3 Advanced GCE in Chemistry 97
Specification Issue 2 March 2016 Pearson Education Limited 2016
Standard electrode potentials
Eo Standard electrode potential of aqueous system at 298 K, that is, standard
emf of electrochemical cell in the hydrogen half-cell forms the left-hand side
electrode system.
Right-hand electrode system Eo/V
1 Na+ + e- Na -2.71
2 Mg2+ + 2e- Mg -2.37
3 Al3+ + 3e- Al -1.66
2+ -
4 V + 2e V -1.18
2+ -
5 Zn + 2e Zn -0.76
6 Cr3+ + 3e- Cr -0.74
7 Fe2+ + 2e- Fe -0.44
3+ - 2+
8 Cr + e Cr -0.41
3+ - 2+
9 V +e V -0.26
10 Ni2+ + 2e- Ni -0.25
11 H+ + e- H2 0.00
12 S4O62- + e S2O3 - 2-
+0.09
2+ - +
13 Cu + e Cu +0.15
14 Cu2+ + 2e- Cu +0.34
15 VO2+ + 2H+ + e- V3+ + H2O +0.34
- -
16 O2 + H2O + 2e 2OH +0.40
17 S2O3 2- +
+ 6H + 4e 2S + 3H2O -
+0.47
18 Cu+ + e- Cu +0.52
19 I2 + e- I- +0.54
+ -
20 3O2 + 2H + 2e H2O2 +0.68
3+ - 2+
21 Fe + e Fe +0.77
22 Ag+ + e- Ag +0.80
23 NO3- + 2H+ + e- NO2 + H2O +0.80
- - - -
24 ClO + H2O + 2e Cl + 2OH +0.89
25 VO2+ + 2H + e VO + - 2+
+ H2O +1.00
26 Br2 + e- Br- +1.09
27 O2 + 2H+ + 2e- H2O +1.23
Cr2O 7 - + 7H+ + 3e- Cr3+ + +1.33
2
28 7
2 H2O
29 Cl2 + e- Cl- +1.36
30 MnO4- + 8H+ + 5e- Mn2+ + 4H2O +1.51
31 H2O2 + H+ + e- H2O +1.77
98 Pearson Edexcel Level 3 Advanced GCE in Chemistry
Specification Issue 2 March 2016 Pearson Education Limited 2016
Pearson Edexcel Level 3 Advanced GCE in Chemistry 99
Specification Issue 2 March 2016 Pearson Education Limited 2016
Anda mungkin juga menyukai
- Organic I Reactions (COMPLETE) PDFDokumen10 halamanOrganic I Reactions (COMPLETE) PDFcztinu88% (50)
- Enter age & weight to calculate pediatric drug dosesDokumen1 halamanEnter age & weight to calculate pediatric drug dosesBruno SantosBelum ada peringkat
- Schaum's Easy Outline of Organic Chemistry, Second EditionDari EverandSchaum's Easy Outline of Organic Chemistry, Second EditionPenilaian: 3.5 dari 5 bintang3.5/5 (2)
- IR spectroscopy guide for organic functional group analysisDokumen24 halamanIR spectroscopy guide for organic functional group analysisakshantratwanBelum ada peringkat
- AMINES Anil HssliveDokumen9 halamanAMINES Anil HssliveRanit MukherjeeBelum ada peringkat
- Haloalkanes WorksheetDokumen4 halamanHaloalkanes WorksheetAnonymous 8VJhV1eI2y100% (4)
- Topic 10 Organic ChemistryDokumen12 halamanTopic 10 Organic ChemistrySiddharth JainBelum ada peringkat
- IR Spectrum Table: Quick Reference for Functional Group IdentificationDokumen5 halamanIR Spectrum Table: Quick Reference for Functional Group IdentificationMike Dinh100% (3)
- Drug MetabolismDokumen46 halamanDrug Metabolismأحمد عاطفBelum ada peringkat
- Chemistry Data BookletDokumen7 halamanChemistry Data Bookletbob turnerBelum ada peringkat
- Edexcel Chemistry Data Sheet AlevelDokumen6 halamanEdexcel Chemistry Data Sheet AlevelManoj oliBelum ada peringkat
- S05 Data BookletDokumen7 halamanS05 Data BookletChi Wang LAWBelum ada peringkat
- FTIR Infrared Spectroscopy Absorption TableDokumen4 halamanFTIR Infrared Spectroscopy Absorption TablePankaj Kumar PalBelum ada peringkat
- Sample: Structure Functional Group Present Peaks (CM)Dokumen2 halamanSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANBelum ada peringkat
- Sample: Structure Functional Group Present Peaks (CM)Dokumen2 halamanSample: Structure Functional Group Present Peaks (CM)MUHAMMAD USMANBelum ada peringkat
- IR Absorption FrequenciesDokumen1 halamanIR Absorption FrequenciesRismayani Miftahul IBelum ada peringkat
- Data Booklet: Physical ConstantsDokumen1 halamanData Booklet: Physical Constantsアフリディ ホセインBelum ada peringkat
- Organic and Inorganic Chemistry Lab NotesDokumen4 halamanOrganic and Inorganic Chemistry Lab NotesJean - Luc BertilloBelum ada peringkat
- Al KanesDokumen12 halamanAl KanesHarsh TyagiBelum ada peringkat
- Infrared Spectroscopy Absorption Table - Chemistry LibreTexts PDFDokumen7 halamanInfrared Spectroscopy Absorption Table - Chemistry LibreTexts PDFNurhayati HasanahBelum ada peringkat
- Bảng phổ IRDokumen5 halamanBảng phổ IRĐan KhanhBelum ada peringkat
- Functional Class Range (CM) Intensity Assignment Alkanes: AlkenesDokumen1 halamanFunctional Class Range (CM) Intensity Assignment Alkanes: AlkenesStoica AlexandruBelum ada peringkat
- IR Spectrum TableDokumen17 halamanIR Spectrum TableAndres Felipe MBelum ada peringkat
- Chem CH 12 NIE Premium NOtesDokumen22 halamanChem CH 12 NIE Premium NOtesansarahmad6931Belum ada peringkat
- Chem 241 242 Formula SheetDokumen2 halamanChem 241 242 Formula SheetClara RyuBelum ada peringkat
- Class 10 Chemistry Chapter Hydrocarbons NotesDokumen28 halamanClass 10 Chemistry Chapter Hydrocarbons Notesnaveedhafiz78612Belum ada peringkat
- IR Spectroyscopy: Pending Region Finger Print 600-1100 Stretching Region 1100-4000Dokumen1 halamanIR Spectroyscopy: Pending Region Finger Print 600-1100 Stretching Region 1100-4000QantLeRabyBelum ada peringkat
- IR Spectrum Table by Frequency RangeDokumen5 halamanIR Spectrum Table by Frequency RangeDiego RodriguezBelum ada peringkat
- Cationic Flotation of Iron Ores AmineDokumen5 halamanCationic Flotation of Iron Ores AmineBismark SarpongBelum ada peringkat
- Reviw RemiDokumen4 halamanReviw RemiBuena QuintinBelum ada peringkat
- Decarboxylative C (sp3) - N Cross-Coupling Via Synergetic Photoredox and Copper CatalysisDokumen7 halamanDecarboxylative C (sp3) - N Cross-Coupling Via Synergetic Photoredox and Copper Catalysislost6taBelum ada peringkat
- Functional GroupsDokumen3 halamanFunctional GroupsManofMiracles89Belum ada peringkat
- Amines: Organic Bases and Their PropertiesDokumen78 halamanAmines: Organic Bases and Their PropertiesWinni TanBelum ada peringkat
- Instrumental Methods Appendix - IrDokumen8 halamanInstrumental Methods Appendix - IrMạnh BùiBelum ada peringkat
- Https WWW - Sigmaaldrich.com Technical-Documents Articles Biology Ir-Spectrum-Table - Printerview.htmlDokumen1 halamanHttps WWW - Sigmaaldrich.com Technical-Documents Articles Biology Ir-Spectrum-Table - Printerview.htmlnissa0% (1)
- 2018 JC2 H2 Nitrogen Compounds Notes (Upload for Students)Dokumen26 halaman2018 JC2 H2 Nitrogen Compounds Notes (Upload for Students)Amelia WongBelum ada peringkat
- Frequency Table For IR & NMRDokumen6 halamanFrequency Table For IR & NMRYogesh PingleBelum ada peringkat
- Hydrocarbons: Ella EH EH ÉDokumen19 halamanHydrocarbons: Ella EH EH ÉIsabella LopezBelum ada peringkat
- IR ChartDokumen2 halamanIR ChartNadiaa SafirraBelum ada peringkat
- Infrared Spectroscopy Absorption TableDokumen7 halamanInfrared Spectroscopy Absorption TableAmalinda Kharisma AdhaniBelum ada peringkat
- Technical Information Chemical Compatibility: Inspired by ChallengeDokumen16 halamanTechnical Information Chemical Compatibility: Inspired by Challengeyrdna nawaiteosBelum ada peringkat
- Curing Epoxy Resins with Citric Acid-Piperazine SaltDokumen6 halamanCuring Epoxy Resins with Citric Acid-Piperazine SaltEloy MijaresBelum ada peringkat
- Frequency Range (CM) Absorption (CM) Appearance Group Compound Class CommentsDokumen3 halamanFrequency Range (CM) Absorption (CM) Appearance Group Compound Class CommentsNazratul NajwaBelum ada peringkat
- 2.03 - Functional GroupsDokumen1 halaman2.03 - Functional GroupsJuan Miguel SalvadorBelum ada peringkat
- 20 PDFDokumen82 halaman20 PDFWalid EbaiedBelum ada peringkat
- Orgarnic Chemistry Functional Group TestDokumen9 halamanOrgarnic Chemistry Functional Group TestShourya veer singhBelum ada peringkat
- Scope of Accreditation: Standards Council of Canada Conseil Canadien Des NormesDokumen10 halamanScope of Accreditation: Standards Council of Canada Conseil Canadien Des NormeswandadwilestariBelum ada peringkat
- (-ENE) (-YNE) (-OL) (-OXY) (Benzene) : ChlorobenzeneDokumen2 halaman(-ENE) (-YNE) (-OL) (-OXY) (Benzene) : ChlorobenzeneyasiraBelum ada peringkat
- Formulary Functional GroupsDokumen1 halamanFormulary Functional Groupsavilaoscarp5Belum ada peringkat
- 17 Carbonyls Notes1 PDFDokumen21 halaman17 Carbonyls Notes1 PDFMary Joy BejeranoBelum ada peringkat
- Hsslive XII Chemistry Amines NoteDokumen4 halamanHsslive XII Chemistry Amines Notetrendzz youtuberBelum ada peringkat
- Chapter 9 AminesDokumen35 halamanChapter 9 AminesHanzalah Iman HamidiBelum ada peringkat
- Organic Chemistry Functional GroupsDokumen6 halamanOrganic Chemistry Functional GroupsDominador RomuloBelum ada peringkat
- Organic GOC Short RevisionDokumen7 halamanOrganic GOC Short Revisionmohitjain0076Belum ada peringkat
- Dr. Laurie S. Starkey, Organic Chemistry LaboratoryDokumen1 halamanDr. Laurie S. Starkey, Organic Chemistry LaboratoryDavid Galiano LatorreBelum ada peringkat
- Chemical Compatibility ChartDokumen16 halamanChemical Compatibility Chartadam_riantoBelum ada peringkat
- Treatment of Emuents From Ammonia Plants - Part III Ozonation of Amines in An Emuent From A Reforming Plant Serving An Ammonia ComplexDokumen8 halamanTreatment of Emuents From Ammonia Plants - Part III Ozonation of Amines in An Emuent From A Reforming Plant Serving An Ammonia ComplexMiguelBelum ada peringkat
- UV SpectrosDokumen25 halamanUV SpectrosokaciaBelum ada peringkat
- Aldehydes and Ketones-01 - TheoryDokumen45 halamanAldehydes and Ketones-01 - TheoryRaju SinghBelum ada peringkat
- 28 HydrocarbonsDokumen6 halaman28 HydrocarbonsDivyansh SinghBelum ada peringkat
- Topic 3: Major Bulk Organic Major Bulk Organic Chemicals From PropyleneDokumen9 halamanTopic 3: Major Bulk Organic Major Bulk Organic Chemicals From PropyleneYong LiBelum ada peringkat
- Ald and Ket Part 1Dokumen3 halamanAld and Ket Part 1Aryan GuptaBelum ada peringkat
- CH—Acids: A Guide to All Existing Problems of CH-Acidity with New Experimental Methods and Data, Including Indirect Electrochemical, Kinetic and Thermodynamic StudiesDari EverandCH—Acids: A Guide to All Existing Problems of CH-Acidity with New Experimental Methods and Data, Including Indirect Electrochemical, Kinetic and Thermodynamic StudiesBelum ada peringkat
- Organo-Transition Metal Compounds and Related Aspects of Homogeneous Catalysis: Pergamon Texts in Inorganic ChemistryDari EverandOrgano-Transition Metal Compounds and Related Aspects of Homogeneous Catalysis: Pergamon Texts in Inorganic ChemistryBelum ada peringkat
- BadgersDokumen2 halamanBadgersJ LevinsBelum ada peringkat
- FoxesDokumen2 halamanFoxesJ LevinsBelum ada peringkat
- Grey SquirrelsDokumen2 halamanGrey SquirrelsJ LevinsBelum ada peringkat
- HedgehogsDokumen2 halamanHedgehogsJ LevinsBelum ada peringkat
- Red SquirrelsDokumen2 halamanRed SquirrelsJ LevinsBelum ada peringkat
- Differentiation SummaryDokumen1 halamanDifferentiation SummaryNishchay BhattBelum ada peringkat
- 05 Gold 1 FP1 Edexcel PDFDokumen17 halaman05 Gold 1 FP1 Edexcel PDFJ LevinsBelum ada peringkat
- Particle Physics Show 1Dokumen29 halamanParticle Physics Show 1J LevinsBelum ada peringkat
- Differentiation SummaryDokumen1 halamanDifferentiation SummaryNishchay BhattBelum ada peringkat
- Tracking SSM AprilDokumen224 halamanTracking SSM AprilIrwan TaufiqBelum ada peringkat
- OpiateConversionDoses (Final) Nov2010Dokumen1 halamanOpiateConversionDoses (Final) Nov2010NajihBelum ada peringkat
- CHMBD 449 - Organic Spectral: AnalysisDokumen22 halamanCHMBD 449 - Organic Spectral: AnalysisIleana ManciuleaBelum ada peringkat
- Enols and EnolatesDokumen44 halamanEnols and Enolatessamocamo 123Belum ada peringkat
- Oxidation Via Iodine and Seo2Dokumen14 halamanOxidation Via Iodine and Seo2Usman GhaniBelum ada peringkat
- Degradation of TG: 1 - Fat catabolism (lipolysis) 2.β-Oxidation of Fatty acids (fatty acid oxidation)Dokumen26 halamanDegradation of TG: 1 - Fat catabolism (lipolysis) 2.β-Oxidation of Fatty acids (fatty acid oxidation)Aboubakar Moalim Mahad moh'dBelum ada peringkat
- Alda Risma CaseDokumen13 halamanAlda Risma CasealdiraBelum ada peringkat
- Product Guide BookDokumen11 halamanProduct Guide BookferdiBelum ada peringkat
- Dissolution MethodsDokumen59 halamanDissolution MethodsShiraz KhanBelum ada peringkat
- 03 Roadmap Oc Eng Student Copy 1595834497 PDFDokumen1 halaman03 Roadmap Oc Eng Student Copy 1595834497 PDFsantosh tripathyBelum ada peringkat
- Pricelist Dec 2014 DpcoDokumen8 halamanPricelist Dec 2014 Dpcohrocking1Belum ada peringkat
- 全民健康保險藥物給付項目及支付標準附件二Dokumen387 halaman全民健康保險藥物給付項目及支付標準附件二Nurfaizah EvaBelum ada peringkat
- Bhen Andrew D. Almodal Crim-201: General Chemistry (Organic)Dokumen5 halamanBhen Andrew D. Almodal Crim-201: General Chemistry (Organic)Benith AlmodalBelum ada peringkat
- Pareto Apotek NuhrintamaDokumen70 halamanPareto Apotek NuhrintamaNur Insani JBelum ada peringkat
- List of Athletes Currently Serving A Period of Ineligibility As A Result of An Anti-Doping Rule Violation Under IAAF RulesDokumen24 halamanList of Athletes Currently Serving A Period of Ineligibility As A Result of An Anti-Doping Rule Violation Under IAAF RulesJon GugalaBelum ada peringkat
- Chemical Test To Distinguish Between Pair of Organic CompoundDokumen11 halamanChemical Test To Distinguish Between Pair of Organic CompoundHishq Dhiman100% (1)
- Carbon chain aldehydes and their structuresDokumen3 halamanCarbon chain aldehydes and their structureslapBelum ada peringkat
- Drugs )Dokumen2 halamanDrugs )Brandon Lingo LeeBelum ada peringkat
- Laporan Perawat Imcu t6 LT 2 Update 260421 Google DriveDokumen46 halamanLaporan Perawat Imcu t6 LT 2 Update 260421 Google DriveNurse HandsomeBelum ada peringkat
- Gomez InventoryAdjustment OCTOBER 2019-ReconciliationDokumen52 halamanGomez InventoryAdjustment OCTOBER 2019-ReconciliationJescilyn Kate MaggayBelum ada peringkat
- Harga Satuan Obat Generik TerbaruDokumen8 halamanHarga Satuan Obat Generik TerbaruBunga LadipaBelum ada peringkat
- Amino Acids BiochemistryDokumen32 halamanAmino Acids BiochemistryKavita MahaseBelum ada peringkat
- Amlodipine CPDokumen2 halamanAmlodipine CPRose EchevarriaBelum ada peringkat
- 4173laporan BTK 26-31 Desember 2017Dokumen128 halaman4173laporan BTK 26-31 Desember 2017tandun nlmBelum ada peringkat
- Apotek Obat Daftar Zat AktifDokumen155 halamanApotek Obat Daftar Zat AktifAnissaBelum ada peringkat
- Perry's Chemical Engineers' Handbook, 8th Edition 238Dokumen1 halamanPerry's Chemical Engineers' Handbook, 8th Edition 238Ooi Chia EnBelum ada peringkat