Test 2 - 2017 PDF
Diunggah oleh
Farouk BassaJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Test 2 - 2017 PDF
Diunggah oleh
Farouk BassaHak Cipta:
Format Tersedia
CHEMICAL ENGINEERING and PULP & PAPER TECHNOLOGY
MULTI-STAGE OPERATIONS 3: CODE: PCCR301
LECTURER: Mr. A F Bassa DATE:25/04/2017
TEST 2
MAX MARKS : 100 (120 minutes)
QUESTION 1 : 45 Marks
A mixture of ethyl alcohol and water with 0.55 mole fraction of alcohol is distilled to give a constant top
product composition of 0.75 mole fraction of alcohol. The column has three ideal plates (four stages)
Using The VLE diagram and equations provided:
a) Determine the initial Reflux ratio
b) Determine the final Reflux ratio if the operation is discontinued when the still has a content of 30
mole per cent alcohol.
c) Determine the final Reflux ratio if the operation is discontinued when 83 per cent of the alcohol
charged has been removed as distillate
HINT: Use three trial values of R = 2.75; 1.5; 0.875
QUESTION 2 : 20 Marks
Consider a multi-component butane-pentane separation process. Composition of the feed, in mole
fraction, is given below (the components are given in order of relative volatility)
Propane iso-Butane n-Butane iso-Pentane n-Pentane
Component
C3 iC4 nC4 iC5 nC5
mol fr in feed 0.05 0.15 0.20 0.25 0.35
The objective of the process is to have 98% of iso-Butane (iC4) in the feed recovered in the
distillate and 95% of n-Butane (nC4) in the feed recovered in the bottoms product. The process
operates at P=8.3 bar. The feed is a saturated liquid.
a) Which component is the heavy key and which is the light key?
b) Identify the HNKs and the LNKs
c) Assuming sharp separation calculate compositions of the distillate and bottoms products
QUESTION 3 : 40 Marks
A vent gas stream in your chemical plant contains 10 mol% of a pollutant, the rest is air. The local
authorities want to reduce the pollutant concentration by absorbing 92 mol% of the pollutant
You have decided to build an absorption tower using water as the absorbent. The inlet water is
pure and the operation is essentially isothermal at 5oC, and isobaric at 10 atm. At 5C your
laboratory has found that the Henrys constant of the pollutant in water at 5oC is 876 atm/mole
fraction
Assume that air is not soluble in water and that water is non-volatile.
a) Find the minimum ratio of water to air (L /G)min on a solute-free basis
b) With (L/G) = 1.5 (L/G)min find the total number of equilibrium stages and the outlet liquid
concentration
HINT: For the graph use the coordinates Y (0 to 0.14) and X (0 to 0.0014)
Anda mungkin juga menyukai
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- Test 2 - 2017 SolnsDokumen7 halamanTest 2 - 2017 SolnsFarouk BassaBelum ada peringkat
- Aegrotat and Make-Up TestDokumen3 halamanAegrotat and Make-Up TestFarouk BassaBelum ada peringkat
- Packed Towers NotesDokumen10 halamanPacked Towers NotesFarouk BassaBelum ada peringkat
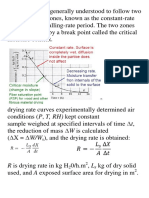
- Drying 2 Class Notes PDFDokumen18 halamanDrying 2 Class Notes PDFFarouk Bassa100% (1)
- Packed Towers - Class Notes 1Dokumen9 halamanPacked Towers - Class Notes 1Farouk BassaBelum ada peringkat
- Introduction To ThermodynamicsDokumen16 halamanIntroduction To ThermodynamicsFarouk BassaBelum ada peringkat
- Packed Towers - Class Notes 1Dokumen9 halamanPacked Towers - Class Notes 1Farouk BassaBelum ada peringkat
- Drying Lecture NotesDokumen18 halamanDrying Lecture NotesFarouk BassaBelum ada peringkat
- Soln ProcedureDokumen1 halamanSoln ProcedureFarouk BassaBelum ada peringkat
- Convective Mass TransferDokumen11 halamanConvective Mass TransferFarouk BassaBelum ada peringkat
- Post Prac QuestionsDokumen1 halamanPost Prac QuestionsFarouk BassaBelum ada peringkat
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- The Otto Schmidt's Inter-Stellar Dust TheoremDokumen1 halamanThe Otto Schmidt's Inter-Stellar Dust TheoremKC CampilanBelum ada peringkat
- NSS Chemistry Part 15 Analytical Chemistry (Structural QuestionsDokumen42 halamanNSS Chemistry Part 15 Analytical Chemistry (Structural QuestionsKelvinNgBelum ada peringkat
- Material Science (Unit 1)Dokumen18 halamanMaterial Science (Unit 1)Gaurav AgarwalBelum ada peringkat
- Egg Egg: Breaks Does Not BreakDokumen3 halamanEgg Egg: Breaks Does Not BreakLily Suhany MahmoodBelum ada peringkat
- Metal Corrosion Causes and PreventionDokumen23 halamanMetal Corrosion Causes and PreventionAman NikhareBelum ada peringkat
- 1 s2.0 S2214785322035441 MainDokumen7 halaman1 s2.0 S2214785322035441 MainMohammad Irfan AliBelum ada peringkat
- Precooled Ahu CalculationDokumen3 halamanPrecooled Ahu CalculationEdmund YoongBelum ada peringkat
- Chapter 2.1Dokumen27 halamanChapter 2.1wendye13Belum ada peringkat
- Jan 23 WCH12 SolvedDokumen28 halamanJan 23 WCH12 Solvedthe dsBelum ada peringkat
- Tut 12 Multiple Char Reactions TutorialDokumen19 halamanTut 12 Multiple Char Reactions TutorialRubén Alfonso Pérez JeldresBelum ada peringkat
- Fan Et Al. - Solids Mixing - Ind. and Eng. Chemistry (1970) Vol 62 NR 7Dokumen17 halamanFan Et Al. - Solids Mixing - Ind. and Eng. Chemistry (1970) Vol 62 NR 7BerndUmmeBelum ada peringkat
- Lee H. Horsley: Azeotropic Data-IIIDokumen638 halamanLee H. Horsley: Azeotropic Data-IIIMátyás Dalnoki100% (1)
- 23 - High Temperature Materials - Torralba PDFDokumen70 halaman23 - High Temperature Materials - Torralba PDFAnish MahajanBelum ada peringkat
- US Crete HF - 2020Dokumen2 halamanUS Crete HF - 2020kemdoBelum ada peringkat
- 02 Diffusivity - PPT (Read Only)Dokumen20 halaman02 Diffusivity - PPT (Read Only)dxdiag97Belum ada peringkat
- Fluid Mechanics - Multiple Choice Questions and Answers (MCQ) - ScholarexpressDokumen4 halamanFluid Mechanics - Multiple Choice Questions and Answers (MCQ) - Scholarexpressnitesh_kumar079976Belum ada peringkat
- Wave Optics Solutions – Fringe Patterns and IntensitiesDokumen15 halamanWave Optics Solutions – Fringe Patterns and Intensitiesashu mishraBelum ada peringkat
- Sika Rep Fine MSDokumen4 halamanSika Rep Fine MSmohghareib80Belum ada peringkat
- Schedule of Rate For Standard Stock Materials Common SR 2021-22 (11 KV System)Dokumen161 halamanSchedule of Rate For Standard Stock Materials Common SR 2021-22 (11 KV System)sagar mukulBelum ada peringkat
- Dr. Kshitij's Seminar on Carbohydrate Metabolism PathwaysDokumen14 halamanDr. Kshitij's Seminar on Carbohydrate Metabolism PathwaysPoonam PandyaBelum ada peringkat
- Flexure Formula Stresses in BeamsDokumen18 halamanFlexure Formula Stresses in BeamsAthena YoungBelum ada peringkat
- Pre-Final Examination Feb 2023Dokumen1 halamanPre-Final Examination Feb 2023Arun AchalamBelum ada peringkat
- Determinación de 3 Alkil 2 Metoxipirazinas en Uvas Mostos y VinosDokumen9 halamanDeterminación de 3 Alkil 2 Metoxipirazinas en Uvas Mostos y VinosEmmanuel BonninBelum ada peringkat
- Determination of Reducing SugarsDokumen5 halamanDetermination of Reducing SugarsrheamaeBelum ada peringkat
- Wondraczek Et Al. - 2022 - Advancing The Mechanical Performance of Glasses PDokumen25 halamanWondraczek Et Al. - 2022 - Advancing The Mechanical Performance of Glasses PAlireza BagherpourBelum ada peringkat
- Applied Physics Question Paper 30Dokumen1 halamanApplied Physics Question Paper 30RA.......VABelum ada peringkat
- Deet1-2 - Lab2 GRP3Dokumen6 halamanDeet1-2 - Lab2 GRP3Jhay lambert MercadoBelum ada peringkat
- Pesticide Analysis From Food Wtih LCMSDokumen473 halamanPesticide Analysis From Food Wtih LCMSberkahBelum ada peringkat
- Material Safety Data Sheet: 1. Identification of The Substance/Preparation and of The Company/UndertakingDokumen7 halamanMaterial Safety Data Sheet: 1. Identification of The Substance/Preparation and of The Company/Undertakingdaniel abiaBelum ada peringkat
- Compact Conductor CalculationDokumen3 halamanCompact Conductor CalculationGautama Chandra PradiptaBelum ada peringkat