CVM Wireframes
Diunggah oleh
api-894731Deskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
CVM Wireframes
Diunggah oleh
api-894731Hak Cipta:
Format Tersedia
Notes:
Text Size
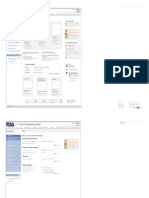
U.S. Food & Drug Administration Search
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
Animal & Veterinary
Animal & Veterinary Most Popular
1 What’s New from CVM
Products &
2 Text here
Procedures
Safety & Health Image Image Image
Report a Problem
Development &
Approvals
Recalls & Alerts
Guidance,
Compliance & Headline Headline Headline Product Approvals
Enforcement Text here Text here Text here
Science & Research LEARN MORE LEARN MORE LEARN MORE
Tools & Resources
News & Events
- Text here
About CVM
- Text here
Contact Information - Text here
Level 3 heading Level 3 heading - Text here
Level 3 keywords... Level 3 keywords...
- Subscribe to CVM Mailing
Resources for You Level 3 heading Level 3 heading Lists
Level 3 keywords... Level 3 keywords...
- Request a Form
Animal Food & Feed
Producers
Animal Drug Producers
Animal Producers Content here
News & Events Contact Us
Pet Owners 800-NNN-NNNN
301-NNN-NNNN
Veterinarians 05/05/08 Pediatric Research Equity Act
En Espanol 05/02/08 51st Annual FDLI ????@cvm.fda.gov
05/02/08 Approval History Center for Veterinary
Medicine
More News Items... 7519 Standish Place
Rockville, MD 20855
Consumers Healthcare Media Inquiries
Industry
Professionals
About CBER
Image Image
Image
Giving Blood, Product
Shortages Report a Problem Submissions,
Guidances
Learn more... Learn more...
Learn more...
Page Last Reviewed:
Page Last Updated:
Email Print Share Updates RSS Content Source:
Project: FDA Template Wireframes
Prepared By: Cari A. Wolfson, Focus on U!
FDA Footer Date Modified: 6/19/2008 by Design for
Context
CVM Topic Page Page Number: 1 of 4
Text Size Notes:
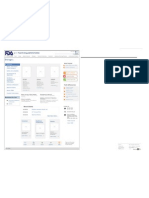
U.S. Food & Drug Administration Search
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
Animal & Veterinary
Animal & Veterinary Animal & Veterinary
Safety & Health Blood, Vaccines & Other Biologics
Report a Problem
Blood & Blood Products
Antimicrobial Overview of Blood Products Advisory Committee
Resistance Report a Problem
Screening Test Kits
Animal Feed Safety More... Recalls & Alerts
System (AFSS)
Product Safety Tissue & Tissue Products Product Approvals
Information
Overview Advisory Committee
Animal Drug Safety Screening Test Kits Safety
More...
MedWatch
Vaccines
Recalls &
Withdrawals Parents Guide Advisory Committee
More...
Resources for You Other Biologics
Animal Food & Feed Gene Therapy
Producers Allergenics
Xenotransplantation
Animal Drug Producers Cellular Products
Therapeutic Biologics
Animal Producers Devices Regulated by CBER
Content hereMore...
Pet Owners
Veterinarians
En Espanol
More [Insert Term]...
Page Last Reviewed:
Page Last Updated:
Email Print Share Updates RSS Content Source:
Project: FDA Template Wireframes
FDA Footer Prepared By: Cari A. Wolfson, Focus on U!
Date Modified: 6/19/2008 by Design for
Context
Products List Page - CWp22 Page Number: 2 of 4
Blood and Blood Products List (Level 4) Page Notes:
Text Size
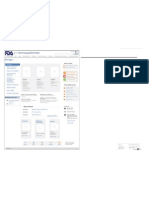
U.S. Food & Drug Administration Search
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
Biologics Biologics > Blood, Vaccines & Other Biologics
Biologics Blood & Blood Products Recalls & Approvals
Blood, Vaccines & The FDA is responsible for ensuring the safety of our nation's blood supply. The
Center for Biologics Evaluation and Research (CBER) regulates the collection of Email Page
Other Biologics
blood and blood components used for transfusion or for the manufacture of Print Page
pharmaceuticals derived from blood and blood components, such as clotting
Blood & Blood factors, and establishes standards for the products themselves. CBER also Bookmark and share
Products regulates related products such as cell separation devices, blood collection
containers and HIV screening tests that are used to prepare blood products or Get email updates
Advisory Committee to ensure the safety of the blood supply. CBER develops and enforces quality Subscribe to RSS
standards, inspects blood establishments and monitors reports of errors,
accidents and adverse clinical events. CBER works closely with other parts of
Approvals the Public Health Service (PHS) to identify and respond to potential threats to
blood safety, to develop safety and technical standards, to monitor blood
Blood Donor supplies and to help industry promote an adequate supply of blood and blood
Screening Test Kits products. While a blood supply with zero risk of transmitting infectious disease
may not be possible, the blood supply is safer than it has ever been.
Questions About Over a period of years, FDA has progressively strengthened the overlapping
Blood safeguards that protect patients from unsuitable blood and blood products.
Blood donors are now asked specific and very direct questions about risk
factors that could indicate possible infection with a transmissible disease. This
"up-front" screening eliminates approximately 90 percent of unsuitable donors.
Resources for You FDA also requires blood centers to maintain lists of unsuitable donors to
prevent the use of collections from them. Also, blood donations are now tested
Consumers & Healthcare for seven different infectious agents. In addition to strengthening these
Providers safeguards, FDA has significantly increased its oversight of the blood industry.
The agency inspects all blood facilities at least every two years, and "problem"
Industry facilities are inspected more often. Blood establishments are now held to
quality standards comparable to those expected of pharmaceutical
manufacturers.
In 1997, the FDA initiated the Blood Action Plan to increase the effectiveness of
its scientific and regulatory actions, and to ensure greater coordination with our
PHS partners. The plan was adopted by DHHS and is revised as new concerns
emerge. As biological products, blood and blood products are likely always to
carry an inherent risk of infectious agents. Therefore, zero risk may be
unattainable. The role of FDA is to drive that risk to the lowest level reasonably
achievable without unduly decreasing the availability of this life saving
resource.
Blood Topics
Blood Product Availability
Compliance & Surveillance
Blood Establishment Registration
Forms
ISBT Label
Product Listings
Publications
Safety
Page Last Reviewed:
Page Last Updated:
Content Source: Project: FDA Template Wireframes
Prepared By: Cari A. Wolfson, Focus on U!
Date Modified: 6/19/2008 by Design for
FDA Footer Context
Blood List Page - CWp57 Page Number: 3 of 4
Notes:
Text Size
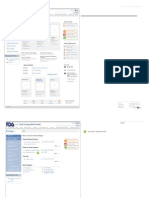
U.S. Food & Drug Administration Search
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
Topic Page Title
Topic Page Title > Level Two > Level Three > Level Four > Level Five
Level Two
Level Three List Page Title
Level Four Email Page
Print Page
Level Five Link
Group List
Level Five Link
List of Links
Image Description of link goes here…Lorem ipsum dolor sit amet,
Level Five Link
consectetuer adipiscing elit. Nam interdum. Donec accumsan.
Level Six Link
List of Links
Level Six Link Image Description of link goes here…Lorem ipsum dolor sit amet,
consectetuer adipiscing elit. Nam interdum. Donec accumsan.
Level Six Link
Level Five Link Group List
Level Five Link
List of Links
List of Links
List of Links
Group List
06/15/08 News item or event goes here
06/15/08 News item or event goes here
06/15/08 News item or event goes here
06/15/08 News item or event goes here
Page Last Reviewed:
Page Last Updated:
Email Print Content Source:
FDA Footer
Project: FDA Template Wireframes
Prepared By: Cari A. Wolfson, Focus on U!
Date Modified: 6/19/2008 by Design for
Context
CVM List Page Page Number: 4 of 4
Anda mungkin juga menyukai
- Giving Blood Scenario v2Dokumen3 halamanGiving Blood Scenario v2api-894731Belum ada peringkat
- Biologics Topic Page OnlyDokumen1 halamanBiologics Topic Page Onlyapi-894731Belum ada peringkat
- Biologics Topic Page OnlyDokumen1 halamanBiologics Topic Page Onlyapi-894731Belum ada peringkat
- Biologics Topic Page OnlyDokumen5 halamanBiologics Topic Page Onlyapi-894731Belum ada peringkat
- Register Blood Bank Scenario v2Dokumen4 halamanRegister Blood Bank Scenario v2api-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- Manufacturer Page Rewritten - HTMDokumen1 halamanManufacturer Page Rewritten - HTMapi-894731Belum ada peringkat
- What Is Cellular Inflammation - Australian NaturalCareDokumen8 halamanWhat Is Cellular Inflammation - Australian NaturalCareSaidu AuduBelum ada peringkat
- Registered Medical DevicesDokumen2 halamanRegistered Medical DevicesDTBelum ada peringkat
- Agfa HealthCare Presentation - tcm561-98342Dokumen22 halamanAgfa HealthCare Presentation - tcm561-98342Pantat babi BersinarBelum ada peringkat
- SOPs - Pharma PathwayDokumen7 halamanSOPs - Pharma PathwayDeepakBelum ada peringkat
- Biologics Topic Page No Left NavDokumen1 halamanBiologics Topic Page No Left Navapi-894731Belum ada peringkat
- Catalogue - Source Medical LTC PDFDokumen216 halamanCatalogue - Source Medical LTC PDFHermeeBelum ada peringkat
- Combination Topic Page OnlyDokumen1 halamanCombination Topic Page Onlyapi-894731Belum ada peringkat
- Marketing Mix: The 4Ps of MarketingDokumen40 halamanMarketing Mix: The 4Ps of MarketingAshutosh GuptaBelum ada peringkat
- Formulating Liquid Laundry Detergents _ HAPPIDokumen13 halamanFormulating Liquid Laundry Detergents _ HAPPIali rezaeiBelum ada peringkat
- PatientPlan Book1 052019-FINAL Digital Update-1Dokumen44 halamanPatientPlan Book1 052019-FINAL Digital Update-1Ryrey Abraham PacamanaBelum ada peringkat
- Reducing Length of Stay Using LeanDokumen42 halamanReducing Length of Stay Using LeanAsiimwe D Pius100% (1)
- Classeuroutils D'estimation Du Besoins COVID-19 (Enregistré Automatiquement)Dokumen45 halamanClasseuroutils D'estimation Du Besoins COVID-19 (Enregistré Automatiquement)ADJAHOUTONONBelum ada peringkat
- N95 Vs FFP3 & FFP2 Masks - What's The DifferenceDokumen1 halamanN95 Vs FFP3 & FFP2 Masks - What's The DifferenceRobert SmoothopBelum ada peringkat
- Asnt CP 189 (2020) PDFDokumen3 halamanAsnt CP 189 (2020) PDFAjay TriguneBelum ada peringkat
- Combination Topic Page OnlyDokumen1 halamanCombination Topic Page Onlyapi-894731Belum ada peringkat
- Dosing & Administration Moderna COVID-19 Vaccine (EUA)Dokumen1 halamanDosing & Administration Moderna COVID-19 Vaccine (EUA)Aung Zayar PaingBelum ada peringkat
- 2021 - Seedtreatment Agropages 2Dokumen1 halaman2021 - Seedtreatment Agropages 2Catherine TangBelum ada peringkat
- 911香港展會攤位傳單 7Dokumen4 halaman911香港展會攤位傳單 7sherry.hsiaoBelum ada peringkat
- Brain Cancer Vaccine Succeeds at Prolonging Survival in Phase 3 TrialDokumen1 halamanBrain Cancer Vaccine Succeeds at Prolonging Survival in Phase 3 TrialGlen RosselliBelum ada peringkat
- 30 Veterinary Practices BufDokumen40 halaman30 Veterinary Practices BufKaKa ManaBelum ada peringkat
- Beginning SAP R - 3 Implementation at Geneva PharmaceuticalsDokumen40 halamanBeginning SAP R - 3 Implementation at Geneva PharmaceuticalsVivekBelum ada peringkat
- Preparing For Dehorning - Here's A Review of Proper Protocol - Bovine VeterinarianDokumen10 halamanPreparing For Dehorning - Here's A Review of Proper Protocol - Bovine Veterinarianvictorm aceropBelum ada peringkat
- 510 (K) Premarket Notification2Dokumen1 halaman510 (K) Premarket Notification2kuttyjBelum ada peringkat
- Bio Meri Eux IncDokumen140 halamanBio Meri Eux IncAbhishek BanerjeeBelum ada peringkat
- Investor Deck - Doctor Insta PDFDokumen28 halamanInvestor Deck - Doctor Insta PDFSamarjit Samanta100% (3)
- HUL - Nabha Proposal - Revised - 3rdjune PDFDokumen13 halamanHUL - Nabha Proposal - Revised - 3rdjune PDFJagdeepSinghBelum ada peringkat
- PCI Naturals Raw Material Resale Supplier QuestionnaireDokumen8 halamanPCI Naturals Raw Material Resale Supplier QuestionnaireJuniorBelum ada peringkat
- Beauty Guide Skin Care PDFDokumen129 halamanBeauty Guide Skin Care PDFnguyen ba trungBelum ada peringkat
- Capstone Chapter1 FinalDokumen5 halamanCapstone Chapter1 FinalSaysain UkayBelum ada peringkat
- Capstone Chapter1 FinalDokumen5 halamanCapstone Chapter1 FinalMei GoBelum ada peringkat
- Capstone Chapter1 FinalDokumen5 halamanCapstone Chapter1 FinalSaysain UkayBelum ada peringkat
- Jk Insurance BrokerDokumen23 halamanJk Insurance BrokerARKAJIT DEY-DMBelum ada peringkat
- "The E-Petvet Card": Marketing Management New Product Development PlanDokumen31 halaman"The E-Petvet Card": Marketing Management New Product Development PlanCharlene Gem BactadBelum ada peringkat
- PPMP 2021Dokumen28 halamanPPMP 2021Jojo JustoBelum ada peringkat
- Tempus RNA Isolation Cms - 042989Dokumen38 halamanTempus RNA Isolation Cms - 042989Alexandru CodreanuBelum ada peringkat
- Animalscience Programofstudy2020Dokumen2 halamanAnimalscience Programofstudy2020api-279666485Belum ada peringkat
- 3B KatalogDokumen108 halaman3B Kataloghv1979Belum ada peringkat
- Complaint Handling KPIsDokumen19 halamanComplaint Handling KPIsDayaBelum ada peringkat
- Medical Device Regulation-USFDADokumen38 halamanMedical Device Regulation-USFDAMADDINENI AVANEESHWARBelum ada peringkat
- PSCI Supplier Conference 2020 India Session 4 - Implementing A Comprehensive Industrial Hygiene Program  - Panel Presentation - Recording and SlidesDokumen34 halamanPSCI Supplier Conference 2020 India Session 4 - Implementing A Comprehensive Industrial Hygiene Program  - Panel Presentation - Recording and SlidesBulent InanBelum ada peringkat
- CAMAF 2024 Electronic BrochureDokumen47 halamanCAMAF 2024 Electronic BrochureLesley ShonhiwaBelum ada peringkat
- 3M India leads in pharmaceuticals, medical devices and diagnosticsDokumen11 halaman3M India leads in pharmaceuticals, medical devices and diagnosticsRaviBelum ada peringkat
- JNJ Cagny 2018Dokumen51 halamanJNJ Cagny 2018Ala BasterBelum ada peringkat
- Veterinary Pet Care System Automates Animal ServicesDokumen5 halamanVeterinary Pet Care System Automates Animal ServicesSaysain UkayBelum ada peringkat
- Safari - Apr 18, 2024 at 12:32 AMDokumen1 halamanSafari - Apr 18, 2024 at 12:32 AM9rznd7tnz8Belum ada peringkat
- Department of Health - National Practice Standards For The Mental Health Workforce 2013Dokumen1 halamanDepartment of Health - National Practice Standards For The Mental Health Workforce 2013catalinauroraBelum ada peringkat
- BHP Formula No 27 Refrence - ALPHA - MS - Motion Sickness and Consequent Problems Like Nausea - Schwabe IndiaDokumen2 halamanBHP Formula No 27 Refrence - ALPHA - MS - Motion Sickness and Consequent Problems Like Nausea - Schwabe IndiaDr. Kazy Habibur RahmanBelum ada peringkat
- BD PlaybookDokumen12 halamanBD PlaybookSudarshan ShettyBelum ada peringkat
- Auspar Eslicarbazepine Acetate - 210909Dokumen28 halamanAuspar Eslicarbazepine Acetate - 210909Anna FlorentinaBelum ada peringkat
- PVMA KeystoneVeterinarian PVMA KeystoneVeterinarianSpring2021Dokumen36 halamanPVMA KeystoneVeterinarian PVMA KeystoneVeterinarianSpring2021Andreea Blanco VeraBelum ada peringkat
- Simplified MELC-Based Budget of Lesson in Animal Production (Swine) NC IIDokumen5 halamanSimplified MELC-Based Budget of Lesson in Animal Production (Swine) NC IImichael hobayanBelum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- To Do CurrentDokumen10 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Page Rewritten EvolutionDokumen2 halamanPage Rewritten Evolutionapi-894731Belum ada peringkat
- Biologics Topic Page EvolutionDokumen3 halamanBiologics Topic Page Evolutionapi-894731Belum ada peringkat
- To Do CurrentDokumen10 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Manufacturer Page Rewritten - HTMDokumen1 halamanManufacturer Page Rewritten - HTMapi-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Manufacturer Page Rewritten - HTMDokumen1 halamanManufacturer Page Rewritten - HTMapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Combination Topic Page OnlyDokumen1 halamanCombination Topic Page Onlyapi-894731Belum ada peringkat
- Combination Topic Page OnlyDokumen1 halamanCombination Topic Page Onlyapi-894731Belum ada peringkat
- Donating Blood Page No CalloutsDokumen1 halamanDonating Blood Page No Calloutsapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Biologics Topic Page No Left NavDokumen2 halamanBiologics Topic Page No Left Navapi-894731Belum ada peringkat
- Biologics Topic Page No Left NavDokumen1 halamanBiologics Topic Page No Left Navapi-894731Belum ada peringkat
- CVM WireframesDokumen4 halamanCVM Wireframesapi-894731Belum ada peringkat
- 1972 East Carolinas EV WBTV-BenJerrys 062519Dokumen2 halaman1972 East Carolinas EV WBTV-BenJerrys 062519Anonymous jeL70dsBelum ada peringkat
- Sepsis: Texts: Text ADokumen18 halamanSepsis: Texts: Text AInsta GlobalBelum ada peringkat
- ReferenceDokumen4 halamanReferenceDaroo D.TBelum ada peringkat
- Compatibility Testing: Dr. Mohammed H Saiem Aldahr Blood Bank 3 Medical TechnologyDokumen44 halamanCompatibility Testing: Dr. Mohammed H Saiem Aldahr Blood Bank 3 Medical TechnologyAnne Lorraine Margarette DulayBelum ada peringkat
- MLS 201 Lecture Note - Introduction To BGSDokumen7 halamanMLS 201 Lecture Note - Introduction To BGSBarry AllenBelum ada peringkat
- Corporate Managerial EconomicsDokumen14 halamanCorporate Managerial EconomicsfaeeezBelum ada peringkat
- QMMC Citizens Charter 11-28-13Dokumen68 halamanQMMC Citizens Charter 11-28-13DocAxi Maximo Jr AxibalBelum ada peringkat
- Muskaan Shah - Internship ReportDokumen5 halamanMuskaan Shah - Internship ReportMuskaanBelum ada peringkat
- 5th Handbook of Transfusion Medicine PDFDokumen186 halaman5th Handbook of Transfusion Medicine PDFreywae100% (3)
- FINAL - Requirements For Transfusion Laboratory Practice Fourth EditionDokumen49 halamanFINAL - Requirements For Transfusion Laboratory Practice Fourth EditionSara M AmeenBelum ada peringkat
- R A - 7719Dokumen3 halamanR A - 7719Osannah Irish InsongBelum ada peringkat
- BloodDokumen7 halamanBloodpravinambadeBelum ada peringkat
- Blood Donor RetentionDokumen17 halamanBlood Donor RetentionAstrud LabradorBelum ada peringkat
- Complications After Heart Transplantation - Hope For The Best, But Prepare For The WorstDokumen11 halamanComplications After Heart Transplantation - Hope For The Best, But Prepare For The WorstJorge AlvarezBelum ada peringkat
- 1639661490148-MCQs Lab Staff Prom Exam, LLHDokumen4 halaman1639661490148-MCQs Lab Staff Prom Exam, LLHobed cudjoeBelum ada peringkat
- Conventional Blood Banking and Blood Component Storage Regulation: Opportunities For ImprovementDokumen7 halamanConventional Blood Banking and Blood Component Storage Regulation: Opportunities For ImprovementKatona imreBelum ada peringkat
- Kotak Mahindra BankDokumen23 halamanKotak Mahindra BankSai VasudevanBelum ada peringkat
- Blood DonationnnDokumen1 halamanBlood DonationnnAina ZhrhBelum ada peringkat
- CiullabbDokumen71 halamanCiullabbMariel AbatayoBelum ada peringkat
- Who Can Donate BloodDokumen4 halamanWho Can Donate BloodsreeramBelum ada peringkat
- Collection of Blood From DonorsDokumen7 halamanCollection of Blood From DonorsBernardoHernandezBelum ada peringkat
- Notice: Grant and Cooperative Agreement Awards: Methotrexate Injection USP Preservative FreeDokumen2 halamanNotice: Grant and Cooperative Agreement Awards: Methotrexate Injection USP Preservative FreeJustia.comBelum ada peringkat
- Blood Component Fractionation: Manual Versus Automatic ProceduresDokumen6 halamanBlood Component Fractionation: Manual Versus Automatic ProceduresAngelBelum ada peringkat
- Albay Province Ordinance Establishing Voluntary Blood Services CouncilDokumen7 halamanAlbay Province Ordinance Establishing Voluntary Blood Services CouncilAlb GuarinBelum ada peringkat
- CC - Lab Manual PDFDokumen152 halamanCC - Lab Manual PDFVishal SindhnoorBelum ada peringkat
- Autologous - OmaDokumen55 halamanAutologous - OmaOmprakashBelum ada peringkat
- Blood TransfusionDokumen21 halamanBlood TransfusionAnup GouravBelum ada peringkat
- 3rd All Surgery Question FileDokumen136 halaman3rd All Surgery Question FileСымбат КулдасоваBelum ada peringkat
- Compendium of Transfusion MedicineDokumen50 halamanCompendium of Transfusion MedicineLudmilla MartinsBelum ada peringkat
- Analyzing Oxyglobin pricing and launch strategyDokumen8 halamanAnalyzing Oxyglobin pricing and launch strategyRajagopal PuttapartiBelum ada peringkat