Combination Topic Page Only
Diunggah oleh
api-894731Deskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Combination Topic Page Only
Diunggah oleh
api-894731Hak Cipta:
Format Tersedia
Notes:
Text Size
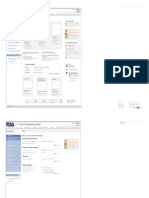
U.S. Food & Drug Administration Search
FDA Homepage Food Drugs Medical Devices Biologics Animal & Veterinary Cosmetics Radiation-Emitting Products Combination Products
Combination Products I would usually include a “Resources for You” section here, with a list of
1 specific audiences. However:
Combination Products Most Popular o This topic has only one audience, industry
About Combination Comment on the o The audience does break down into specific types – pharmaceutical
Products
1 “Report a Problem” manufacturers, device manufacturers, and academic researchers, to name
procedure three
Safety Image Comment on the o But their information needs do not differ.
2
application procedure So there is no reason to specify different audiences.
Jurisdictional
Information I pulled this from the “What’s New” section of the current Combination
3 2 Products site. Ideally, you would have something more recent, and text that
RFD Process Report a Problem highlighted the combination aspect of the product. This is just an example.
2
Transdermal Patch for Treatment of Parkinson's Disease Approved
Guidance & Regulatory On May 9, 2007, The U.S. Food and Drug Administration (FDA) today Product Approvals
Information There is a standard button for “Recalls & Alerts” but I did not see any data of
announced the approval of Neupro (rotigotine transdermal system), a 3
skin patch designed to treat symptoms of early Parkinson's disease.
that type on this site. I am adding this comment so you know about the button,
Requests for Comment should you need it in the future.
LEARN MORE Related Reports &
News & Events
Articles 5 4 Because there is just one audience, I needed to put something else here.
About Combination Products Request for Designation (RFD)
Examples, Questions about Process - Report to Congress: OCP
Combination Products How to Write an RFD, Questions about Performance Report for
I didn’t have anything for the Tools & Resources modules, because there are
RFDs 2006 5
Jurisdictional Information no online tools. So I adapted the “Related Resources” module.
Jurisdictional Transfers, Request Guidance & Regulatory Information - Article: “Combination
for Designation (RFD) Regulation Overview, Enabling Act, Products: Challenges and
Jurisdictional Decisions Acts, Rules & Regulations, Guidances Progress” (Regulatory Affairs
1 Focus Magazine, 8/05)
News & Events - Editorial: “FDA’s Office of
Combination Products:
2/08 Office of Combination Products (OCP) Review Roles, Progress &
Performance Updated challenges” (Journall of
Medical Dvice Regulation, 8/
3/07 Combination Product Approved: Absorbable Collagen 05)
Sponge with Genetically Engineered Human Protein
2/07 Guidance: Devices Used to Process Human Cells,
Contact Us
Tissues, and Cellular and Tissue-Based Products
301-427-1934
(HCT/P’s)
More News Items...
combination@fda.gov
4 Office of Combination
Products
Regulatory Information Jurisdictional Updates 15800 Crabbs Branch Way
(HFG-3), Suite 200
Rockville, MD 20855
Final Rule: Definition of the Breath Test Combination
Primary Mode of Action of a Products (9/06) Media Inquiries
Combination Product (Federal
Register, 8/25/05) Minimal Manipulation of About OCP
Structural Tissue (9/06) [pdf file,
Definition of a Combination 49KB]
Product from 21 CFR § 3.2(e)
Heparin Catheter Lock-Flush
Final Rule: Assignment of solutions: Transfer from DCER-
Combination Products/Product CDRH (9/17/06) [pdf file, 51KB]
Jurisdiction Program (6/23/05)
Page Last Reviewed:
Page Last Updated:
Email Print Share Updates RSS Content Source: Project: FDA Template Wireframes
Prepared By: Cari A. Wolfson, Focus on U!
Date Modified: 6/27/2008 by Design for
FDA Footer Context
VetMed Topic Page Only Page Number: 1 of 1
Anda mungkin juga menyukai
- Topical and Transdermal Drug Delivery: Principles and PracticeDari EverandTopical and Transdermal Drug Delivery: Principles and PracticeHeather A. E. BensonPenilaian: 5 dari 5 bintang5/5 (1)
- Combination Topic Page OnlyDokumen1 halamanCombination Topic Page Onlyapi-894731Belum ada peringkat
- Manufacturer Page Rewritten - HTMDokumen1 halamanManufacturer Page Rewritten - HTMapi-894731Belum ada peringkat
- Medical Device Classification FormDokumen9 halamanMedical Device Classification FormsouidiBelum ada peringkat
- Biologics Topic Page No Left NavDokumen1 halamanBiologics Topic Page No Left Navapi-894731Belum ada peringkat
- Product ComplaintsDokumen9 halamanProduct ComplaintsAlejandro LlccBelum ada peringkat
- OeMedical Documentation Release 0.1b Medical DocsDokumen14 halamanOeMedical Documentation Release 0.1b Medical DocsEvans CorpBelum ada peringkat
- Date of Publication 17 May 2011Dokumen47 halamanDate of Publication 17 May 2011adasdasBelum ada peringkat
- PD-400424 Rev B HolterCare 11.0.1 Release NotesDokumen8 halamanPD-400424 Rev B HolterCare 11.0.1 Release NotesJain BabuBelum ada peringkat
- A Simple Approach To Forecasting Correlated Products: by Larry LapideDokumen3 halamanA Simple Approach To Forecasting Correlated Products: by Larry LapideNguyen NguyenBelum ada peringkat
- Date of Publication 17 May 2011Dokumen48 halamanDate of Publication 17 May 2011jibran khanBelum ada peringkat
- 5 10 (K) SUMMARY: Contact Person:FDDokumen5 halaman5 10 (K) SUMMARY: Contact Person:FDHadanBelum ada peringkat
- BRD - Cims in Webhistree UpdatedDokumen13 halamanBRD - Cims in Webhistree UpdatedRashika GuptaBelum ada peringkat
- Running Head: Nexus Cardiac Products LTD Case Study 1Dokumen23 halamanRunning Head: Nexus Cardiac Products LTD Case Study 1waqarBelum ada peringkat
- 16 Kundeninfo enDokumen7 halaman16 Kundeninfo enanil nsBelum ada peringkat
- Solving Pharmas Quality Unit Identity CrisisDokumen3 halamanSolving Pharmas Quality Unit Identity CrisisAYMEN GOODKidBelum ada peringkat
- Achieving ''Zero'' Defects For Visible Particles in InjectablesDokumen13 halamanAchieving ''Zero'' Defects For Visible Particles in InjectablesmmmmmBelum ada peringkat
- Irjet V6i4319Dokumen5 halamanIrjet V6i4319ves projectBelum ada peringkat
- The Original Probiotic PLUS: SA Cannabis Script ApprovalDokumen2 halamanThe Original Probiotic PLUS: SA Cannabis Script ApprovalpharmacydailyBelum ada peringkat
- The eCTD Regulatory Dossier Regulatory Pathways Training, DCVMN WorkshopDokumen42 halamanThe eCTD Regulatory Dossier Regulatory Pathways Training, DCVMN WorkshopAdriana ABelum ada peringkat
- Giving Blood Scenario v2Dokumen3 halamanGiving Blood Scenario v2api-894731Belum ada peringkat
- Looking Into The Future Biosimilar Landscape: A Case StudyDokumen9 halamanLooking Into The Future Biosimilar Landscape: A Case StudyshashankBelum ada peringkat
- Pharmaceutical Dosage Forms - Parenteral Medications, Vol 3Dokumen728 halamanPharmaceutical Dosage Forms - Parenteral Medications, Vol 3Goodbye Cruel WorldBelum ada peringkat
- Edma Classification ReagentsDokumen82 halamanEdma Classification ReagentsHarun GanićBelum ada peringkat
- Your Life Science Digital Transformation With Iot: Safer, Happier and HealthierDokumen33 halamanYour Life Science Digital Transformation With Iot: Safer, Happier and Healthierbru_ribBelum ada peringkat
- Volume Viewer Innova 15.0 ext4 OMDokumen28 halamanVolume Viewer Innova 15.0 ext4 OMluiz.artur.filhoBelum ada peringkat
- Case No. 1: Botox: Almost Trouble-Free New Faces: Laguna State Polytechnic UniversityDokumen12 halamanCase No. 1: Botox: Almost Trouble-Free New Faces: Laguna State Polytechnic UniversityMia Joyce Roselada PanesaBelum ada peringkat
- Bio Electronics Report (BIEL) - Sizing The OpportunityDokumen54 halamanBio Electronics Report (BIEL) - Sizing The OpportunityMatt100% (3)
- Process Validation Case StudiesDokumen5 halamanProcess Validation Case StudiesKonisbell Alcántara UreñaBelum ada peringkat
- 2009 - IPEC Excipient Information PackageDokumen24 halaman2009 - IPEC Excipient Information Packagepascal candillonBelum ada peringkat
- Nexus Cardiac Case StudyDokumen16 halamanNexus Cardiac Case Studywaqar0% (2)
- CVM WireframesDokumen4 halamanCVM Wireframesapi-894731Belum ada peringkat
- ISPE PQLI Guide Part 2 Product Realization QbDDokumen233 halamanISPE PQLI Guide Part 2 Product Realization QbDBraulio Muñoz GutierrezBelum ada peringkat
- DelWorks DR Console Version 3.0 - Quick Reference GuideDokumen15 halamanDelWorks DR Console Version 3.0 - Quick Reference GuideBio HGEBelum ada peringkat
- Sample Medical Equipment Business PlanDokumen3 halamanSample Medical Equipment Business Planmalik_saleem_akbarBelum ada peringkat
- Ultimate Guide To CAPA - MedicalDeviceDokumen19 halamanUltimate Guide To CAPA - MedicalDeviceanilsamuel0077418100% (1)
- Understanding Cleanliness Classifications for Life Science Facilities _ Pharmaceutical EngineeringDokumen16 halamanUnderstanding Cleanliness Classifications for Life Science Facilities _ Pharmaceutical Engineeringsidparikh254Belum ada peringkat
- Isp Isg 453564935121Dokumen346 halamanIsp Isg 453564935121shahine.joudBelum ada peringkat
- Digital Health Marketplace Summary For Canada 1635193267Dokumen28 halamanDigital Health Marketplace Summary For Canada 1635193267arushi166Belum ada peringkat
- Latin America: Understanding Regulatory Compliance Requirements Across The Life Science Industry (Pharmaceuticals, Biologics, Medical Devices, IVDs)Dokumen4 halamanLatin America: Understanding Regulatory Compliance Requirements Across The Life Science Industry (Pharmaceuticals, Biologics, Medical Devices, IVDs)ComplianceOnlineBelum ada peringkat
- FDA Approval Process for Generic Drugs Seminar TranscriptDokumen16 halamanFDA Approval Process for Generic Drugs Seminar TranscriptTawfeeq BA AbbadBelum ada peringkat
- Abbott PDFDokumen1 halamanAbbott PDFAna Célia PereiraBelum ada peringkat
- Warehouse Temperature Management and ControlDokumen20 halamanWarehouse Temperature Management and ControlPhan ChaugiangBelum ada peringkat
- Analyst Consensus Rating: About Neothetics, IncDokumen8 halamanAnalyst Consensus Rating: About Neothetics, IncLiew Chee KiongBelum ada peringkat
- Verified Market Research - Updated Customized Sample Report - Canada Dry Brewers Yeast MarketDokumen66 halamanVerified Market Research - Updated Customized Sample Report - Canada Dry Brewers Yeast MarketnikhilpampatwarBelum ada peringkat
- Safety Data Sheet for EcoSMART Insect Killer Granules 2 for Lawns & LandscapesDokumen9 halamanSafety Data Sheet for EcoSMART Insect Killer Granules 2 for Lawns & LandscapesFaizan NazirBelum ada peringkat
- Mobile Application AProposalforthe Inventory Managementof PharmaceutDokumen12 halamanMobile Application AProposalforthe Inventory Managementof PharmaceutHamdala tamiratBelum ada peringkat
- Cleaning Validation For The 21St Century: Acceptance Limits For Active Pharmaceutical Ingredients (Apis) - Part IiDokumen9 halamanCleaning Validation For The 21St Century: Acceptance Limits For Active Pharmaceutical Ingredients (Apis) - Part IiMechaheb MassinissaBelum ada peringkat
- Market Research - Internship ProjectDokumen8 halamanMarket Research - Internship ProjectShraddha HegannaBelum ada peringkat
- Significance of Pharmaceutica ExcipientDokumen12 halamanSignificance of Pharmaceutica ExcipientRusyda Humaira Arumaisha100% (1)
- Beamex White Paper - Data Integrity ENG-1Dokumen4 halamanBeamex White Paper - Data Integrity ENG-1pobicsayBelum ada peringkat
- Genexpert Error Codes:An Evaluation of Their Definitions and Its Implications On Program Strengthening EffortsDokumen2 halamanGenexpert Error Codes:An Evaluation of Their Definitions and Its Implications On Program Strengthening EffortsKiem Adi BudimanBelum ada peringkat
- Guide to Classifying Medical Devices by RegulationDokumen18 halamanGuide to Classifying Medical Devices by RegulationRajeshBelum ada peringkat
- RN 81952831Dokumen5 halamanRN 81952831Roopa SBelum ada peringkat
- Software Testing For Medical DevicesDokumen8 halamanSoftware Testing For Medical Devicesandrewd420% (1)
- Email Worksheet OverviewDokumen27 halamanEmail Worksheet OverviewIsabel PeraltaBelum ada peringkat
- FDA Easy on-PCDokumen5 halamanFDA Easy on-PCVictor CuellarBelum ada peringkat
- GROUP3 - Tic Tac ToeDokumen1 halamanGROUP3 - Tic Tac ToeMarz CollinsBelum ada peringkat
- ANSI-A156!28!2000 Keying SystemDokumen16 halamanANSI-A156!28!2000 Keying SystemAhmedBelum ada peringkat
- Helping Pharmas Manage Compliance Risks For Speaker ProgramsDokumen9 halamanHelping Pharmas Manage Compliance Risks For Speaker ProgramsCognizantBelum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- To Do CurrentDokumen10 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Page Rewritten EvolutionDokumen2 halamanPage Rewritten Evolutionapi-894731Belum ada peringkat
- Biologics Topic Page EvolutionDokumen3 halamanBiologics Topic Page Evolutionapi-894731Belum ada peringkat
- To Do CurrentDokumen10 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- To Do CurrentDokumen5 halamanTo Do Currentapi-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Register Blood Bank Scenario v4 Blood Topic PageDokumen5 halamanRegister Blood Bank Scenario v4 Blood Topic Pageapi-894731Belum ada peringkat
- Donating Blood Page No CalloutsDokumen1 halamanDonating Blood Page No Calloutsapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- Giving Blood Scenario Blood Topic PageDokumen4 halamanGiving Blood Scenario Blood Topic Pageapi-894731Belum ada peringkat
- CVM WireframesDokumen4 halamanCVM Wireframesapi-894731Belum ada peringkat
- Biologics Topic Page No Left NavDokumen2 halamanBiologics Topic Page No Left Navapi-894731Belum ada peringkat
- Epilepsy and LevetiracetamDokumen53 halamanEpilepsy and LevetiracetamHasan Ahmed KhanBelum ada peringkat
- Paul B. Bishop, DC, MD, PHD, Jeffrey A. Quon, DC, PHD, FCCSC, Charles G. Fisher, MD, MHSC, FRCSC, Marcel F.S. Dvorak, MD, FRCSCDokumen10 halamanPaul B. Bishop, DC, MD, PHD, Jeffrey A. Quon, DC, PHD, FCCSC, Charles G. Fisher, MD, MHSC, FRCSC, Marcel F.S. Dvorak, MD, FRCSCorlando moraBelum ada peringkat
- OPD Network ListDokumen354 halamanOPD Network ListSHAIKH ABDUL AZIZ salim bashaBelum ada peringkat
- Immunology Serology Blood BankingDokumen5 halamanImmunology Serology Blood BankingEdsss Villar100% (3)
- Sustainability ReportDokumen84 halamanSustainability ReportBhavan YadavBelum ada peringkat
- Artikel 3Dokumen23 halamanArtikel 3Hadian UwuoBelum ada peringkat
- Wirmen Beautycare Cloth Pad SDN - BHDDokumen9 halamanWirmen Beautycare Cloth Pad SDN - BHDadilahBelum ada peringkat
- Journal On The Impact of Nursing Informatics To Clinical PracticeDokumen2 halamanJournal On The Impact of Nursing Informatics To Clinical PracticeLhara Vhaneza CuetoBelum ada peringkat
- Legal Medicine 2020 2021Dokumen4 halamanLegal Medicine 2020 2021Zie DammiBelum ada peringkat
- Reviews of Two Works by Dr. Amy Baker.Dokumen9 halamanReviews of Two Works by Dr. Amy Baker.Talia SchwartzBelum ada peringkat
- Bio-Oil® Product ManualDokumen60 halamanBio-Oil® Product ManualguitarristaclasicosdnBelum ada peringkat
- Yoga Your Home Practice CompanionDokumen257 halamanYoga Your Home Practice Companionjohncoltrane97% (33)
- Introduction To Public Health... 1stDokumen37 halamanIntroduction To Public Health... 1stNELSONJD20195100% (3)
- Ten Trends To Watch in The Coming Year - The EconomistDokumen1 halamanTen Trends To Watch in The Coming Year - The EconomistAnonymous KaoLHAkt100% (1)
- Nursing Assignment SampleDokumen12 halamanNursing Assignment Sampleswetha swethaBelum ada peringkat
- Risk for Infection AssessmentDokumen7 halamanRisk for Infection AssessmentLouis RoderosBelum ada peringkat
- 31congenital GlaucomasDokumen12 halaman31congenital GlaucomasShari' Si WahyuBelum ada peringkat
- Care Plan SummaryDokumen5 halamanCare Plan Summaryapi-541785084Belum ada peringkat
- Function: What Is The Skeletal System?Dokumen6 halamanFunction: What Is The Skeletal System?Mr. Christian ParabuacBelum ada peringkat
- Journal Club Presentation: DR Waleed AhmadDokumen30 halamanJournal Club Presentation: DR Waleed Ahmadkaram aliBelum ada peringkat
- College of Physicians and Surgeons of Alberta QB1803-01472 Certified Record of Proceedings - Illegal sabotage of Alberta Cancer Therapy Programs and abuse (physical violence, harassment, verbal abuse) of frontline healthcare staff by Alberta NDP and their AHS and CPSA Officials, covered-up by CPSADokumen908 halamanCollege of Physicians and Surgeons of Alberta QB1803-01472 Certified Record of Proceedings - Illegal sabotage of Alberta Cancer Therapy Programs and abuse (physical violence, harassment, verbal abuse) of frontline healthcare staff by Alberta NDP and their AHS and CPSA Officials, covered-up by CPSAWilliam MakisBelum ada peringkat
- Respiration 3... Pulmonary Function TestsDokumen26 halamanRespiration 3... Pulmonary Function Testsapi-19641337Belum ada peringkat
- Prevention Strategies For Periodontal Disease - Chapter 16Dokumen10 halamanPrevention Strategies For Periodontal Disease - Chapter 16Daniah MBelum ada peringkat
- Table : Number of Population, Hospitals and Beds in All Over JordanDokumen8 halamanTable : Number of Population, Hospitals and Beds in All Over JordanjBelum ada peringkat
- عقد خدمDokumen2 halamanعقد خدمtasheelonlineBelum ada peringkat
- Behavorial Methods IIDokumen18 halamanBehavorial Methods IImehak727Belum ada peringkat
- Undulating Periodization For BodybuildingDokumen24 halamanUndulating Periodization For BodybuildingPete Puza89% (9)
- Use of Essential Oils Following Traumatic Burn Injury A Case StudyDokumen12 halamanUse of Essential Oils Following Traumatic Burn Injury A Case StudyShandaPrimaDewiBelum ada peringkat
- AMD UpdatedDokumen258 halamanAMD UpdatedmcolossodBelum ada peringkat
- GENETIC DISORDERS AND CYTOGENETICSDokumen7 halamanGENETIC DISORDERS AND CYTOGENETICSsmilechance8Belum ada peringkat