T.ni Insect Cell Line
Diunggah oleh
AlleleBiotechHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
T.ni Insect Cell Line
Diunggah oleh
AlleleBiotechHak Cipta:
Format Tersedia
T.
ni Insect Cells,
Expression significantly higher levels of secreted recombinant proteins compared to other insect cells.
he T.ni Insect Cell Line, derived from ovarian cells of cabbage looper, Trichoplusia ni, have been shown to be capable of expressing significantly higher levels of secreted recombinant proteins compared to other insect cells. In addition, it also offers faster doubling time when compared to Sf9 or Sf21. Alleles T.ni Insect Cells have been adapted for growth in serum free medium.
5. After 48 hours, count cells, determine their viability and subculture them. 6. Split the cultures 1:3 when cells become 90% confluent. 7. Displace cells from the flasks surface by rapping the flask sharply against your hand 3 or 4 times (>75% of the cells should be detached from the surface of the flask). 8. Transfer the cell suspension into a microcentrifuge tube. Determine viability using the trypan blue exclusion method and determine cell density electronically using a Coulter Counter or manually using a hemocytometer chamber. 9. Seed appropriate amount of cells in appropriate vessels (4 - 7 x 105 cells to a T75 tissue culture flask or 6-9 x 105 cells to a T150 tissue culture flask). For suspension culture, start culture in a tissue culture flask for several passages, then transferring the cells to a suspension flask of suitable size at a minimum density of 1 x 105 cells/ml. Suspension culture should be diluted to 5 x 104 cells/ml when cells reach a density of 2 x 105 cells/ml. If suspension cultures are grown in shaker flasks, addition of 0.1% plutonic F68 is necessary to prevent cell shear.
Box 1 | Basic Info Cat. No. ABP-CEL-10008
Contents >1 x 10 viable T.ni cells in 1 ml Serum-Free Insect Medium containing 7.5% DMSO. (Frozen)
6
Storage
Store at -80C for up to one month. For longterm stability, store in liquid nitrogen.
Cat. No. ABP-CEL-10005
Contents >1 x 106 T.ni cells in 70 ml of Serum-Free Insect Culture Medium. (Culture)
Storage
Cells are shipped at room temperature. Process cells immediately upon receipt.
All procedures should be carried out under strict aseptic conditions in a sterile hood. Cells are propagated in TNM-FH Medium (Cat. No. ABP-MED-10001) 1. Transfer a small aliquot of the cell suspension to a microcentrifuge tube.
ABP-CEL-10005 Cell Culture
Protocols
All procedures should be carried out under strict aseptic conditions in a sterile hood. 1. Thaw T.ni cells by placing cryovial in a 27C waterbath with vigorous agitation (do not immerse cap). 2. Spray cryovial with 70% ethanol, wipe it dry. Transfer contents to 15 ml sterile tube containing 10 ml of insect medium (Serum-Free Insect Medium, Cat.# ABPMED-10002 for T.ni cells). 3. Spin down the cells at 1,200 rpm for 3 minutes. Discard the supernatant and re-suspend the cell pellet in 10 ml of fresh insect cell medium. Repeat this cell washing once again to remove DMSO completely. 4. Discard the supernatant and re-suspend the cell pellet in 20 ml of fresh insect cell medium. Transfer cell suspension to sterile 10 cm tissue culture dish or T75 tissue culture flask. Incubate at 27C. No CO2 required.
ABP-CEL-10008 Frozen Cells
2. Determine viability using the trypan blue exclusion method. 3. Determine cell density electronically using a Coulter Counter or manually using a hemocytometer chamber. 4. Transfer the 4 - 7 x 105 cells to a T75 tissue culture flask or 6-9 x 105 cells to a T150 tissue culture flask and allow the cells to attach for 30 minutes at room temperature. 5. Replace the medium with fresh TNM-FH medium: 15 ml for a T75 flask or 30 ml for a T150 flask. 6. Propagate at 27C. Cells should begin dividing within 2 days. 7. Split the cultures 1:3 when cells become 90% confluent. 8. Displace cells from the flasks surface by rapping the flask sharply against your hand 3 or 4 times (>75% of the cells should be detached from the surface of the flask).
Continued on Next Page
Allele Biotech-Introducing Cost Effectiveness to Research
8. Displace cells from the flasks surface by rapping the flask sharply against your hand 3 or 4 times (>75% of the cells should be detached from the surface of the flask). 9. Transfer the cell suspension into a microcentrifuge tube and determine cell count and viability (repeat Steps 1-3). 10. Seed appropriate amount of cells in appropriate vessels (4 7 x 105 cells to a T75 tissue culture flask or 6-9 x 105 cells to a T150 tissue culture flask). For suspension culture, start culture in a tissue culture flask for several passages, then transferring the cells to a suspension flask of suitable size at a minimum density of 1 x 105 cells/ ml. Suspension culture should be diluted to 5 x 104 cells/ml when cells reach a density of 2 x 105 cells/ml. If suspension cultures are grown in shaker flasks, addition of 0.1% plutonic F68 is necessary to prevent cell shear.
or Research Use Only. Not for Diagnostic or Therapeutic Use. Purchase does not include or carry any right to resell or transfer this product either as a stand-alone product or as a component of another product. Any use of this product other than the permitted use without the express written authorization of Allele Biotech is strictly prohibited
References
1. Smith, G.E. et al. Proc. Nat. Acad. Sci., USA (1985) 82: 8404-8; 2. Vaughn, J.L. et al. (1977) In Vitro 13: 213-217.
Website: www.allelebiotech.com Call: 1-800-991-RNAi/858-587-6645 (Pacific Time: 9:00AM~5:00PM) Email: oligo@allelebiotech.com For Technical Support: Email:oligo@allelebiotech.com
Welcome to Join in the Discussion at Alleles Network: Allele News: news.allelebiotech.com Allele Blog: blogs.allelebiotech.com Allele Facebook: http://www.facebook.com/ Allele Twitter: http://www.twitter.com/allele_biotech Allele Myspace: http://www.myspace.com/allelebiotech
Related Products
Sapphire Baculovirus DNA High level expression. The p10 promoter is partially deactivated and the lytic p10 gene is deleted so that transcription levels are higher due to reduced interference and healthier insect cells. TNM-FH Insect Culture Medium Fully supplemented Grace's medium including trace metals, lactalbumin hydrolysate, yeastolate and 10% heat inactivated fetal bovine serum. Sapphire Insect Transfection Kit Ideal for easy and efficient transfection of DNAs into insect cells.
Serum Free Insect Culture Medium Specifically developed for the Baculovirus Expression Vector System (BEVS) technology. It supports excellent growth and propagation of Sf9,Sf21, T.ni and High Five.
Allele Biotech-Introducing Cost Effectiveness to Research
Anda mungkin juga menyukai
- M13KO7 Helper Phage for phage displayDokumen1 halamanM13KO7 Helper Phage for phage displayAlleleBiotechBelum ada peringkat
- Neural Induction FactorsDokumen2 halamanNeural Induction FactorsAlleleBiotechBelum ada peringkat
- Cre Recombinase IVT PCR TemplateDokumen1 halamanCre Recombinase IVT PCR TemplateAlleleBiotechBelum ada peringkat
- Modified UTPDokumen1 halamanModified UTPAlleleBiotechBelum ada peringkat
- Omni-Tube PCRDokumen3 halamanOmni-Tube PCRAlleleBiotechBelum ada peringkat
- High Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionDokumen1 halamanHigh Quality, Standard rATP Suitable For Large Scale IVT.: DescriptionAlleleBiotechBelum ada peringkat
- PNCS dLanYFPDokumen1 halamanPNCS dLanYFPAlleleBiotechBelum ada peringkat
- Luciferase PCR Template For IVTDokumen1 halamanLuciferase PCR Template For IVTAlleleBiotechBelum ada peringkat
- ARCADokumen1 halamanARCAAlleleBiotechBelum ada peringkat
- Modified CTPDokumen1 halamanModified CTPAlleleBiotechBelum ada peringkat
- Anti-RFP (3F5) Monoclonal AntibodyDokumen1 halamanAnti-RFP (3F5) Monoclonal AntibodyAlleleBiotechBelum ada peringkat
- Custom Sub Cloning ServiceDokumen1 halamanCustom Sub Cloning ServiceAlleleBiotechBelum ada peringkat
- High Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionDokumen1 halamanHigh Quality, Standard RGTP Suitable For Large Scale IVT.: DescriptionAlleleBiotechBelum ada peringkat
- Anti-RFP (5F8) Monoclonal AntibodyDokumen1 halamanAnti-RFP (5F8) Monoclonal AntibodyAlleleBiotechBelum ada peringkat
- RNA Purification SystemDokumen3 halamanRNA Purification SystemAlleleBiotechBelum ada peringkat
- RNA Purification SystemDokumen3 halamanRNA Purification SystemAlleleBiotechBelum ada peringkat
- T7 RNA PolymeraseDokumen1 halamanT7 RNA PolymeraseAlleleBiotechBelum ada peringkat
- Sapphire Insect Transfection ReagentDokumen1 halamanSapphire Insect Transfection ReagentAlleleBiotechBelum ada peringkat
- Surface Bind RNA Purification SystemDokumen3 halamanSurface Bind RNA Purification SystemAlleleBiotechBelum ada peringkat
- ChromoTek GFP-multiTrapDokumen3 halamanChromoTek GFP-multiTrapAlleleBiotechBelum ada peringkat
- iPS Induction IVT TemplatesDokumen2 halamaniPS Induction IVT TemplatesAlleleBiotechBelum ada peringkat
- Mwasabi PCR Template For IVTDokumen1 halamanMwasabi PCR Template For IVTAlleleBiotechBelum ada peringkat
- Allele AgaroseDokumen1 halamanAllele AgaroseAlleleBiotechBelum ada peringkat
- Luciferase PCR Template For IVTDokumen1 halamanLuciferase PCR Template For IVTAlleleBiotechBelum ada peringkat
- AvantGene Transfection KitDokumen2 halamanAvantGene Transfection KitAlleleBiotechBelum ada peringkat
- ChromoTek RFP BoosterDokumen1 halamanChromoTek RFP BoosterAlleleBiotechBelum ada peringkat
- pmTFP1 ClathrinDokumen4 halamanpmTFP1 ClathrinAlleleBiotechBelum ada peringkat
- mClavGR2 Fusion VectorsDokumen3 halamanmClavGR2 Fusion VectorsAlleleBiotechBelum ada peringkat
- High Titer Luc Lentiviral ParticlesDokumen1 halamanHigh Titer Luc Lentiviral ParticlesAlleleBiotechBelum ada peringkat
- Rex1-Oct3/4-Nanog iPS Methylation Detection PrimersDokumen1 halamanRex1-Oct3/4-Nanog iPS Methylation Detection PrimersAlleleBiotechBelum ada peringkat
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5782)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (587)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (72)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (265)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (119)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Pilocarpine Eye DropDokumen3 halamanPilocarpine Eye DropSidahmed SiDo BouchenakBelum ada peringkat
- Every Child Matters PDF 2003Dokumen2 halamanEvery Child Matters PDF 2003AshleyBelum ada peringkat
- 1235-Article Text-3235-1-10-20201111Dokumen13 halaman1235-Article Text-3235-1-10-20201111ikhwan dwikesumaBelum ada peringkat
- Know Your Council PDFDokumen28 halamanKnow Your Council PDFAnonymous LH6vOd9IqBelum ada peringkat
- Chison User Manual of Q9-V2.0-20140814Dokumen232 halamanChison User Manual of Q9-V2.0-20140814WaleedBelum ada peringkat
- IM-Generalized Peritonitis Concept MapDokumen21 halamanIM-Generalized Peritonitis Concept MapTrisBelum ada peringkat
- Taryn ZadunayskiDokumen2 halamanTaryn Zadunayskiapi-278580762Belum ada peringkat
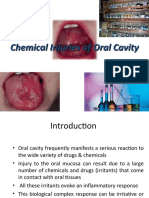
- Chemical Injuries of Oral CavityDokumen58 halamanChemical Injuries of Oral CavityShalini SoniBelum ada peringkat
- KDIGO Diabetes 2022 Guideline Slide SetDokumen68 halamanKDIGO Diabetes 2022 Guideline Slide SetBigPharma HealtcareBelum ada peringkat
- Eagle2019 Article IncreasedRiskOfMusculoskeletalDokumen9 halamanEagle2019 Article IncreasedRiskOfMusculoskeletaljosé_farinha_4Belum ada peringkat
- Air PollutionDokumen16 halamanAir Pollutionvgs127350% (2)
- A Summary of Freud's Psychosexual Stages of DevelopmentDokumen3 halamanA Summary of Freud's Psychosexual Stages of Developmentscaremoon50% (2)
- Experience Calm and Balance with the Four Directions Tai-Chi FormDokumen21 halamanExperience Calm and Balance with the Four Directions Tai-Chi Formmikelee555100% (3)
- Pharma CompaniesDokumen62 halamanPharma CompaniesRajneesh SehgalBelum ada peringkat
- Endocrine System Diagnostic Text and Some DiseasesDokumen3 halamanEndocrine System Diagnostic Text and Some Diseasesevangelo22656Belum ada peringkat
- P504 Work PackDokumen16 halamanP504 Work PackFernando SantosBelum ada peringkat
- Pakistan Orthopaedic Assocition: Memorandum and ArticlesDokumen14 halamanPakistan Orthopaedic Assocition: Memorandum and ArticlesSobia TahirBelum ada peringkat
- Chapter 2 Social Demography PPT (Autosaved)Dokumen35 halamanChapter 2 Social Demography PPT (Autosaved)Mannat BindraBelum ada peringkat
- Claim ReceiptDokumen3 halamanClaim ReceiptsheerazaliBelum ada peringkat
- European Guidelines On DRLs For Paediatric Imaging PDFDokumen105 halamanEuropean Guidelines On DRLs For Paediatric Imaging PDFPapadoveBelum ada peringkat
- Name: - Grade and SectionDokumen8 halamanName: - Grade and SectionRodel AgustinBelum ada peringkat
- Rekap Resep - 2021-03-01T160848.881Dokumen2.906 halamanRekap Resep - 2021-03-01T160848.881Dhita AnaleptaBelum ada peringkat
- Abnormal Endoscopic Pharyngeal and LaryngealDokumen7 halamanAbnormal Endoscopic Pharyngeal and LaryngealLilia ScutelnicBelum ada peringkat
- Antibacterial Activities of Essential Oils from Zingiber officinale var. rubrumDokumen4 halamanAntibacterial Activities of Essential Oils from Zingiber officinale var. rubrumAkil LadzinrankBelum ada peringkat
- Form 3 Meitheal Planning ReviewDokumen3 halamanForm 3 Meitheal Planning ReviewtqsnrBelum ada peringkat
- Second Edition, 2017: Untold Stories of CourageDokumen32 halamanSecond Edition, 2017: Untold Stories of CouragenandumallidiBelum ada peringkat
- Skills Assessment Checklist: MODULE 6: Massive Hemorrhage Control in TFCDokumen7 halamanSkills Assessment Checklist: MODULE 6: Massive Hemorrhage Control in TFCSae TumBelum ada peringkat
- I. Ethico-Moral NursingDokumen20 halamanI. Ethico-Moral NursingriffyjeanBelum ada peringkat
- Force-Feeding in Mauritania, Shanyah McCluneyDokumen8 halamanForce-Feeding in Mauritania, Shanyah McCluneyShanyah McCluneyBelum ada peringkat
- Engaging Veteran Students: Presentation by Corrie Hawes, Michelle Staggs & Judith Vasquez-PerezDokumen26 halamanEngaging Veteran Students: Presentation by Corrie Hawes, Michelle Staggs & Judith Vasquez-Perezapi-441528268Belum ada peringkat