Optimizing Treatment Approaches in Seborrheic Dermatitis: (Literature Review)
Diunggah oleh
Andika Anjani AgustinJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Optimizing Treatment Approaches in Seborrheic Dermatitis: (Literature Review)
Diunggah oleh
Andika Anjani AgustinHak Cipta:
Format Tersedia
Goldenberg_Seb_Derm copy_Layout 1 2/13/13 2:45 PM Page 44
[LITERATURE REVIEW]
Optimizing Treatment Approaches
in Seborrheic Dermatitis
GARY GOLDENBERG, MD
Mount Sinai School of Medicine, Department of Dermatology, New York, New York
ABSTRACT
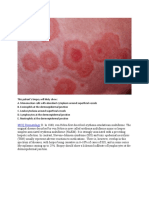
Seborrheic dermatitis is a chronic, recurring, cutaneous condition that causes erythema and flaking, sometimes
appearing as macules or plaques with dry white or moist oily scales. In adults, it commonly occurs in areas with high
concentrations of sebaceous glands. The face and scalp are the most frequently affected areas, and involvement of multiple
sites is common. Dandruff is regarded as a mild noninflammatory form of seborrheic dermatitis. There is a high incidence
of seborrheic dermatitis among persons with human immunodeficiency virus infection or Parkinson’s disease. The cause
of seborrheic dermatitis is not well understood, but appears to be related to the composition of the sebaceous gland
secretions, the proliferation of Malessezia yeasts, and the host immune response. Treatment options for nonscalp and
scalp seborrheic dermatitis include topical agents and shampoos containing antifungal agents, anti-inflammatory agents,
keratolytic agents, and calcineurin inhibitors. Because multiple body sites are usually involved, the physician should
examine all commonly affected areas. Patients should be made aware that seborrheic dermatitis is a chronic condition that
will probably recur even after successful treatment. (J Clin Aesthet Dermatol. 2013;6(2):44–49.)
S
eborrheic dermatitis (SD), a chronic, recurrent, than half of patients with facial SD, the scalp is affected as
inflammatory condition characterized by erythema well.3 On the face, SD commonly occurs in the nasolabial
and skin flaking, may be resistant to treatment and folds, eyebrows, anterior hairline, and glabella.1,6 On the
often has a substantial negative impact on quality of life.1–3 scalp, the lesions may range from mild desquamation to
It affects approximately six million people in the United brownish crusts affixed to the skin and hair.5 Lesions on
States and is associated with direct and indirect medical the central chest may have a petaloid appearance.7 Some
costs of approximately $230 million per year.4 patients report pruritus, particularly if the scalp is
Although the causes of SD are not completely affected.2,5,6 It generally is not accompanied by papules or
understood, progress has been made in this area, and pustules.2 Secondary bacterial infection may occur,
several effective treatment options are available. This aggravating erythema and exudate and causing local
article will review the clinical presentation of SD and the discomfort.5
current understanding of its etiology and discuss currently In adults, SD is a chronic, recurrent condition marked
available treatment options. by periods of exacerbation occurring at variable intervals.6
Patients may report that outbreaks are triggered by
CLINICAL PRESENTATION emotional stress, depression, fatigue, exposure to air
Seborrheic dermatitis may appear as macules or thin conditioning or damp or dry conditions in the workplace,
plaques with a reddish or yellow appearance and dry white systemic infections, use of certain medications, or other
or moist oily scales.5 In adults, it most often occurs in areas factors.3
with a high concentration of sebaceous glands, including The infantile form of SD is a self-limited condition
the face, scalp, ears, chest, and body folds.5 It usually generally resolving by age three or four months.6 The adult
affects multiple body areas, occurring on the face in 88 form usually appears first around the time of puberty,
percent of patients, the scalp in 70 percent, the chest in 27 when sebaceous glands become more active, sometimes
percent, and the arms or legs in 1 to 2 percent.3 In more lasting until young adulthood.1 The condition increases
DISCLOSURE: Dr. Goldenberg reports no relevant conflicts of interest. Manuscript development was supported by Promius Pharma, LLC.
ADDRESS CORRESPONDENCE TO: Gary Goldenberg, MD, 5 East 98th St, Box 1048, New York, NY 10029; E-mail: Garygoldenbergmd@gmail.com
44 [February 2013 • Volume 6 • Number 2] 44
Goldenberg_Seb_Derm copy_Layout 1 2/13/13 2:45 PM Page 45
again in prevalence after age 50.1 It affects approximately 1 The role of the host immune response in the
to 5 percent of immunocompetent adults and as many as pathogenesis of SD is uncertain. Some researchers have
20 to 83 percent of human immunodeficiency virus (HIV)- reported increased numbers of natural killer cells, CD16
positive individuals.5,6 Other populations at risk include cells, and inflammatory interleukins and activation of
persons with Parkinson’s disease or other neurological complement in the lesional skin of patients with SD
disorders, mood disorders, significant life stress, or low compared with their own nonlesional skin or the skin of
exposure to sunlight.2 More men than women have SD, but healthy controls.6 Nevertheless, total antibody levels are no
it shows no preference for any racial or ethnic group.6 It higher in SD patients than in controls and a host response
may occur in association with atopic dermatitis or other specific to Malassezia yeasts has not been identified.9 The
skin disorders, complicating its diagnosis.8 prevalence of SD in persons infected with HIV suggests
Some controversy has surrounded the relationship that the condition is mediated by the immune system;
between SD and dandruff. Most authors now agree that however, the response of SD to successful retroviral
dandruff is a mild, noninflammatory form of SD.2,6,9 therapy is variable.5
Dandruff is extremely common, with a prevalence as high Thus, a definitive understanding of the pathophysiology
as 50 percent of the population.2 of SD awaits further research, but the role of Malassezia
yeasts as causative or contributing agents appears to be
CAUSES OF SEBORRHEIC DERMATITIS well established.
Although the causes of SD are not completely
understood, it appears to result from a combination of the DIAGNOSIS
following three factors: sebaceous gland secretion, The differential diagnosis of SD should include psoriasis,
presence of Malassezia yeast, and the host immune rosacea, Demodex dermatitis, atopic eczema, pityriasis
response.6 versicolor, contact dermatitis, and tinea infections.2 SD
Sebum is an important component of skin surface lipids may also resemble Langerhans cell histiocytosis or
and contains high amounts of squalene, wax esters, and secondary syphilis.2,5 The diagnosis is usually clinical, but
triglycerides.10 Persons with SD do not necessarily have candidiasis, tinea infection, and Demodex dermatitis may
excess sebaceous gland activity, but the composition of be ruled out with a negative potassium hydroxide test.2 It
their skin surface lipid may be altered, creating a more should be kept in mind that SD may be accompanied by
supportive environment for growth of lipid-dependent other dermatological disorders.
micro-organisms.10 Care should be taken to differentiate SD from psoriasis
The role of Malassezia yeasts in SD is somewhat vulgaris.15 Early SD has a spongiform appearance that
controversial, although most researchers believe they play distinguishes it from psoriasis, but in later stages these
an important role.9 Malassezia yeasts are normally conditions are more difficult to tell apart. Some patients
commensal species found primarily in follicular infundibula present with sebopsoriasis, which includes features of both
and commonly isolated from sebum-rich areas of the body, disease states.2 Lesions on the elbows or knees and nail
such as the face, scalp, trunk, and back.11 They produce pitting suggest psoriasis, which may spare the face.15
abundant lipases that hydrolyze triglycerides and free
saturated fatty acids on which the yeast is dependent.12 TREATMENT
These fatty acids may have irritant effects that induce The primary goals of therapy for SD are to clear the
scaling or may cause release of arachidonic acid, which visible signs of disease and reduce bothersome symptoms,
promotes inflammation in skin.9 There are seven primary especially pruritus.6 Because the face and scalp are the
species: M. globosa, M. restricta, M. obtusa, M. slooffiae, most commonly affected areas, itching or redness on the
M. sympodialis, M. furfur, and M. pachydermatis (the scalp in a patient with facial SD indicates the need for
last occurs only on animals).9 M. globosa and M. restricta treatment at both sites.3 Patients should be informed that
are thought to be the species most commonly associated SD is a chronic, relapsing condition and that they should
with SD, although M. furfur and other species have also anticipate future outbreaks.16 Patients should also be
been implicated.9,13,14 Some studies have found high advised to avoid triggers of SD symptoms to the extent
numbers of Malassezia yeasts on the scalp of persons with possible and not to irritate the lesions by excessive
SD, but others have found no difference in the density of scratching or use of potent keratolytic preparations.16,17
these yeasts between the skin of persons with SD and that
of persons without it.1 Differing sampling methods may NONSCALP SEBORRHEIC DERMATITIS
contribute to these contradictory findings. Malassezia Antifungal agents, anti-inflammatory agents, and
exist not only on the skin surface, but also within the layers keratolytic agents are available in a variety of formulations
of the stratum corneum, and a true count would require for treatment of SD on areas other than the scalp. Table 1
examining the full thickness of the skin squama.1 Support lists commonly used treatments for nonscalp SD and
for the role of Malassezia in SD comes from studies indicates the level of evidence that supports their use.
demonstrating that use of various antifungal treatments Antifungal agents. With the understanding of the role
results in reduction of Malassezia, which is accompanied of Malassezia in SD, antifungal agents have taken on an
by improvement in symptoms.6,9 important role in its treatment. Ketoconazole 2% cream
[ February 2013 • Volume 6 • Number 2] 45
Goldenberg_Seb_Derm copy_Layout 1 2/13/13 2:45 PM Page 46
cream in treatment of facial SD, with a similar side effect
TABLE 1. Treatments for nonscalp seborrheic dermatitis profile.23
For patients with persistent SD resistant to topical
LEVEL OF EVIDENCE* agents, oral antifungals may be an option. Oral itraconazole
given in a dose of 200mg/day for one week, followed by a
ANTIFUNGAL AGENTS maintenance dose, resulted in clinical improvement of SD
symptoms in two open-label trials.24,25
Ketoconazole A
Corticosteroids. Hydrocortisone and a wide variety of
Ciclopiroxolamine A
other low- to mid-potency corticosteroids have been used
successfully in the treatment of SD. A double-blind study
Sertaconazole C that compared hydrocortisone 1% cream with ketoconazole
2% cream in 72 patients with mild-to-moderate SD found
Metronidazole A that the two agents produced similar rates of response and
similar reductions in scaling, redness, itching, and papules.26
Itraconazole (oral) C In a 12-week, single-blind, randomized, comparative trial,
Lithium succinate/lithium gluconate A hydrocortisone 1% ointment was found to be equally as
effective as tacrolimus 0.1% ointment in reducing the
CORTICOSTEROIDS symptoms of facial SD by physician assessment, although
tacrolimus was superior by patient assessment.27
Hydrocortisone A Combination antifungal/anti-inflammatory. Promiseb®
Topical Cream (Promius Pharma, LLC, Bridgewater, New
ANTI-INFLAMMATORY/ANTIFUNGAL COMBINATION
Jersey) is a nonsteroidal prescription medical device with
Promiseb® Topical Cream B anti-inflammatory and antifungal activity approved for
treatment of SD.28 In an investigator-blind, parallel-group
CALCINEURIN INHIBITORS study, 77 patients with mild or moderate SD of the face
were randomized to combination antifungal/anti-
Tacrolimus B
inflammatory cream or desonide 0.05% cream twice daily
Pimecrolimus B for up to 28 days.29 Severity of symptoms declined
significantly from baseline to Day 14 and Day 28 in both
*Levels of evidence: A=randomized, double-blind, controlled trial(s); groups.29 Treatment was successful (clear or almost clear)
B=randomized single-blind trial(s); C=open-label studies in 85 percent of patients using combination antifungal/anti-
inflammatory cream and 92 percent of patients using
applied twice daily for four weeks has been shown to be as desonide cream (P=not significant) and the two products
effective as hydrocortisone 1% cream in treatment of SD at had similar safety profiles.29
multiple body sites.18 In a randomized, double-blind trial of Calcineurin inhibitors. Topical calcineurin inhibitors
459 patients with SD treated with ketoconazole 2% gel or have immunomodulatory and anti-inflammatory properties
vehicle once daily for 14 days, there was a significantly that make them useful in the treatment of SD.27
higher rate of successful treatment (25.3% vs. 13.9%, Tacrolimus 0.1% ointment was found to be as effective
P=0.0014) and significantly greater reductions in as hydrocortisone 1% ointment in the treatment of SD,
erythema, pruritus, and scaling in ketoconazole-treated required fewer applications during the 12-week study
patients.19 A 2% foam formulation of ketoconazole has been period because of clearing of symptoms, and was rated
shown to be significantly more effective than vehicle for more favorably by patients.27
treatment of SD on the face, scalp, and body, and equally In a randomized, open-label trial, pimecrolimus 1% cream
as effective as ketoconazole 2% cream.20 was compared with betamethasone 0.1% cream in 20
Ciclopiroxolamine 1% cream, twice daily for 28 days patients with SD who were instructed to discontinue
followed by once daily for 28 days, was compared with treatment when symptoms cleared.30 By Day 9, all patients
vehicle for the treatment of SD in a randomized, double- had discontinued treatment.30 The two drugs were equally
blind trial that enrolled 129 patients.21 At the end of the effective at reducing symptoms of erythema, scaling, and
maintenance phase, complete disappearance of erythema pruritus, but symptom relief was sustained longer in the
and scaling was found in 63 percent of the pimecrolimus group.30 In comparative trials, pimecrolimus
ciclopiroxolamine-treated group and 34 percent of the 1% cream has been shown to be as effective as
vehicle-treated group (P<0.007).21 hydrocortisone 1% cream and ketoconazole 2% cream in the
In an open-label study of sertaconazole nitrate 2% treatment of SD, with higher rates of adverse effects.31,32
cream, 59 percent of 20 subjects with mild-to-severe SD Pimecrolimus 1% cream was found to be significantly more
were successfully treated, with improvements in scaling, effective for treatment of facial SD than methylprednisolone
erythema, induration, and pruritus.22 0.1% cream or metronidazole 0.75% gel when applied twice
A randomized, double-blind study demonstrated that daily for eight weeks, with fewer adverse effects and a lower
metronidazole 0.75% gel is as effective as ketoconazole 2% rate of recurrence than metronidazole.33
46 [February 2013 • Volume 6 • Number 2] 46
Goldenberg_Seb_Derm copy_Layout 1 2/13/13 2:45 PM Page 47
SCALP SEBORRHEIC DERMATITIS
Seborrheic dermatitis of the scalp is most conveniently TABLE 2. Treatments for seborrheic dermatitis of the scalp
treated with shampoos containing antifungal agents,
corticosteroids, or keratolytic agents; products are also LEVEL OF EVIDENCE*
available that combine drugs from these different classes.
Table 2 lists commonly used treatments for SD of the scalp ANTIFUNGAL AGENTS
and indicates the level of evidence that supports their use.
Ketoconazole shampoo A
Antifungal shampoos. Ketoconazole 2% shampoo was
compared with selenium sulfide 2.5% shampoo in a four-
Ciclopiroxolamine shampoo A
week, randomized, double-blind trial of patients with
moderate-to-severe dandruff.34 Twice-weekly use of either CORTICOSTEROIDS
shampoo was superior to placebo, but not significantly
different from each other.34 There was a significantly higher Clobetasol propionate B
incidence of adverse effects among patients using selenium
sulfide shampoo.34 COMBINATION PRODUCTS
Ciclopirox 1% shampoo used once or twice weekly for Promiseb® Plus Scalp Wash C
four weeks was shown to be superior to vehicle for
treatment of SD in a randomized, double-blind, controlled Ciclopiroxolamine/salicyclic acid B
study that recruited 949 patients.35 Subsequent
prophylactic use of ciclopirox shampoo once weekly or Ciclopiroxolamine/zinc pyrithione B
once every two weeks reduced the relapse rate.35
KERATOLYTIC PRODUCTS
Ciclopirox shampoo and ketoconazole shampoo were
compared in a double-blind study of 350 patients with SD.36 Lipohydroxy acid A
The two treatments were equally effective and both better
than placebo, although patients rated the ciclopirox Propylene glycol A
shampoo more favorably.36
*Levels of evidence: A=randomized, double-blind, controlled trial(s);
Corticosteroid shampoos. In a randomized, single-
B=randomized single-blind trial(s); C=open-label studies
blind study of 326 subjects with moderate-to-severe scalp
SD, clobetasol propionate 0.05% shampoo twice weekly for
four weeks produced a significantly greater reduction in subjects with scalp SD.41 After four weeks of treatment, the
symptoms than ketoconazole 2% shampoo.37 Alternating tolerance, global efficacy, and cosmetic effects of the
use of clobetasol shampoo and ketoconazole shampoo was lipohydroxy acid shampoo were significantly superior to
also superior to ketoconazole shampoo alone.37 those of the ciclopiroxolamine shampoo.41
Combination products. Promiseb® Plus Scalp Wash A topical solution of urea, propylene glycol, and lactic
(Promius Pharma, LLC) contains surfactants and skin acid, applied daily for four weeks then three times per
conditioning agents, which remove excess sebum as well as week for four weeks, was compared with placebo for
lactoferrin and piroctone olamine, which may reduce the treatment of mild-to-severe SD of the scalp.42 Erythema
proliferation of Malassezia.38 In an open-label trial, 25 and desquamation were improved at Weeks 2 and 4, but
subjects with SD used this proprietary wash an average of the improvements were not maintained at eight weeks.42
twice weekly for two weeks.38 All 25 had a positive response
and more than 90 percent reported improvement in CONCLUSION
seborrhea, dandruff, pruritus, and redness.38 Seborrheic dermatitis is a common, chronic, inflammatory
In a single-blind study, a shampoo containing cutaneous condition characterized by erythema and skin
ciclopiroxolamine 1.5% and salicylic acid 3% was shown to flaking that tends to recur even after successful treatment
have efficacy similar to that of ketoconazole 2% shampoo and has a significant negative impact on quality of life. Its
for the treatment of dandruff/SD.39 For both groups, occurrence appears to be related to the proliferation of
improvement was sustained for 14 days after treatment commensal Malassezia species. Occurrence at multiple body
ended.39 sites is common; the face and scalp are the most frequently
A shampoo containing ciclopiroxolamine 1.5% and zinc affected areas. Numerous antifungal, anti-inflammatory,
pyrithione 1% was found to be as effective as ketoconazole keratolytic, and immunomodulatory agents have been shown
2% foaming gel in a single-blind study of 189 patients with to be effective in the treatment of SD, but patients should be
scalp SD, with a greater reduction in pruritus during the informed that recurrence is common and that ongoing
early treatment phase and more favorable ratings from treatment may be necessary.
patients.40
Keratolytic products. A randomized, double-blind REFERENCES
study compared a shampoo containing lipohydroxy acid 1. Bikowski J. Facial seborrheic dermatitis: a report on current
0.1% and salicylic acid 1.3% with a shampoo containing status and therapeutic horizons. J Drugs Dermatol.
ciclopiroxolamine 1.5% and salicylic acid 3% in 100 2009;8(2):125–133.
[ February 2013 • Volume 6 • Number 2] 47
Goldenberg_Seb_Derm copy_Layout 1 2/13/13 2:45 PM Page 48
2. Gupta AK, Bluhm R, Cooper EA, et al. Seborrheic dermatitis. ciclopiroxolamine 1% cream in facial seborrhoeic dermatitis. Br
Dermatol Clin. 2003;21:401–412. J Dermatol. 2001;144:1033–1037.
3. Peyri J, Lleonart M, and the Spanish Group of the SEBDERM 22. Elewski BE, Cantrell WC. An open-label study of the safety and
Study. Clinical and therapeutic profile and quality of life of efficacy of sertaconazole nitrate in the treatment of seborrheic
patients with seborrheic dermatitis. Actas Dermosifiliogr. dermatitis. J Drugs Dermatol. 2011;10(8):895–899.
2007;98:476–482. 23. Seckin D, Gurbuz O, Akin O. Metronidazole 0.75% gel vs.
4. Bickers DR, Lim HW, Margolis D, et al. The burden of skin ketoconazole 2% cream in the treatment of facial seborrheic
diseases: 2004. J Am Acad Dermatol. 2006;55:490–500. dermatitis: a randomized, double-blind study. J Eur Acad
5. Sampaio AL, Mameri AC, Vargas TJ, et al. Seborrheic dermatitis. Dermatol Venereol. 2007;21:345–350.
An Bras Dermatol. 2011;86:1061–1074. 24. Shemer A, Kaplan B, Nathansohn N, et al. Treatment of moderate
6. Del Rosso JQ. Adult seborrheic dermatitis. A status report on to severe facial seborrheic dermatitis with itraconazole: an open
practical topical management. J Clin Aesthet Dermatol. non-comparative study. Isr Med Assoc J. 2008;10:417–418.
2011;4(5):32–38. 25. Kose O, Erbil H, Gur AR. Oral itraconazole for the treatment of
7. Reider N, Fritsch PO. Seborrheic dermatitis. In: Bolognia JL, seborrhoeic dermatitis: an open, noncomparative trial. J Eur
Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia: Acad Dermatol Venereol. 2005;19:172–175.
Penn: Elsevier; 2012. 26. Stratigos JD, Antoniou C, Katsambas A, et al. Ketoconazole 2%
8. Del Rosso JQ, Kim GK. Seborrheic dermatitis and Malassezia cream versus hydrocortisone 1% cream in the treatment of
species: how are they related? J Clin Aesthet Dermatol. seborrheic dermatitis. A double-blind comparative study. J Am
2009:2(11):14–17. Acad Dermatol. 1988;19:850–853.
9. Gupta AK, Batra R, Bluhm R, et al. Skin diseases associated with 27. Papp KA, Papp A, Dahmer B, Clark CS. Single-blind, randomized
Malassezia species. J Am Acad Dermatol. 2004;51:785–798. controlled trial evaluating the treatment of facial seborrheic
10. De Luca C, Valacchi G. Surface lipids as multifunctional dermatitis with hydrocortisone 1% ointment compared with
mediators of skin responses to environmental stimuli. Mediators tacrolimus 0.1% ointment in adults. J Am Acad Dermatol.
Inflamm. 2010;2010:321494. 2012;67:e11–e15.
11. Hay RJ. Malassezia, dandruff and seborrhoeic dermatitis: an 28. Kircik L. An open-label, single-center pilot study to determine
overview. Br J Dermatol. 2011;165(Suppl 2):2–8. the antifungal activity of a new nonsteroidal cream (Promiseb
12. Dawson TL Jr. Malassezia globosa and restrica: breakthrough Topical Cream) after 7 days of use in healthy volunteers. Clin
understanding of the etiology and treatment of dandruff and Dermatol. 2009;27(Suppl):S44–S47.
seborrheic dermatitis through whole-genome analysis. J Invest 29. Elewski B. An investigator-blind, randomized, 4-week, parallel-
Dermatol Symp Proc. 2007;12:15–19. group, multicenter pilot study to compare the safety and efficacy
13. Lee YW, Byun HJ, Kim BJ, et al. Distribution of Malassezia of a nonsteroidal cream (Promiseb Topical Cream) and desonide
species on the scalp in Korean seborrheic dermatitis patients. cream 0.05% in the twice-daily treatment of mild to moderate
Ann Dermatol. 2011;23(2):156–161. seborrheic dermatitis of the face. Clin Dermatol.
14. Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia 2009;27(Suppl):S48–S53.
species isolated from patients with seborrheic dermatitis, atopic 30. Rigopoulos D, Ioannides D, Kalogeromitros D, et al. Pimecrolimus
dermatitis, pityriasis versicolor and normal subjects. Med Mycol. cream 1% vs. betamethasone 17-valerate 0.1% cream in the
2000;38:337–341. treatment of seborrhoeic dermatitis. A randomized open-label
15. Habif TP. Psoriasis and other papulosquamous diseases. In: Habif clinical trial. Br J Dermatol. 2004;151:1071–1075.
TP. Clinical Dermatology: A Color Guide to Diagnosis and 31. Firooz A, Solhpour A, Gorouhi F, et al. Pimecrolimus cream, 1%,
Therapy. 5th ed. Philadelphia: Penn: Elsevier; 2010. vs. hydrocortisone acetate cream, 1%, in the treatment of facial
16. Faergemann J. Management of seborrheic dermatitis and seborrheic dermatitis: a randomized, investigator-blind, clinical
pityriasis versicolor. Am J Clin Dermatol. 2000;1(2):75–80. trial. Arch Dermatol. 2006;142(8):1065–1086.
17. Stefanaki I, Katsambasa A. Therapeutic update on seborrheic 32. Koc E, Arca E, Kose O, Akar A. An open, randomized,
dermatitis. Skin Therapy Lett. 2010;15(5):1–4. prospective, comparative study of topical pimecrolimus 1%
18. Katsambas A, Antoniou CH, Frangouli E, et al. A double-blind cream and topical ketoconazole 2% cream in the treatment of
trial of treatment of seborrheic dermatitis with 2% ketoconazole seborrheic dermatitis. J Dermatol Treat. 2009;20(1):4–9.
cream compared with 1% hydrocortisone cream. Br J Dermatol. 33. Cicek D, Kandi B, Bakar S, Turgut D. Pimecrolimus 1% cream,
1989;121:353–357. methylprednisolone aceponate 0.1% cream and metronidazole
19. Elewski B, Ling MR, Phillips TJ. Efficacy and safety of a new 0.75% gel in the treatment of seborrheic dermatitis: a
once-daily topical ketoconazole 2% gel in the treatment of randomized clinical study. J Dermatol Treat. 2009;20:344–349.
seborrheic dermatitis: a phase III trial. J Drugs Dermatol. 34. Danby FW, Maddin WS, Margesson LJ, Rosenthal D. A
2006;5(7):646–650. randomized, double-blind, placebo-controlled trial of
20. Elewski BE, Abramovits W, Kempers S, et al. A novel foam ketoconazole 2% shampoo versus selenium sulfide 2.5%
formulation of ketoconazole 2% for the treatment of seborrheic shampoo in the treatment of moderate to severe dandruff. J Am
dermatitis on multiple body regions. J Drugs Dermatol. Acad Dermatol. 1993;29:1008–1012.
2007;6(10):1001–1008. 35. Shuster S, Meynadier J, Kerl H, Nolting S. Treatment and
21. Dupuy P, Maurette C, Amoric JC, et al. Randomized, placebo- prophylaxis of seborrheic dermatitis of the scalp with
controlled, double-blind study on clinical efficacy of antipityrosporal 1% ciclopirox shampoo. Arch Dermatol.
48 [February 2013 • Volume 6 • Number 2]
Goldenberg_Seb_Derm copy_Layout 1 2/13/13 2:45 PM Page 49
2005;141:47–52. containing ciclopirox olamine (1.5%) and salicylic acid (3%), or
36. Ratnavel RC, Squire RA, Boorman GC. Clinical efficacies of ketoconazole (2%, Nizoral®) for the treatment of dandruff/
shampoos containing ciclopirox olamine (1.5%) and seborrhoeic dermatitis. J Dermatol Treat. 2002;13:51–60.
ketoconazole (2.0%) in the treatment of seborrhoeic dermatitis. 40. Lorette G, Ermosilla V, Study Investigators Group. Clinical
J Dermatol Treat. 2007;18:88–96. efficacy of a new ciclopiroxolamine/zinc pyrithione shampoo in
37. Ortonne J-P, Nikkels AF, Reich K, et al. Efficacious and safe scalp seborrheic dermatitis treatment. Eur J Dermatol.
management of moderate to severe scalp seborrhoeic dermatitis 2006;16(5):558–564.
using clobetasol propionate shampoo 0.05% combined with 41. Seite S, Rougier A, Talarico S. Randomized study comparing the
ketoconazole shampoo 2%: a randomized, controlled study. Br J efficacy and tolerance of a llipohydroxy acid shampoo to a
Dermatol. 2011;165:171–176. ciclopiroxolamine shampoo in the treatment of scalp seborrheic
38. Kircik L. Subject evaluation of the treatment of seborrheic dermatitis. J Cosmet Dermatol. 2009;8:249–253.
dermatitis of the scalp with a non-steroidal scalp wash. Poster 42. Emtestam L, Svensson A, Rensfeldt K. Treatment of seborrheic
presented at: 2012 Winter Clinical Dermatology Conference; dermatitis of the scalp with a topical solution of urea, lactic acid,
January 14–19, 2012; Kaanapali, Hawaii. and propylene glycol (K301): results of two double-blind,
39. Squire RA, Goode K. A randomised, single-blind, single-centre randomised, placebo-controlled studies. Mycoses. 2012;55(5):
clinical trial to evaluate comparative clinical efficacy of shampoos 393–403.
[ February 2013 • Volume 6 • Number 2] 49
Anda mungkin juga menyukai
- Raj Sekhar PfizerDokumen53 halamanRaj Sekhar PfizerRaj Sekhar67% (3)
- CubeX - Back To The Roots - Landscaping OTC Opportunities in The Indian Ayurveda Market-HighlightsDokumen16 halamanCubeX - Back To The Roots - Landscaping OTC Opportunities in The Indian Ayurveda Market-Highlightscubex_consultingBelum ada peringkat
- Rosacea: Clinical PracticeDokumen11 halamanRosacea: Clinical PracticeLusy Dwitama LeryBelum ada peringkat
- API and Intermediates of GujratDokumen16 halamanAPI and Intermediates of GujratSamyak JainBelum ada peringkat
- Background: National Rosacea SocietyDokumen18 halamanBackground: National Rosacea SocietyPanda OnlineShopBelum ada peringkat
- Bipolar Children-Cutting-Edge Controversy Insights and ResearchDokumen176 halamanBipolar Children-Cutting-Edge Controversy Insights and Researchdariaevelin100% (1)
- Cns MCQDokumen11 halamanCns MCQAjay YadavBelum ada peringkat
- Muscles of the Scapula and ShoulderDokumen6 halamanMuscles of the Scapula and ShoulderAndika Anjani AgustinBelum ada peringkat
- Psoriasis A Case StudyDokumen14 halamanPsoriasis A Case StudyYayin PestañoBelum ada peringkat
- Seborrheic Dermatitis EmedicineDokumen10 halamanSeborrheic Dermatitis EmedicineIkram IkramBelum ada peringkat
- Principles of Wound HealingDokumen8 halamanPrinciples of Wound HealingTracy100% (6)
- ASHP Best Practices 2015-2016 PDFDokumen793 halamanASHP Best Practices 2015-2016 PDFAhmad MakhloufBelum ada peringkat
- 7 Common Myths About QP Training Debunked: A Guide For Senior ManagersDokumen15 halaman7 Common Myths About QP Training Debunked: A Guide For Senior ManagersSupriya KapasBelum ada peringkat
- Handbook Titration 6.0-MB PDF-EnglishDokumen190 halamanHandbook Titration 6.0-MB PDF-EnglishRahul100% (1)
- Benefits and Risks of Topical CorticosteroidsDokumen3 halamanBenefits and Risks of Topical CorticosteroidsIrene Djuardi100% (1)
- AP CHHGH Full Final ListDokumen23 halamanAP CHHGH Full Final ListYakshit JainBelum ada peringkat
- Optimizing Treatment Approaches in Seborrheic DermatitisDokumen17 halamanOptimizing Treatment Approaches in Seborrheic DermatitisShinta Restyana WidyaBelum ada peringkat
- Adalsteinsson2020 Inmunologia de La Dermatitis SeborreicaDokumen9 halamanAdalsteinsson2020 Inmunologia de La Dermatitis SeborreicaLaura GarciaBelum ada peringkat
- Seborrheic Dermatitis of The Scalp Etiology and TreatmentDokumen9 halamanSeborrheic Dermatitis of The Scalp Etiology and TreatmentAndi WahyuniBelum ada peringkat
- Chapter 26:: Seborrheic Dermatitis:: Dae Hun Suh: Clinical FeaturesDokumen10 halamanChapter 26:: Seborrheic Dermatitis:: Dae Hun Suh: Clinical FeaturesmyztBelum ada peringkat
- Jurnal Dermatitis Seboroik 3 (Easti)Dokumen8 halamanJurnal Dermatitis Seboroik 3 (Easti)Easti vishiara amdelyBelum ada peringkat
- Seborrheic Dermatitis in Adolescents and AdultsDokumen51 halamanSeborrheic Dermatitis in Adolescents and AdultsCosmina GeorgianaBelum ada peringkat
- Psoriasis: PathophysiologyDokumen23 halamanPsoriasis: PathophysiologyImran KhanBelum ada peringkat
- Seborrheic Dermatitis From JurnalDokumen6 halamanSeborrheic Dermatitis From JurnalindciwBelum ada peringkat
- 103.dermatological Manifestations in Diabetes JBADDokumen20 halaman103.dermatological Manifestations in Diabetes JBADhaytham aliBelum ada peringkat
- Diagnosisandmanagementof Psoriasisinchildren: Megha M. TollefsonDokumen17 halamanDiagnosisandmanagementof Psoriasisinchildren: Megha M. TollefsonMasithaBelum ada peringkat
- Atopic DermatitisDokumen8 halamanAtopic DermatitiskoesantoBelum ada peringkat
- Article of Demodecosis in Dog-DikonversiDokumen12 halamanArticle of Demodecosis in Dog-DikonversiSosial EkonomiBelum ada peringkat
- Seborrheic Dermatitis UpdateDokumen7 halamanSeborrheic Dermatitis Updatescaindrianie2903Belum ada peringkat
- Diagnosis and Treatment of Rosacea: Aaron F. Cohen, MD, and Jeffrey D. Tiemstra, MDDokumen4 halamanDiagnosis and Treatment of Rosacea: Aaron F. Cohen, MD, and Jeffrey D. Tiemstra, MDbookaholic7Belum ada peringkat
- Diagnosa Dan Terapi SSSS PDFDokumen6 halamanDiagnosa Dan Terapi SSSS PDFni putu.comsurya dianaBelum ada peringkat
- 887 1445 1 SM PDFDokumen7 halaman887 1445 1 SM PDFazrinaBelum ada peringkat
- (Serbian Journal of Dermatology and Venereology) National Guidelines For The Treatment of Atopic DermatitisDokumen25 halaman(Serbian Journal of Dermatology and Venereology) National Guidelines For The Treatment of Atopic DermatitisFebtri IRnawitaBelum ada peringkat
- Jadotte Pityriasis AlbaDokumen8 halamanJadotte Pityriasis Albapene asoyBelum ada peringkat
- Skin Manifestations of Systemic Disease: Key PointsDokumen7 halamanSkin Manifestations of Systemic Disease: Key PointsJUAN DAVID RESTREPO MARINBelum ada peringkat
- Pics MCQSDokumen22 halamanPics MCQStaniaBelum ada peringkat
- Rashid 2015Dokumen6 halamanRashid 2015Francesca BizzocoBelum ada peringkat
- Seborrehic DermatitisDokumen25 halamanSeborrehic DermatitisHallidayBelum ada peringkat
- Wa0017.Dokumen9 halamanWa0017.CHAMBI MAMANI JESSICA BETTYBelum ada peringkat
- Lamb 2017Dokumen6 halamanLamb 2017Carolina González RiveraBelum ada peringkat
- Psoriasis: Posted: 02 Aug 2010 11:18 PM PDTDokumen5 halamanPsoriasis: Posted: 02 Aug 2010 11:18 PM PDTScamb TrekBelum ada peringkat
- Research Paper On EczemaDokumen7 halamanResearch Paper On Eczemauziianwgf100% (1)
- 0610 - Peds Acne FeaDokumen9 halaman0610 - Peds Acne FeaReginaBelum ada peringkat
- Assessment of Lipid Profile Among Patients With Seborrheic DermatitisDokumen7 halamanAssessment of Lipid Profile Among Patients With Seborrheic DermatitisAinun Aii NoorBelum ada peringkat
- Robinson HC CT105003132Dokumen5 halamanRobinson HC CT105003132Francesca BizzocoBelum ada peringkat
- 3 PDFDokumen6 halaman3 PDFsandra magdalenaBelum ada peringkat
- Veterinary Clinics: Update On Canine DemodicosisDokumen13 halamanVeterinary Clinics: Update On Canine Demodicosisanthon lunBelum ada peringkat
- Gangguan Kulit Pada LansiaDokumen28 halamanGangguan Kulit Pada LansianoviBelum ada peringkat
- COLGATE Oral - Lesions - BookDokumen89 halamanCOLGATE Oral - Lesions - BookVijetha RaiBelum ada peringkat
- DBST Dlja INO Studentov (PDF - Io)Dokumen6 halamanDBST Dlja INO Studentov (PDF - Io)eidBelum ada peringkat
- Dermatitis SeborreicaDokumen6 halamanDermatitis SeborreicaDiego Eraso IncaBelum ada peringkat
- BJD 17896Dokumen8 halamanBJD 17896JeromeBelum ada peringkat
- articulo_ingles_2_ginaDokumen8 halamanarticulo_ingles_2_ginassariedmartinezBelum ada peringkat
- Dermatitis Atopik PDFDokumen11 halamanDermatitis Atopik PDFmlengseBelum ada peringkat
- Levy 2012Dokumen20 halamanLevy 2012Anonymous ujOv31SBelum ada peringkat
- Rosacea - Pathogenesis, Clinical Features, and Diagnosis - UpToDateDokumen56 halamanRosacea - Pathogenesis, Clinical Features, and Diagnosis - UpToDateJorge Leonardo BedoyaBelum ada peringkat
- FileVT0310 PR HorneCEDokumen6 halamanFileVT0310 PR HorneCEHassim M H SBelum ada peringkat
- Pityriasis AlbaDokumen7 halamanPityriasis AlbaAri Kurniawan100% (1)
- Childhood Granulomatous Periorificial DermatitisDokumen4 halamanChildhood Granulomatous Periorificial DermatitisNiaUlfaAngreiniTambunanBelum ada peringkat
- 127407Dokumen6 halaman127407ecleftikBelum ada peringkat
- Skin in Clinical DiagnosisDokumen64 halamanSkin in Clinical Diagnosisadamu mohammad0% (1)
- Demodicosis in Different Age Groups and AlternativDokumen23 halamanDemodicosis in Different Age Groups and AlternativJuliana FreitasBelum ada peringkat
- Perioral DermatitisDokumen5 halamanPerioral Dermatitissusanti michellinaBelum ada peringkat
- Atopic Dermatitis:: Dr. Francisco Eizayaga, MD PHDDokumen18 halamanAtopic Dermatitis:: Dr. Francisco Eizayaga, MD PHDAngelaBelum ada peringkat
- Cap Area 2 DermatoDokumen169 halamanCap Area 2 DermatoSaul RivasBelum ada peringkat
- 19411-Article Text-62419-1-10-20180329 PDFDokumen10 halaman19411-Article Text-62419-1-10-20180329 PDFGreice BelfortBelum ada peringkat
- Tinea Versicolor: Pathophysiology, Etiology in <40 CharsDokumen4 halamanTinea Versicolor: Pathophysiology, Etiology in <40 Charswahyuni taslimBelum ada peringkat
- Dry Eye Disease: Consideration For Women's HealthDokumen13 halamanDry Eye Disease: Consideration For Women's HealthIntan N HBelum ada peringkat
- Cosmetics 09 00078 v2Dokumen14 halamanCosmetics 09 00078 v2Dara PitraBelum ada peringkat
- Bahan Crs 2Dokumen6 halamanBahan Crs 2Andika Anjani AgustinBelum ada peringkat
- NervusDokumen8 halamanNervusAndika Anjani AgustinBelum ada peringkat
- Fisiologi PernafasanDokumen10 halamanFisiologi PernafasanAndika Anjani AgustinBelum ada peringkat
- SkizofreniaDokumen54 halamanSkizofreniaAndika Anjani AgustinBelum ada peringkat
- Muscle Origin Insertion Innervation Blood Supply Action: Hip and ThighDokumen6 halamanMuscle Origin Insertion Innervation Blood Supply Action: Hip and ThighAndika Anjani AgustinBelum ada peringkat
- Intra Uterine Fetal Death and Some Related Factors A Silent Tragedy in Southeastern Iran 2167 0846.1000129Dokumen3 halamanIntra Uterine Fetal Death and Some Related Factors A Silent Tragedy in Southeastern Iran 2167 0846.1000129mayaBelum ada peringkat
- Fetomaternal Hemorrhage A Review After A Case Report 2376 127X 1000197Dokumen3 halamanFetomaternal Hemorrhage A Review After A Case Report 2376 127X 1000197mayaBelum ada peringkat
- A Case of Atopic Dermatitis Coexisting With Psoriasis Vulgaris 2155 9554 1000386Dokumen3 halamanA Case of Atopic Dermatitis Coexisting With Psoriasis Vulgaris 2155 9554 1000386Andika Anjani AgustinBelum ada peringkat
- 56.ArifKulit OfloxacinLeprae AJCEM 01072015 PDFDokumen5 halaman56.ArifKulit OfloxacinLeprae AJCEM 01072015 PDFAndika Anjani AgustinBelum ada peringkat
- KDIGO 2017 Guideline Update Summary: Diagnosis and Treatment of CKD-MBDDokumen71 halamanKDIGO 2017 Guideline Update Summary: Diagnosis and Treatment of CKD-MBDAndika Anjani AgustinBelum ada peringkat
- Aria Rhinitis Allergic Pocket GuideDokumen8 halamanAria Rhinitis Allergic Pocket GuideAlfani FajarBelum ada peringkat
- Dec - 09.friedman-Headache Patient PDFDokumen13 halamanDec - 09.friedman-Headache Patient PDFAndika Anjani AgustinBelum ada peringkat
- Efektivitas Pregabalin Untuk Terapi Nyeri Kronis-Evidence-based ReviewDokumen4 halamanEfektivitas Pregabalin Untuk Terapi Nyeri Kronis-Evidence-based ReviewMuhammad AsrizalBelum ada peringkat
- Acute Sinusitis in AdultsDokumen9 halamanAcute Sinusitis in AdultsAndika Anjani AgustinBelum ada peringkat
- 10 5923 J Otolaryn 20120101 01Dokumen5 halaman10 5923 J Otolaryn 20120101 01CON287Belum ada peringkat
- Current and Future Directions in Pediatric Allergic Rhinitis PDFDokumen13 halamanCurrent and Future Directions in Pediatric Allergic Rhinitis PDFAndika Anjani AgustinBelum ada peringkat
- JClinNeonatol34183-8487701 233437 PDFDokumen8 halamanJClinNeonatol34183-8487701 233437 PDFAndika Anjani AgustinBelum ada peringkat
- Acute Sinusitis in AdultsDokumen9 halamanAcute Sinusitis in AdultsAndika Anjani AgustinBelum ada peringkat
- Rhinitis and Sinusitis PDFDokumen13 halamanRhinitis and Sinusitis PDFAndika Anjani AgustinBelum ada peringkat
- A New Clinical Scoring System For Adenoid Hypertrophy in Children PDFDokumen7 halamanA New Clinical Scoring System For Adenoid Hypertrophy in Children PDFAndika Anjani AgustinBelum ada peringkat
- Chronic Rhinosinusitis With Nasal PolypsDokumen8 halamanChronic Rhinosinusitis With Nasal PolypsAndika Anjani AgustinBelum ada peringkat
- Clinicotherapeutic Profile of PatientsDokumen5 halamanClinicotherapeutic Profile of PatientsAndika Anjani AgustinBelum ada peringkat
- Inflammatory Septal Nasal Polyp PDFDokumen5 halamanInflammatory Septal Nasal Polyp PDFAndika Anjani AgustinBelum ada peringkat
- Chronic Rhinosinusitis Phenotypes: An Approach to Better Medical CareDokumen4 halamanChronic Rhinosinusitis Phenotypes: An Approach to Better Medical CareAndika Anjani AgustinBelum ada peringkat
- Herbesser 90 SR and 180 SR capsules for hypertension and anginaDokumen2 halamanHerbesser 90 SR and 180 SR capsules for hypertension and anginaAqleedasBelum ada peringkat
- Intercalation of Drugs in LDH and Their Controlled Release, A ReviewDokumen31 halamanIntercalation of Drugs in LDH and Their Controlled Release, A ReviewSebastian Pala100% (1)
- C C CCCCCCCCCC C C CCC CC C CCC CDokumen46 halamanC C CCCCCCCCCC C C CCC CC C CCC CSyed AhmadBelum ada peringkat
- Gpat 2014 SolvedDokumen12 halamanGpat 2014 Solvedjhansi100% (1)
- Original Papers: The Efficacy of Oral Ivermectin vs. Sulfur 10% Ointment For The Treatment of ScabiesDokumen8 halamanOriginal Papers: The Efficacy of Oral Ivermectin vs. Sulfur 10% Ointment For The Treatment of ScabieserwinBelum ada peringkat
- Contents of Supplement 84Dokumen2 halamanContents of Supplement 84shri_palaniBelum ada peringkat
- Pharmacology 1: Pharmacodynamics and ReceptorsDokumen19 halamanPharmacology 1: Pharmacodynamics and ReceptorsBin HipBelum ada peringkat
- Pediatric Dosing For OTCsDokumen5 halamanPediatric Dosing For OTCsCareyTranBelum ada peringkat
- The Word Spring 2014 FinalDokumen23 halamanThe Word Spring 2014 Finalapi-276783597Belum ada peringkat
- (Derita, Marcos G. Rai, Mahendra Zacchino, Susan (B-Ok - Xyz) PDFDokumen327 halaman(Derita, Marcos G. Rai, Mahendra Zacchino, Susan (B-Ok - Xyz) PDFKima MadBelum ada peringkat
- Clinical Research Site ProfileDokumen3 halamanClinical Research Site ProfileDeepan55Belum ada peringkat
- Tugas 2Dokumen272 halamanTugas 2viviBelum ada peringkat
- Niper NotesDokumen9 halamanNiper NotesVizit DubeyBelum ada peringkat
- LearnEnglish Writing B2 A Letter of ComplaintDokumen4 halamanLearnEnglish Writing B2 A Letter of ComplaintCas TanBelum ada peringkat
- 230703Dokumen8 halaman230703ciejhae3111Belum ada peringkat
- Drug and Price Control Order: Sajal Kumar Chowdhury B.Pharm 4 YearDokumen14 halamanDrug and Price Control Order: Sajal Kumar Chowdhury B.Pharm 4 YearSiva PrasadBelum ada peringkat
- SalesDokumen2 halamanSalesapi-77262028Belum ada peringkat
- AmlodipineDokumen2 halamanAmlodipineRph AinBelum ada peringkat
- Final Bio Report - Raproxain (R)Dokumen39 halamanFinal Bio Report - Raproxain (R)Nafis MehranBelum ada peringkat
- Quantitative Determination of Ketotifen in Drug Dosage Forms by Spectrophotometric MethodDokumen5 halamanQuantitative Determination of Ketotifen in Drug Dosage Forms by Spectrophotometric MethodKhovivah IpBelum ada peringkat
- H Pylori - Scheme de TratamentDokumen4 halamanH Pylori - Scheme de Tratamentgaspe1999Belum ada peringkat