New England Journal Medicine: The of
Diunggah oleh
Dias PradikaJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
New England Journal Medicine: The of
Diunggah oleh
Dias PradikaHak Cipta:
Format Tersedia
new england
The
journal of medicine
established in 1812 february 11, 2010 vol. 362 no. 6
Childhood Obesity, Other Cardiovascular Risk Factors,
and Premature Death
Paul W. Franks, Ph.D., Robert L. Hanson, M.D., M.P.H., William C. Knowler, M.D., Dr.P.H., Maurice L. Sievers, M.D.,
Peter H. Bennett, M.B., F.R.C.P., and Helen C. Looker, M.B., B.S.
A b s t r ac t
Background
The effect of childhood risk factors for cardiovascular disease on adult mortality is From the Diabetes Epidemiology and Clini-
poorly understood. cal Research Section, National Institute of
Diabetes and Digestive and Kidney Diseas-
es, National Institutes of Health, Phoenix,
Methods AZ (P.W.F., R.L.H., W.C.K., M.L.S., P.H.B.,
In a cohort of 4857 American Indian children without diabetes (mean age, 11.3 years; H.C.L.); the Genetic Epidemiology and
Clinical Research Group, Department of
12,659 examinations) who were born between 1945 and 1984, we assessed whether Public Health and Clinical Medicine, Sec-
body-mass index (BMI), glucose tolerance, and blood pressure and cholesterol levels tion for Medicine, Umeå University Hos-
predicted premature death. Risk factors were standardized according to sex and pital, Umeå, Sweden (P.W.F.); the Medi-
cal Research Council Epidemiology Unit,
age. Proportional-hazards models were used to assess whether each risk factor was Institute of Metabolic Sciences, University
associated with time to death occurring before 55 years of age. Models were ad- of Cambridge, Cambridge, United Kingdom
justed for baseline age, sex, birth cohort, and Pima or Tohono O’odham Indian (P.W.F.); and Mount Sinai School of Med-
icine, New York (H.C.L.). Address reprint
heritage. requests to Dr. Franks at the Genetic Epi-
demiology and Clinical Research Group,
Results Department of Public Health and Clinical
Medicine, Section for Medicine, Umeå
There were 166 deaths from endogenous causes (3.4% of the cohort) during a me- University Hospital, Umeå 901 87, Sweden,
dian follow-up period of 23.9 years. Rates of death from endogenous causes among or at paul.franks@medicin.umu.se.
children in the highest quartile of BMI were more than double those among chil-
N Engl J Med 2010;362:485-93.
dren in the lowest BMI quartile (incidence-rate ratio, 2.30; 95% conf idence interval Copyright © 2010 Massachusetts Medical Society.
[CI], 1.46 to 3.62). Rates of death from endogenous causes among children in the
highest quartile of glucose intolerance were 73% higher than those among children
in the lowest quartile (incidence-rate ratio, 1.73; 95% CI, 1.09 to 2.74). No significant
associations were seen between rates of death from endogenous or external causes
and childhood cholesterol levels or systolic or diastolic blood-pressure levels on a
continuous scale, although childhood hypertension was signif icantly associated
with premature death from endogenous causes (incidence-rate ratio, 1.57; 95% CI,
1.10 to 2.24).
Conclusions
Obesity, glucose intolerance, and hypertension in childhood were strongly associated
with increased rates of premature death from endogenous causes in this population.
In contrast, childhood hypercholesterolemia was not a major predictor of prema-
ture death from endogenous causes.
n engl j med 362;6 nejm.org february 11, 2010 485
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
The ne w e ngl a nd jour na l of me dicine
D
espite recent increases in life ex- Study Examinations
pectancy, the rising global prevalence of We assessed the extent to which childhood body-
obesity may reverse this trend.1 The rising mass index (BMI), 2-hour plasma glucose level
rates and increasingly early onset of other chronic during a 75-g oral glucose-tolerance test, and
diseases such as type 2 diabetes may also affect blood pressure and total cholesterol levels pre-
mortality rates.2 dicted premature death. The baseline examina-
Cardiovascular risk factors are common in tion was the f irst examination at which all these
children.3,4 Although early-onset diabetes has variables were measured. The analyses included
been shown to raise mortality rates,2 and the data from the date of the baseline examination
relation between cardiovascular risk factors dur- until the person’s death, the person’s 55th birth-
ing adulthood and early death is well def ined,5-7 day, or the end of 2003, whichever came f irst.
little is known about the way in which cardiovas- Vital status was ascertained as of December 31,
cular risk factors that are present during child- 2003. Death records for community residents
hood affect life span. Defining such relationships were maintained throughout the study period.
may help predict the long-term human and eco- Copies of death certif icates were obtained. The
nomic costs of cardiovascular risk factors in underlying cause of death was classif ied as en-
childhood and might justify interventions that dogenous or external. We def ined deaths due to
are intended to improve health and reduce the endogenous causes as those in which the proxi-
rates of premature death. mate cause was disease or self-inf licted injury,
In this study, we assessed the extent to which such as acute alcohol intoxication or drug use,
obesity, glucose intolerance, hypertension, and and deaths due to external causes as those that
hypercholesterolemia in children without diabe- resulted from such causes as accidents or homi-
tes predicted premature death (def ined as death cide. These def initions are consistent with those
before 55 years of age) in American Indians used in previous mortality studies undertaken
from Arizona. in this cohort.10 The cause of death was deter-
mined from a review of available clinical autopsy
M E TH O DS records and death certif icates. (For a list of the
specif ic causes of death and the corresponding
Study Population International Classif ication of Diseases, 9th Revision
We invited residents in a well-def ined geographic [ICD-9] codes, see the Supplementary Appen-
area of the Gila River Indian Community in Ari- dix, available with the full text of this article at
zona, most of whom were Pima or Tohono NEJM.org.)
O’odham Indians,8,9 to participate in a longitudi- All participants underwent a 75-g oral glucose-
nal study of diabetes and related disorders. Pima tolerance test; results were interpreted accord-
or Tohono O’odham Indian heritage was def ined ing to World Health Organization diagnostic
by the heritage of each of the child’s parents, criteria.11 We considered diabetes to be present
grandparents, and great-grandparents, as report- if the fasting plasma glucose concentration was
ed by the parents of the participating children. more than 7.0 mmol per liter (126 mg per deci-
Included in the study were 4857 children and liter), if the 2-hour plasma glucose concentra-
adolescents (5 to <20 years of age) who had at tion was 11.1 mmol per liter (200 mg per deci-
least 4/8 Pima or Tohono O’odham Indian heri- liter) or more, or if a previous clinical diagnosis
tage, did not have diabetes, and underwent one was documented. Blood pressure was measured
or more research examinations between February and standard anthropometric data were obtained
1966 and December 2003. Participants were born while participants were wearing lightweight
between 1945 and 1984 and resided on the reser- clothing and no shoes; the data were collected
vation during the study. Participants who were 18 by trained study personnel.8,9,12 No measures of
years of age or older gave written informed con- puberty were available. Blood assays were per-
sent; those younger than 18 years of age gave formed as described previously.8,9,12 Alcohol
written assent and a parent or guardian gave writ- dependence in adulthood (for which data were
ten informed consent. The institutional review available from 2672 of the participants) was
board of the National Institute of Diabetes and Di- estimated with the use of the CAGE question-
gestive and Kidney Diseases approved the study. naire.13
486 n engl j med 362;6 nejm.org february 11, 2010
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Childhood obesit y a nd pr em at ur e de ath
Statistical Analysis Table 1. Baseline Characteristics of the Participants and Prevalence of Death
before 55 Years of Age.*
Analyses were performed with the use of SAS soft-
ware, version 9.1 (SAS Institute). The characteristics Variable Value
of the participants are presented as arithmetic Sex — no. (%)
means (±SD) or, in the case of characteristics with Male 2397 (49.4)
skewed distributions, as medians and ranges. The Female 2460 (50.6)
z scores, which were standardized within sex and Age — yr
1-year age strata, were computed for use in regres- Mean 11.3±3.7
sion analyses. Age-standardized and sex-standard- Range 5–19
ized incidence was calculated by the direct method Age group — no. (%)
with the use of the total study population as the 5–9 2075 (42.7)
reference group. Incidence-rate ratios were calcu- 10–14 1913 (39.4)
lated from the incidence data with the use of Pois- 15–19 869 (17.9)
son regression controlled for age, sex, and Pima
Body-mass index†
or Tohono O’odham Indian heritage. For incidence
Mean 21.9±6.1
analyses, follow-up was truncated at 55 years of
Range 12.4–55.3
age, since there were few person-years beyond that
Obesity — no. (%)‡ 1394 (28.7)
point. Cox proportional-hazards models were
2-hr glucose — mmol/liter
used to test for associations between the baseline
Mean 5.5±1.2
childhood risk factors and time to death, with
Range 1.3–11.0
adjustment for baseline age, sex, Pima or Tohono
Impaired glucose tolerance — no. (%) 198 (4.1)
O’odham Indian heritage, and birth year, since
birth year was correlated with many variables of Blood pressure — mm Hg
interest (e.g., r = 0.36 for the correlation between Systolic
BMI and birth year). We tested the validity of the Mean 106±16
proportionality assumption for each variable by Range 58–196
including a time-dependent interaction term in Diastolic
the baseline models.14 When this assumption Mean 59±11
was violated, stratified proportional-hazards mod- Range 6–110
els were f itted and a summarized incidence-rate Hypertension — no. (%)§ 607 (12.5)
ratio was calculated across strata; no material dif- Total cholesterol — mmol/liter
ferences in death rates were observed across sex Mean 3.8±0.7
and baseline-age strata (data not shown). Range 1.6–11.2
Hypercholesterolemia — no. (%)¶ 182 (3.7)
R ESULT S Follow-up period — yr
Premature Death among Study Participants Median 23.9
Table 1 shows the baseline characteristics of the Range 0.04–37.9
participants. During the follow-up period, 559 of Death — no. (%)
the 4857 participants (11.5%) died before they From all causes 559 (11.5)
reached 55 years of age. A total of 166 deaths From endogenous causes 166 (3.4)
were from endogenous causes: 59 were attributed From external causes 393 (8.1)
to alcoholic liver disease, 22 to cardiovascular dis- * Plus–minus values are means ±SD. To convert values for glucose to milligrams
ease, 21 to infections, 12 to cancer, 10 to diabe- per deciliter, multiply by 18.01. To convert values for cholesterol to milligrams
tes or diabetic nephropathy, 9 to acute alcoholic per deciliter, multiply by 38.67.
† The body-mass index is the weight in kilograms divided by the square of the
poisoning or drug overdose, and 33 to other height in meters.
causes (see the Supplementary Appendix for a list ‡ Obesity was defined as a body-mass index in the 95th percentile or higher of
of ICD-9 codes). Table 2 shows the rates of pre- the U.S. population according to the 2000 database of the Centers for Disease
Control and Prevention.15
mature death by 10-year age strata. § Hypertension was defined according to the criteria of the National High Blood
Pressure Education Program.16
Childhood Obesity and Premature Death ¶ Hypercholesterolemia was defined as a cholesterol level higher than the cutoff
point designated by the American Heart Association (total cholesterol, 5.18 mmol
BMI was positively associated with the risk of pre- per liter [200 mg per deciliter]).17
mature death from endogenous causes (incidence-
n engl j med 362;6 nejm.org february 11, 2010 487
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
The ne w e ngl a nd jour na l of me dicine
Table 2. Premature Death among Study Participants, According to Age at Study Entry.*
Person-Years
Age of Follow-up Premature Death
All Causes External Causes Endogenous Causes
no./1000 no./1000 no./1000 % of all
no. person-yr no. person-yr no. person-yr deaths
5–14 yr 20,066 6 0.30 6 0.30 0 0 0
15–24 yr 43,081 190 4.41 181 4.20 9 0.21 4.7
25–34 yr 31,163 190 6.10 144 4.62 46 1.48 24.2
35–44 yr 17,154 108 6.30 47 2.74 61 3.56 56.5
45–54 yr 4,646 65 13.99 15 3.23 50 10.76 76.9
* Participants were 5 to 19 years of age when they entered the study. Person-years of follow-up were partitioned according to age over the
course of the follow-up period. The incidence analysis was stratified according to decade of age and truncated at the age of 55 years.
rate ratio per 1 unit of BMI z score, 1.40; 95% 1.31 (95% CI, 1.10 to 1.57) for premature death
conf idence interval [CI], 1.20 to 1.63). BMI was from all causes, 1.90 (95% CI, 1.37 to 2.65) for
positively, but not signif icantly, associated with death from endogenous causes, and 1.14 (95% CI,
death from external causes (incidence-rate ratio 0.92 to 1.41) for death from external causes.
per 1 SD of standardized BMI, 1.19; 95% CI, 1.00
to 1.42). Childhood Glucose, Cholesterol, and Blood-
Children in the highest quartile of age-stan- Pressure Levels and Premature Death
dardized and sex-standardized BMI had signif i- The 2-hour plasma glucose level during a 75-g oral
cantly higher rates of death than did children in glucose-tolerance test, expressed in age-standard-
the lowest quartile (Fig. 1 and Table 3). The rates ized and sex-standardized units, was not associ-
of death from endogenous causes among chil- ated with premature death from either endoge-
dren in the highest quartile of BMI were more nous or external causes. However, children in the
than double those among children in the lowest highest quartile of glucose level had a 73% higher
quartile (incidence-rate ratio, 2.30; 95% CI, 1.46 risk of premature death from endogenous causes
to 3.62) (Table 3). This finding could not be ex- than children in the lowest quartile (Table 3).
plained just by the presence of extremely obese Adjustment for childhood BMI reduced the mag-
children in the highest quartile, however, since nitude of the association (incidence-rate ratio,
none of the 51 extremely obese children (BMI 1.24; 95% CI, 0.79 to 1.96).
z score >3) died during the follow-up period, In models of impaired glucose tolerance (i.e.,
possibly because these participants were young- 2-hour glucose level of 7.8 to 11.0 mmol per liter
er and from more recent birth cohorts (median [140 to 199 mg per deciliter])18 as compared with
follow-up, 21.4 years) than participants who were normal glucose tolerance as the predictor vari-
less obese. The association between BMI and pre- able, the incidence-rate ratios were 0.90 (95% CI,
mature death from endogenous causes was attenu- 0.63 to 1.30) for all-cause premature death, 0.81
ated but remained significant after adjustment for (95% CI, 0.39 to 1.65) for death from endoge-
baseline glucose level, cholesterol level, and blood nous causes, and 0.94 (95% CI, 0.62 to 1.43) for
pressure (incidence-rate ratio for the highest BMI death from external causes. Children with im-
quartile vs. the lowest quartile, 1.41; 95% CI, paired glucose tolerance accounted for 15% of
1.19 to 1.67) (Table 3). the children in the highest quartile of plasma
A total of 1394 of the children (28.7%) were glucose levels and were all in the top decile of
obese, which was def ined as a BMI in the 95th the standardized 2-hour glucose distribution.
percentile or higher on the Centers for Disease No significant associations were observed be-
Control and Prevention (CDC) growth charts.15 tween death rates and childhood cholesterol lev-
Among the obese children as compared with the els or blood pressure (Table 3). In models in
nonobese children, the incidence-rate ratios were which hypercholesterolemia, as def ined by the
488 n engl j med 362;6 nejm.org february 11, 2010
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Childhood obesit y a nd pr em at ur e de ath
BMI quartile 1 BMI quartile 2 BMI quartile 3 BMI quartile 4
A Premature Death from All Causes B Premature Death from All Causes C Premature Death from All Causes
(5 to <10 yr) (10 to <15 yr) (15 to <20 yr)
1.00 1.00 1.00
0.95 0.95 0.95
Survival (%)
Survival (%)
Survival (%)
0.90 0.90 0.90
0.85 0.85 0.85
0.80 0.80 0.80
0.75 0.75 0.75
0.00 0.00 0.00
0 10 20 30 40 50 60 0 10 20 30 40 50 60 0 10 20 30 40 50 60
Age (Yr) Age (Yr) Age (Yr)
D Premature Death from External Causes E Premature Death from External Causes F Premature Death from External Causes
(5 to <10 yr) (10 to <15 yr) (15 to <20 yr)
1.00 1.00 1.00
0.95 0.95 0.95
Survival (%)
Survival (%)
Survival (%)
0.90 0.90 0.90
0.85 0.85 0.85
0.80 0.80 0.80
0.75 0.75 0.75
0.00 0.00 0.00
0 10 20 30 40 50 60 0 10 20 30 40 50 60 0 10 20 30 40 50 60
Age (Yr) Age (Yr) Age (Yr)
G Premature Death from Endogenous Causes H Premature Death from Endogenous Causes I Premature Death from Endogenous Causes
(5 to <10 yr) (10 to <15 yr) (15 to <20 yr)
1.00 1.00 1.00
0.95 0.95 0.95
Survival (%)
Survival (%)
Survival (%)
0.90 0.90 0.90
0.85 0.85 0.85
0.80 0.80 0.80
0.75 0.75 0.75
0.00 0.00 0.00
0 10 20 30 40 50 60 0 10 20 30 40 50 60 0 10 20 30 40 50 60
Age (Yr) Age (Yr) Age (Yr)
Figure 1. Kaplan–Meier Curves for Premature Death.
The graphs show the rates of premature death from all causes, external causes, and endogenous causes according to quartiles of age-
standardized and sex-standardized body-mass index at different baseline ages during childhood and adolescence. Plots were computed
with the use of baseline data. Age at baseline for each age group was taken as the midpoint of the age range.
American Heart Association cutoff point (total nif icant association with rates of death from all
cholesterol level, 5.18 mmol per liter [200 mg per causes (incidence-rate ratio, 1.15; 95% CI, 0.93 to
deciliter]), was used as the predictor variable,17 1.43) or from external causes (incidence-rate ratio,
the incidence-rate ratios were 1.33 (95% CI, 0.95 0.98; 95% CI, 0.75 to 1.29). However, childhood
to 1.88) for all-cause premature death, 1.70 (95% hypertension was strongly associated with the
CI, 0.96 to 3.01) for death from endogenous rate of death from endogenous causes (incidence-
causes, and 1.18 (95% CI, 0.77 to 1.80) for death rate ratio, 1.57; 95% CI, 1.10 to 2.24).
from external causes.
With hypertension def ined according to the Potential Mediators of the Association
criteria of the National High Blood Pressure Edu- between Obesity and Death
cation Program16 in the case of children and as Most deaths occurred in study participants who
140/90 mm Hg or higher in the case of partici- were not known to have diabetes. Of the 559 par-
pants 18 years of age or older, there was no sig- ticipants in whom diabetes developed, 79 died:
n engl j med 362;6 nejm.org february 11, 2010 489
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
The ne w e ngl a nd jour na l of me dicine
Table 3. Incidence-Rate Ratios for Premature Death, According to Quartile of Variables.*
Premature Death Premature Death Premature Death from
Variable from All Causes from External Causes Endogenous Causes
IRR (95% CI) P value IRR (95% CI) P value IRR (95% CI) P value
Body-mass index 0.02 0.35 <0.01
Q2 vs. Q1 0.91 (0.72–1.16) 0.82 (0.62–1.09) 1.24 (0.77–2.00)
Q3 vs. Q1 1.01 (0.80–1.24) 0.94 (0.71–1.23) 1.28 (0.78–2.09)
Q4 vs. Q1 1.31 (1.04–1.66) 1.06 (0.80–1.40) 2.30 (1.46–3.62)
2-Hour glucose 0.37 0.78 0.12
Q2 vs. Q1 1.10 (0.86–1.40) 1.00 (0.76–1.32) 1.43 (0.88–2.30)
Q3 vs. Q1 0.97 (0.75–1.24) 0.88 (0.66–1.18) 1.24 (0.75–2.04)
Q4 vs. Q1 1.17 (0.93–1.48) 1.02 (0.77–1.39) 1.73 (1.09–2.74)
Systolic blood pressure 0.19 0.80 0.09
Q2 vs. Q1 0.92 (0.71–1.21) 0.98 (0.73–1.33) 0.75 (0.43–1.30)
Q3 vs. Q1 1.15 (0.90–1.48) 1.11 (0.83–1.49) 1.26 (0.77–2.07)
Q4 vs. Q1 1.16 (0.91–1.48) 1.08 (0.81–1.45) 1.34 (0.83–2.15)
Diastolic blood pressure 0.72 0.96 0.35
Q2 vs. Q1 1.08 (0.84–1.38) 1.07 (0.80–1.42) 1.09 (0.66–1.82)
Q3 vs. Q1 0.99 (0.78–1.27) 0.99 (0.75–1.32) 1.00 (0.61–1.64)
Q4 vs. Q1 1.11 (0.88–1.41) 1.01 (0.76–1.34) 1.40 (0.89–2.19)
Total cholesterol 0.14 0.21 0.77
Q2 vs. Q1 1.08 (0.85–1.37) 1.07 (0.81–1.42) 1.11 (0.71–1.75)
Q3 vs. Q1 1.04 (0.81–1.32) 1.01 (0.76–1.34) 1.11 (0.71–1.75)
Q4 vs. Q1 1.30 (1.02–1.65) 1.30 (0.99–1.72) 1.28 (0.81–2.02)
* Incidence-rate ratios (IRRs) were calculated by means of Poisson regression according to quartiles of variables standardized by age and sex.
P values are for linear trends across quartiles. Q denotes quartile.
40 from endogenous causes and 39 from external hood increase mortality rates. We conducted the
causes. Adjusting the BMI prediction models for present study to determine whether the presence
incident diabetes did not signif icantly alter the of these risk factors in childhood predicts pre-
risk estimates (incidence-rate ratio for the high- mature death. The rate of death from endoge-
est BMI quartile vs. the lowest quartile, 2.70; 95% nous causes in the highest quartile of childhood
CI, 1.70 to 4.31). In contrast, inclusion of diabe- BMI was more than double that in the lowest
tes in the 2-hour glucose model reduced the risk quartile, and the rate in the highest quartile of
estimate for the highest quartile of 2-hour glucose childhood two-hour plasma glucose levels during
levels, and the association between the highest a 75-g oral glucose-tolerance test was 73% higher
and lowest quartiles was not signif icant (inci- than that in the lowest quartile. Although neither
dence-rate ratio, 1.10; 95% CI, 0.72 to 1.68). In Cox
blood pressure nor cholesterol level in childhood,
proportional-hazards models that included 2672 when included as a continuous variable, signif i-
participants, there were no signif icant associa- cantly predicted premature death, childhood hy-
tions between childhood BMI and alcohol depen- pertension increased the risk of premature death
dency in adulthood (incidence-rate ratio per unit from endogenous causes by 57%.
of BMI z score, 1.01; 95% CI, 0.96 to 1.07). The absence of an association between pre-
mature death and cholesterol levels may be due
DI S C U S S I O N partly to the low proportion of deaths due to
cardiovascular disease in this cohort (13.3%).
It is well known that obesity, glucose intolerance, Treatment for any of the predictor traits during
hypertension, and hypercholesterolemia in adult- childhood or during adulthood did not appear to
490 n engl j med 362;6 nejm.org february 11, 2010
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Childhood obesit y a nd pr em at ur e de ath
explain the pattern of association (data not shown). Stockholm between 1921 and 1947, weight gain
No childhood risk factor that was examined sig- between puberty and young adulthood was asso-
nif icantly predicted rates of premature death ciated with cardiovascular disease, diabetes, and
from external causes. death from all causes.22 A limitation of these
Childhood obesity predicted premature death studies is that obesity was uncommon during the
from endogenous, but not external, causes. The study period. For example, of the 2299 children
study was not powered to analyze effects on more in the Welsh study,21 only 92 (4.0%) had a BMI
specif ic categories of cause of death. Including above the 90th percentile for the age-specif ic
only liver-related causes of death in the analysis and sex-specif ic distributions of the 1990 British
reduced the magnitude of the association of pre- population, and British children in 1990 were
mature death with childhood BMI and with the leaner than their contemporary counterparts.24
2-hour glucose level, but the direction and pattern In the Arizona Pima Indians, unlike most
of associations were similar to those observed other ethnic groups, childhood obesity has been
when all endogenous causes of death were in- common for decades.25 It has been estimated that
cluded. at the turn of the 21st century, approximately
We considered whether the relationship be- 15% of U.S. children between the ages of 6 and
tween childhood BMI and premature death re- 19 years (11 million children) were overweight
f lects associations with adiposity or some other or obese,26 a prevalence that is unlikely to de-
component of body mass. Our study began be- cline in the near future27 and that is triple the
fore the availability of modern adiposity measures prevalence among children of the same age in
such as dual-energy x-ray absorptiometry. How- the 1960s.28,29 In the present study, 1394 chil-
ever, we previously reported relationships be- dren (28.7%) were obese (BMI, ≥95th percentile
tween BMI and adipose mass and between adi- on the 2000 CDC growth charts). This prevalence
pose mass and the cardiovascular risk factors in is similar to that observed in contemporary His-
this population19; in that study, BMI and adipos- panic and African-American children.27 Thus, al-
ity were strongly correlated (r>0.96), varying little though we studied a population with high rates
with age and sex, and BMI and adipose mass of obesity and diabetes, our f indings may ref lect
were similarly correlated with the cardiovascular the future burden of premature death among
risk factors. Thus, the observations for childhood contemporary children from other ethnic groups
BMI reported here are likely to ref lect a positive and may be more generalizable than the findings
association between adiposity and rates of pre- in previous studies.
mature death. In this study, we compared mortality rates
In a study involving 508 U.S. adolescents (13 with several clinical risk factors as variables. Ad-
to 18 years of age) who were born between 1922 justing the obesity models for the development of
and 1935, overweight (>75th percentile of the diabetes in adulthood did not signif icantly alter
sample distribution) was associated with increased the risk estimates, whereas adjusting the glucose
rates of death due to coronary heart disease.20 models for subsequent diabetes did attenuate the
Two studies have assessed the relationship be- association between childhood glucose levels and
tween body weight and mortality in European premature death. Hence, dysregulated glucose
birth cohorts from the early 20th century.21,22 In metabolism in childhood may be a mediator of
a study of 2299 Welsh children born between the effects of childhood obesity on mortality
1937 and 1939, there was no association between rates, but it does not appear to be the sole or
childhood BMI and death from cardiovascular dominant factor; however, the association be-
causes.21 However, there was an association be- tween childhood glucose intolerance and prema-
tween childhood BMI and death from all causes; ture death does appear to be mediated by the
the lowest rate of death was seen in the next-to- development of subsequent diabetes.
lowest BMI quartile and the highest rate of death The pattern of the relationships between the
in the highest quartile, suggesting that, as in the risk factors and observed mortality supports the
case of adult Pima Indians,23 a U-shaped relation- view that childhood obesity is an early metabolic
ship exists between obesity and mortality. In the derangement, whereas most of the other risk
second European study, involving 504 overweight factors evolve later. In fact, the predictive power
children and adolescents admitted to hospitals in of a risk score for type 2 diabetes (including
n engl j med 362;6 nejm.org february 11, 2010 491
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
The ne w e ngl a nd jour na l of me dicine
measures of obesity and insulin, blood-pressure, genetic factors have pleiotropic effects on BMI
glucose, and lipid levels) in children is almost and mortality.
entirely dependent on abdominal obesity, whereas Childhood obesity is predictive of excess mor-
in adolescents, the risk profile has evolved to in- tality in several divergent settings,20-22 indicating
clude obesity, hyperglycemia, and dyslipidemia.30 that obesity itself is causally related to either
Our f indings complement those in our previous death or other commonly related factors. Even if
study, which showed that type 2 diabetes, when preventing childhood obesity does not affect the
it occurs during adolescence in this population, risk of death, increased physical activity and
strongly predicts subsequent renal failure and modif ication of diet are likely to have long-term
death.2 benefits. The lack of specific data on such factors
Although there was no signif icant association is a limitation of this study.
between childhood hypercholesterolemia and In summary, obesity in children who do not
death before 55 years of age in this young co- have diabetes is associated with an increased rate
hort, an elevated cholesterol level in childhood of death from endogenous causes during early
may emerge as a significant risk factor and other adulthood, an association that may be partially
causes of death may predominate if the cohort is mediated by the development of glucose intoler-
followed to older ages. Cholesterol levels, how- ance and hypertension in childhood. In contrast,
ever, are lower in American Indians than they the cholesterol level in childhood is not a major
are in most other ethnic groups,31 a f inding that determinant of premature death in this popula-
may partially explain the absence of association tion. Childhood obesity is becoming increasing-
for this trait. The relationship between BMI and ly prevalent around the globe. Our observations,
high-density lipoprotein (HDL) cholesterol is rela- combined with those of other investigators, sug-
tively strong in Pima children (r = −0.3 to −0.6), gest that failure to reverse this trend may have
but the relationship between BMI and total cho- wide-reaching consequences for the quality of
lesterol is weaker (r = 0.1).19 The effect of BMI on life and longevity. Such evidence underscores the
premature death might be attributable in part to importance of preventing obesity starting in the
low HDL-cholesterol concentrations, which were early years of life.
not measured in most of the study participants.
Nevertheless, we speculate that low HDL-choles- Supported by the National Institute of Diabetes and Digestive
and Kidney Diseases (NIDDK) intramural research program. Dr.
terol levels are likely to mediate rather than con- Franks was supported in part by grants from the Swedish Diabe-
found this relationship. tes Association, the Swedish Heart Lung Foundation, the Swedish
It is possible that the relationship between Research Council, Umeå University (Career Development Award),
and the Västerbotten regional health authorit y (Strategic Ap-
childhood BMI and mortality is confounded by pointment 2006-09).
unmeasured lifestyle factors. Nevertheless, obe- No potential conf lict of interest relevant to this article was
sity can be both the cause and the consequence reported.
We thank members of the Gila River Indian Community for
of adverse lifestyle factors such as physical in- participating in this study and for the profound commitment
activity, excessive caloric intake, and specif ic this community has made over the past half century to studies
nutrient preferences. Thus, such factors may be that seek to further our understanding of human health and dis-
ease; the staff of the Diabetes Epidemiology and Clinical Research
important components of the causal pathway be- Section of the NIDDK for conducting the examinations; and Joy
tween obesity and death. It is also possible that C. Bunt, M.D., Ph.D., for comments on the manuscript.
Reference s
1. Olshansk y SJ, Passaro DJ, Hershow U.S. adolescents: the Third National Health 6. Malik S, Wong ND, Franklin SS, et al.
RC, et al. A potential decline in life expec- and Nutrition Examination Survey. Diabe- Impact of the metabolic syndrome on mor-
tancy in the United States in the 21st cen- tes Care 2001;24:834-7. tality from coronary heart disease, cardio-
tury. N Engl J Med 2005;352:1138-45. 4. Andersen LB, Harro M, Sardinha LB, vascular disease, and all causes in United
2. Pavkov ME, Bennett PH, Knowler WC, et al. Physical activity and clustered car- States adults. Circulation 2004;110:1245-
Krakoff J, Sievers ML, Nelson RG. Effect diovascular risk in children: a cross-sec- 50.
of youth-onset type 2 diabetes mellitus on tional study (The European Youth Heart 7. McTigue K, Larson JC, Valoski A, et al.
incidence of end-stage renal disease and Study). Lancet 2006;368:299-304. Mortality and cardiac and vascular out-
mortality in young and middle-aged Pima 5. Lakka HM, Laaksonen DE, Lakka TA, comes in extremely obese women. JAMA
Indians. JAMA 2006;296:421-6. et al. The metabolic syndrome and total 2006;296:79-86.
3. Fagot-Campagna A, Saaddine JB, Fle- and cardiovascular disease mortality in 8. Bennett PH, Burch TA, Miller M. Dia-
gal KM, Beckles GL. Diabetes, impaired middle-aged men. JAMA 2002;288:2709- betes mellitus in American (Pima) Indi-
fasting glucose, and elevated HbA1c in 16. ans. Lancet 1971;2:125-8.
492 n engl j med 362;6 nejm.org february 11, 2010
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Childhood obesit y a nd pr em at ur e de ath
9. Knowler WC, Bennett PH, Hamman Panel on Detection, Evaluation, and Treat- Off icer 2002. London: Department of
RF, Miller M. Diabetes incidence and prev- ment of High Blood Cholesterol in Adults Health, 2002.
alence in Pima Indians: a 19-fold greater (Adult Treatment Panel III): f inal report. 25. Pettitt DJ, Baird HR, Aleck KA, Ben-
incidence than in Rochester, Minnesota. Circulation 2002;106:3143-421. nett PH, Knowler WC. Excessive obesity in
Am J Epidemiol 1978;108:497-505. 18. American Diabetes Association: clini- offspring of Pima Indian women with di-
10. Sievers ML, Nelson RG, Bennett PH. cal practice recommendations 2002. Dia- abetes during pregnancy. N Engl J Med
Adverse mortality experience of a south- betes Care 2002;25:Suppl:S1-S147. 1983;308:242-5.
western American Indian communit y: 19. Lindsay RS, Hanson RL, Roumain J, 26. Child health USA 2004. Rockville, MD:
overall death rates and underlying causes Ravussin E, Knowler WC, Tataranni PA. Department of Health and Human Ser-
of death in Pima Indians. J Clin Epidemiol Body mass index as a measure of adipos- vices, 2004.
1990;43:1231-42. ity in children and adolescents: relation- 27. Ogden CL, Carroll MD, Flegal KM.
11. Report of a WHO consultation: def i- ship to adiposity by dual energy x-ray ab- High body mass index for age among US
nition, diagnosis and classif ication of sorptiometry and to cardiovascular risk children and adolescents, 2003-2006.
diabetes mellitus and its complications. factors. J Clin Endocrinol Metab 2001;86: JAMA 2008;299:2401-5.
Part 1: diagnosis and classif ication of dia- 4061-7. 28. Ogden CL, Carroll MD, Curtin LR,
betes mellitus. Geneva: World Health Or- 20. Must A, Jacques PF, Dallal GE, Bajema McDowell MA, Tabak CJ, Flegal KM. Prev-
ganization, Department of Noncommuni- CJ, Dietz WH. Long-term morbidity and alence of overweight and obesity in the
cable Disease Surveillance, 1999. mortality of overweight adolescents: a fol- United States, 1999-2004. JAMA 2006;295:
12. Franks PW, Looker HC, Kobes S, et al. low-up of the Harvard Growth Study of 1549-55.
Gestational glucose tolerance and risk of 1922 to 1935. N Engl J Med 1992;327: 29. Hedley AA, Ogden CL, Johnson CL,
type 2 diabetes in young Pima Indian off- 1350-5. Carroll MD, Curtin LR, Flegal KM. Preva-
spring. Diabetes 2006;55:460-5. 21. Gunnell DJ, Frankel SJ, Nanchahal K, lence of overweight and obesity among US
13. Ewing JA. Detecting alcoholism: the Peters TJ, Davey Smith G. Childhood obe- children, adolescents, and adults, 1999-
CAGE questionnaire. JAMA 1984;252: sit y and adult cardiovascular mortalit y: 2002. JAMA 2004;291:2847-50.
1905-7. a 57-y follow-up study based on the Boyd 30. Franks PW, Hanson RL, Knowler WC,
14. Prentice RL, Kalbf leisch JD. Hazard Orr cohort. Am J Clin Nutr 1998;67:1111-8. et al. Childhood predictors of young-
rate models with covariates. Biometrics 22. DiPietro L, Mossberg HO, Stunkard AJ. onset type 2 diabetes. Diabetes 2007;56:
1979;35:25-39. A 40-year history of overweight children 2964-72.
15. Center for Disease Control and Pre- in Stockholm: life-time overweight, mor- 31. Howard BV, Davis MP, Pettitt DJ,
vention. CDC growth charts. Washington, bidity, and mortality. Int J Obes Relat Me- Knowler WC, Bennett PH. Plasma and
DC: National Center for Health Statistics, tab Disord 1994;18:585-90. lipoprotein cholesterol and triglyceride
2000 (publication no. 314). 23. Hanson RL, McCance DR, Jacobsson concentrations in the Pima Indians: dis-
16. The Fourth Report on the Diagnosis, LT, et al. The U-shaped association be- tributions differing from those of Cauca-
Evaluation, and Treatment of High Blood tween body mass index and mortality: sians. Circulation 1983;68:714-24.
Pressure in Children and Adolescents. Pe- relationship with weight gain in a Native Copyright © 2010 Massachusetts Medical Society.
diatrics 2004;114:555-76. American population. J Clin Epidemiol
17. Third Report of the National Choles- 1995;48:903-16.
terol Education Program (NCEP) Expert 24. Annual report of the Chief Medical
full text of all journal articles on the world wide web
Access to the complete contents of the Journal on the Internet is free to all subscribers. To use this Web site, subscribers should
go to the Journal’s home page (NEJM.org) and register by entering their names and subscriber numbers as they appear on their
mailing labels. After this one-time registration, subscribers can use their passwords to log on for electronic access to the entire
Journal from any computer that is connected to the Internet. Features include a library of all issues since January 1993 and
abstracts since January 1975, a full-text search capacity, and a personal archive for saving articles and search results of interest.
All articles can be printed in a format that is virtually identical to that of the typeset pages. Beginning 6 months after
publication, the full text of all Original Articles and Special Articles is available free to nonsubscribers.
n engl j med 362;6 nejm.org february 11, 2010 493
The New England Journal of Medicine
Downloaded from nejm.org on July 27, 2017. For personal use only. No other uses without permission.
Copyright © 2010 Massachusetts Medical Society. All rights reserved.
Anda mungkin juga menyukai
- Jurnal Observasional AnalitikDokumen9 halamanJurnal Observasional AnalitikJethro BudimanBelum ada peringkat
- New England Journal Medicine: The ofDokumen9 halamanNew England Journal Medicine: The ofegimaruBelum ada peringkat
- Genetic Risk, Adherence To A Healthy Lifestyle, and Coronary DiseaseDokumen10 halamanGenetic Risk, Adherence To A Healthy Lifestyle, and Coronary DiseaseNurul Falah KalokoBelum ada peringkat
- Orthostatic Hypotension Predicts Mortality in Middle-Aged AdultsDokumen8 halamanOrthostatic Hypotension Predicts Mortality in Middle-Aged AdultsTaufiqur Rohman ChimeraBelum ada peringkat
- Orginal Healthy Aging Index 2014Dokumen7 halamanOrginal Healthy Aging Index 2014bin linBelum ada peringkat
- Characterization of Thyroid Abnormalities in A Large Cohort ofDokumen17 halamanCharacterization of Thyroid Abnormalities in A Large Cohort ofyoyo sajaBelum ada peringkat
- Copd JournalDokumen8 halamanCopd JournalKeanuBelum ada peringkat
- Fetal Origins Research Study (Prelim Exam)Dokumen8 halamanFetal Origins Research Study (Prelim Exam)raymund pabilarioBelum ada peringkat
- Dietary Factors and Risk of Non-Hodgkin Lymphoma in Men and WomenDokumen10 halamanDietary Factors and Risk of Non-Hodgkin Lymphoma in Men and WomenJose Angel AbadíaBelum ada peringkat
- Stress MortalitiyDokumen11 halamanStress Mortalitiyerik gunawanBelum ada peringkat
- Metabolic Syndrome and Risk of Mortality in Middle-Aged Versus Elderly Individuals: The Nord-Trøndelag Health Study (HUNT)Dokumen8 halamanMetabolic Syndrome and Risk of Mortality in Middle-Aged Versus Elderly Individuals: The Nord-Trøndelag Health Study (HUNT)Eriekafebriayana RBelum ada peringkat
- Scaglione 2015Dokumen7 halamanScaglione 2015Marlyn SuciningtiasBelum ada peringkat
- Is There A Relationship Between Periodontal Disease and Causes of Death? A Cross Sectional StudyDokumen6 halamanIs There A Relationship Between Periodontal Disease and Causes of Death? A Cross Sectional StudyYaarit IustainBelum ada peringkat
- Prospective Investigation of Major Dietary PatternsDokumen7 halamanProspective Investigation of Major Dietary PatternsJohn SammutBelum ada peringkat
- Nihms 98009Dokumen19 halamanNihms 98009folkloriantaroBelum ada peringkat
- Ni Hms 366201Dokumen17 halamanNi Hms 366201Habiburrahman EffendyBelum ada peringkat
- Serum Taurine and Risk of Coronary Heart Disease: A Prospective, Nested Case-Control StudyDokumen10 halamanSerum Taurine and Risk of Coronary Heart Disease: A Prospective, Nested Case-Control StudyThalia KarampasiBelum ada peringkat
- Suicide Risk in Primary Care Patients With Major Physical DiseasesDokumen9 halamanSuicide Risk in Primary Care Patients With Major Physical DiseasesJ_LOOBelum ada peringkat
- KWF 113Dokumen11 halamanKWF 113Residentes RadiologiaHCEBelum ada peringkat
- Martini2012 PDFDokumen10 halamanMartini2012 PDFSofia ImaculataBelum ada peringkat
- 1918 FullDokumen10 halaman1918 Fulljacopo pruccoliBelum ada peringkat
- Sleep Medicine: Brianna L. Pergola, Sheniz Moonie, Jennifer Pharr, Timothy Bungum, Julia L. AndersonDokumen8 halamanSleep Medicine: Brianna L. Pergola, Sheniz Moonie, Jennifer Pharr, Timothy Bungum, Julia L. AndersonPatricio RodríguezBelum ada peringkat
- NIH Public Access: Placental Histomorphometry in Gestational Diabetes MellitusDokumen13 halamanNIH Public Access: Placental Histomorphometry in Gestational Diabetes MellitusMayra PereiraBelum ada peringkat
- Alzheimer S Dementia - 2022 - Zhang - Midlife Lipid and Glucose Levels Are Associated With Alzheimer S DiseaseDokumen13 halamanAlzheimer S Dementia - 2022 - Zhang - Midlife Lipid and Glucose Levels Are Associated With Alzheimer S DiseaseMarianaBelum ada peringkat
- Godfrey2000 Godfrey, K. M., & Barker, D. J. (2000) - Fetal Nutrition and Adult Disease.Dokumen9 halamanGodfrey2000 Godfrey, K. M., & Barker, D. J. (2000) - Fetal Nutrition and Adult Disease.Nancy Aidée Reyes MéndezBelum ada peringkat
- Epidemiological Study of Clinical and Laboratory Profiles of Patients Woth ALL at DR SoetomoDokumen5 halamanEpidemiological Study of Clinical and Laboratory Profiles of Patients Woth ALL at DR SoetomoJulia Intan Permata SariBelum ada peringkat
- Original Contribution Increased Prevalence of Sleep-Disordered Breathing in AdultsDokumen12 halamanOriginal Contribution Increased Prevalence of Sleep-Disordered Breathing in AdultsAsti Sauna MentariBelum ada peringkat
- Morrison Metabolic Syndrome in Childhood Predicts Adult Cardiovascular Disease 25 Years LateDokumen8 halamanMorrison Metabolic Syndrome in Childhood Predicts Adult Cardiovascular Disease 25 Years LatePaty BritoBelum ada peringkat
- The Relation of Overweight To Cardiovascular Risk Factors Among Children and Adolescents: The Bogalusa Heart StudyDokumen10 halamanThe Relation of Overweight To Cardiovascular Risk Factors Among Children and Adolescents: The Bogalusa Heart StudyaocuBelum ada peringkat
- Dieta y CancerDokumen11 halamanDieta y CancerJose Angel AbadíaBelum ada peringkat
- Risk Factors For Necrotizing Enterocolitis Associated MortalityDokumen7 halamanRisk Factors For Necrotizing Enterocolitis Associated MortalityvaleriaBelum ada peringkat
- Melinder - 2015 - Fitness-Bowel Disease RiskDokumen8 halamanMelinder - 2015 - Fitness-Bowel Disease RiskCLC Area 9Belum ada peringkat
- Relation of Sleep-Disordered Breathing To Cardiovascular Disease RiskDokumen10 halamanRelation of Sleep-Disordered Breathing To Cardiovascular Disease RiskRBelum ada peringkat
- Risk Factors For Sleep-Disordered Breathing in Children: Associations With Obesity, Race, and Respiratory ProblemsDokumen6 halamanRisk Factors For Sleep-Disordered Breathing in Children: Associations With Obesity, Race, and Respiratory ProblemsMiral BassBelum ada peringkat
- ARO SOCRE Predictor Mortalidad 2015Dokumen13 halamanARO SOCRE Predictor Mortalidad 2015raul serranoBelum ada peringkat
- 1993 - BARKER Et Al - Fetal Nutrition and Cardiovascular Disease in Adult LifeDokumen4 halaman1993 - BARKER Et Al - Fetal Nutrition and Cardiovascular Disease in Adult LifeSamanta MonteiroBelum ada peringkat
- Risk Factors For Autism. Perinatal Factors Parental Psychiatric History and Socioeconomic StatusDokumen10 halamanRisk Factors For Autism. Perinatal Factors Parental Psychiatric History and Socioeconomic StatusJulio CesarBelum ada peringkat
- Ni Hms 690786Dokumen17 halamanNi Hms 690786Elaine IllescasBelum ada peringkat
- DZ 11Dokumen5 halamanDZ 11licenteBelum ada peringkat
- Peters 2014Dokumen10 halamanPeters 2014Andrew E P SunardiBelum ada peringkat
- Dietary Patterns, Subclinical InflammationDokumen9 halamanDietary Patterns, Subclinical InflammationJohn SammutBelum ada peringkat
- Fresh Fruit Consumption and Major Cardiovascular Disease in ChinaDokumen12 halamanFresh Fruit Consumption and Major Cardiovascular Disease in ChinaTito Tri SaputraBelum ada peringkat
- Depression and Increased Mortality in Diabetes: Unexpected Causes of DeathDokumen8 halamanDepression and Increased Mortality in Diabetes: Unexpected Causes of DeathAna LemesBelum ada peringkat
- The Genetics of Sudden Infant Death SyndromeDokumen19 halamanThe Genetics of Sudden Infant Death SyndromeBoroghina StelutaBelum ada peringkat
- Ioi70171 2461 2468Dokumen8 halamanIoi70171 2461 2468jimmyneutron1337Belum ada peringkat
- Diarrhoea Morbidity and Mortality in Older Children Adolescents and AdultsDokumen12 halamanDiarrhoea Morbidity and Mortality in Older Children Adolescents and AdultsDelia KrisnasariBelum ada peringkat
- Cardiovascular Risk Factors Among Young AdultsDokumen8 halamanCardiovascular Risk Factors Among Young AdultsJuan Alejandro Bastidas VejarBelum ada peringkat
- Pa Nag Iot Akos 2015Dokumen7 halamanPa Nag Iot Akos 2015Gabriel ParizotoBelum ada peringkat
- Namasitean 124Dokumen6 halamanNamasitean 124Eriekafebriayana RBelum ada peringkat
- Lifestyle, not genetics, drives most early heart diseaseDokumen3 halamanLifestyle, not genetics, drives most early heart diseasechloe a tBelum ada peringkat
- A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and ValidationDokumen11 halamanA New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and ValidationindriBelum ada peringkat
- Joc15132 2120 2127Dokumen8 halamanJoc15132 2120 2127caio personalBelum ada peringkat
- Endokrin4 PDFDokumen6 halamanEndokrin4 PDFAdelina Wahyuni LubisBelum ada peringkat
- Disparities in Periodontitis Prevalence Among Chronic Kidney Disease PatientsDokumen5 halamanDisparities in Periodontitis Prevalence Among Chronic Kidney Disease PatientsKristelle HernandezBelum ada peringkat
- 4 - Health Consequences of Obesity 2012Dokumen5 halaman4 - Health Consequences of Obesity 2012Mateus CarvalhoBelum ada peringkat
- Am J Clin Nutr 2004 Caulfield 193 8Dokumen6 halamanAm J Clin Nutr 2004 Caulfield 193 8Seik Soi100% (1)
- Foreign Body Asphyxia - A Pereventable Cause of Death in HR ElderlyDokumen5 halamanForeign Body Asphyxia - A Pereventable Cause of Death in HR ElderlyTarek Hefz Al RahmanBelum ada peringkat
- Journal Childhood ObecityDokumen10 halamanJournal Childhood ObecitysiscaBelum ada peringkat
- Pregnancy SmokingDokumen9 halamanPregnancy SmokingSex & Gender Women's Health CollaborativeBelum ada peringkat
- Complementary and Alternative Medical Lab Testing Part 12: NeurologyDari EverandComplementary and Alternative Medical Lab Testing Part 12: NeurologyBelum ada peringkat
- Farmakologi: Allopurinol Dan AsetaminofenDokumen2 halamanFarmakologi: Allopurinol Dan AsetaminofenDias PradikaBelum ada peringkat
- Farmakologi: Allopurinol Dan AsetaminofenDokumen2 halamanFarmakologi: Allopurinol Dan AsetaminofenDias PradikaBelum ada peringkat
- BAB IpermenDokumen14 halamanBAB IpermenDias PradikaBelum ada peringkat
- Nej MP 1300375Dokumen9 halamanNej MP 1300375Dias PradikaBelum ada peringkat
- 2 CDokumen8 halaman2 Cdyah sekar ayuBelum ada peringkat
- Urin 24 Jam Pada Ibu HamilDokumen8 halamanUrin 24 Jam Pada Ibu HamilellunelinBelum ada peringkat
- H1N1 Influenza A Disease - Information For Health ProfessionalsDokumen2 halamanH1N1 Influenza A Disease - Information For Health ProfessionalsDias PradikaBelum ada peringkat
- Perspective: New England Journal MedicineDokumen4 halamanPerspective: New England Journal MedicineDias PradikaBelum ada peringkat
- H1N1 Influenza A Disease - Information For Health ProfessionalsDokumen2 halamanH1N1 Influenza A Disease - Information For Health ProfessionalsDias PradikaBelum ada peringkat
- Perspective: New England Journal MedicineDokumen4 halamanPerspective: New England Journal MedicineDias PradikaBelum ada peringkat
- Prevention and Treatment of Seasonal Influenza: Clinical PracticeDokumen7 halamanPrevention and Treatment of Seasonal Influenza: Clinical PracticeDias PradikaBelum ada peringkat
- Innate Immunity in Asthma: EditorialDokumen3 halamanInnate Immunity in Asthma: EditorialDias PradikaBelum ada peringkat
- Vaksin InfluenzaDokumen14 halamanVaksin InfluenzaBellavia FransiscaBelum ada peringkat
- Innate Immunity in Asthma: EditorialDokumen3 halamanInnate Immunity in Asthma: EditorialDias PradikaBelum ada peringkat
- Stalking Influenza Diversity With A Universal Antibody: Clinical Implications of Basic ResearchDokumen2 halamanStalking Influenza Diversity With A Universal Antibody: Clinical Implications of Basic ResearchDias PradikaBelum ada peringkat
- An Obesity-Associated FTO Gene Variant and Increased Energy Intake in ChildrenDokumen13 halamanAn Obesity-Associated FTO Gene Variant and Increased Energy Intake in ChildrenDias PradikaBelum ada peringkat
- Perspective: New England Journal MedicineDokumen4 halamanPerspective: New England Journal MedicineDias PradikaBelum ada peringkat
- Nej MP 1400034Dokumen3 halamanNej MP 1400034Dias PradikaBelum ada peringkat
- Hameed - Heart Failure in PregnancyDokumen26 halamanHameed - Heart Failure in PregnancyDias PradikaBelum ada peringkat
- PhysioEx Exercise 3-123Dokumen16 halamanPhysioEx Exercise 3-123NicoleBelum ada peringkat
- Post-Operative Care, DSTC Online, Jun.2021Dokumen38 halamanPost-Operative Care, DSTC Online, Jun.2021tepat rshsBelum ada peringkat
- HER CCB in Crisis HypertensionDokumen22 halamanHER CCB in Crisis HypertensionHandi Wijaya HasanBelum ada peringkat
- Biology Chapter 3 Notes PDFDokumen5 halamanBiology Chapter 3 Notes PDFLisa KylBelum ada peringkat
- Encyclopedia of Biological Chemistry - Vol - 1Dokumen895 halamanEncyclopedia of Biological Chemistry - Vol - 1aishbiyaBelum ada peringkat
- Two-Dimensional Inverse DynamicsDokumen22 halamanTwo-Dimensional Inverse DynamicsEric Urbina SantibañezBelum ada peringkat
- FULLY REVISED EDITION: NEET RANK EDGE SERIES BIOLOGYDokumen111 halamanFULLY REVISED EDITION: NEET RANK EDGE SERIES BIOLOGYSam MishraBelum ada peringkat
- Wanted: On The Organelle TrailDokumen5 halamanWanted: On The Organelle TrailMOISES DEANDABelum ada peringkat
- Fermented Milk Products May Improve HealthDokumen7 halamanFermented Milk Products May Improve Healthmilu1312Belum ada peringkat
- Isospora SuisDokumen35 halamanIsospora SuisAfif DihanBelum ada peringkat
- Nervous System 4 PDF FreeDokumen15 halamanNervous System 4 PDF FreeZedy GullesBelum ada peringkat
- Respiratory SystemhjhuDokumen23 halamanRespiratory SystemhjhuSara ABelum ada peringkat
- 1 s2.0 S0923181121000840 MainDokumen16 halaman1 s2.0 S0923181121000840 Mainleon4009Belum ada peringkat
- General - GITDokumen90 halamanGeneral - GITAbdallah GamalBelum ada peringkat
- Emile Coue's - Law of Reversed EffortDokumen2 halamanEmile Coue's - Law of Reversed Effortkevin100% (1)
- Monosaccharides: AlpineDokumen12 halamanMonosaccharides: AlpineReigner Jay B. EscartinBelum ada peringkat
- Tissues and Cells Part 1 - Grade 7 - BiologyDokumen4 halamanTissues and Cells Part 1 - Grade 7 - BiologyYasmina Jamal GholoumBelum ada peringkat
- 09 Tusk MaskDokumen6 halaman09 Tusk MaskaksinuBelum ada peringkat
- Author 'S Accepted ManuscriptDokumen46 halamanAuthor 'S Accepted ManuscriptRaul DoctoBelum ada peringkat
- OCR Biology F211 Cells, Exchange and Transport Revision NotesDokumen4 halamanOCR Biology F211 Cells, Exchange and Transport Revision Notes24wrightphilip100% (1)
- NS Conduction and NeurotransmissionDokumen23 halamanNS Conduction and Neurotransmissionbav92100% (1)
- USMLE Flashcards: Behavioral Science - Side by SideDokumen96 halamanUSMLE Flashcards: Behavioral Science - Side by SideMedSchoolStuffBelum ada peringkat
- Case Study Group 2Dokumen40 halamanCase Study Group 2Mary Grace MasBelum ada peringkat
- Photosynthesis Science Presentation in Green Beige Illustrative Style - 20230920 - 192536 - 0000Dokumen11 halamanPhotosynthesis Science Presentation in Green Beige Illustrative Style - 20230920 - 192536 - 0000Rei B.XBelum ada peringkat
- 1987 NBDE 1 ASDA (Biochemistry & Physiology)Dokumen63 halaman1987 NBDE 1 ASDA (Biochemistry & Physiology)Karol BryantBelum ada peringkat
- PNLE Sample Test QuestionsDokumen12 halamanPNLE Sample Test QuestionsHam-Ham LootBelum ada peringkat
- Hematology UnitDokumen8 halamanHematology UnitMary CabalceBelum ada peringkat
- J. Biol. Chem.-1989-Boggaram-11421-7Dokumen7 halamanJ. Biol. Chem.-1989-Boggaram-11421-7Patrick RamosBelum ada peringkat
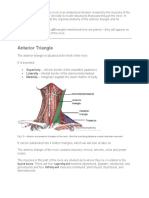
- The Anterior and Posterior Triangles of The NeckDokumen10 halamanThe Anterior and Posterior Triangles of The NeckClair TomBelum ada peringkat
- UG022524 International GCSE in Human Biology 4HB0 For PrintDokumen60 halamanUG022524 International GCSE in Human Biology 4HB0 For Printneoiq5719Belum ada peringkat