Polar Bears and Penguins
Diunggah oleh
Prueba3Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Polar Bears and Penguins
Diunggah oleh
Prueba3Hak Cipta:
Format Tersedia
Polar Bears and Penguins Lesson Guide: Investigation IV – Lesson 4
Lesson Guide: Polar Bears and Penguins
Investigation IV – Lesson 4
In this lesson you will be exploring polarity and bonding between
atoms in greater detail. A comic book will provide new information
about these topics and will introduce you to the concept of electronegativity, which
helps us to understand partial charges.
Answer the following questions:
Consider the following illustration:
∑ Draw the Lewis dot structure for HCl.
∑ If the penguin represents a hydrogen atom and the polar bear represents a
chlorine atom, what does the ice cream represent in the drawing? What do you
think the picture is trying to illustrate?
∑ Would HCl be attracted to the charged wand? Explain your thinking.
Purpose: In this lesson you will be exploring polarity and bonding between atoms in
greater detail. A comic book will provide new information about these topics and will
introduce you to the concept of electronegativity, which helps us to understand partial
charges.
Use the comic book called “The Bare Essentials of Polarity” to answer the following
questions.
Smells © UC Regents, LHS Living by Chemistry, 2003. 85
Lesson Guide: Investigation IV – Lesson 4 Polar Bears and Penguins
1. How does the comic book define a “polar molecule?”
2. Define electronegativity as you understand it, after reading the first two pages of the
comic book.
3. Interpret the picture at the bottom of page 1. Explain how the iceberg, penguins,
and polar bears represent trends in electronegativity.
4. What is the artist trying to represent when there are two polar bears arm wrestling
together, or two penguins arm wrestling together?
5. What three types of bonds are represented on page 3 of the comic book? What
happens to the bonding electrons in each type of bond?
6. Explain why there are four scoops of ice cream in the illustration of O2 on page 3.
7. What do the six scoops of ice cream represent in the illustration of N2 on page 4?
8. Describe what you think is happening to the penguin in the CO2 molecule in the
picture on page 4.
9. Name three things that the picture of CO2 on page 4 illustrates about the molecule.
10. Describe what you think is happening to the penguins in the illustration of H2O on
page 4.
11. Explain what you think the crossed arrow represents in the comic book.
12. What are the two definitions of “dipole” given in the comic book?
Making Sense Question:
What does electronegativity have to do with polarity?
If you finish early. . .
Using polar bears and penguins, create an illustration showing a hydrogen sulfide
molecule, H2S. (Hint: You may wish to start with a Lewis dot structure.)
86 Smells © UC Regents, LHS Living by Chemistry, 2003.
Polar Bears and Penguins Lesson Guide: Investigation IV – Lesson 4
When two atoms with different electronegativities are bonded together, they tend to
attract the bonded electrons to different degrees. This causes the electrons to spend
more time around one of the atoms, resulting in a partial negative charge on this atom.
This tendency of an atom to attract electrons shared between two atoms is called
electronegativity. An atom that strongly attracts the shared electrons is considered
highly electronegative. The atom with lower electronegativity will end up with a partial
positive charge on it. The result is a polar bond. Chemists have a specific name for a
molecule that has two poles - it is called a dipole.
Polar molecules are also called dipoles. The prefix di- means two. A dipole is a molecule
with two partially charged ends, or poles. Chemists refer to polar molecules as dipoles
and they also say that molecules with polar bonds have dipoles. These multiple
definitions can be a bit confusing.
Answer the following questions:
HI molecule
I H
∑ Is the bond between these atoms polar? Explain your reasoning.
∑ How would the atoms be portrayed in the comic book – as polar bears, penguins,
or both? Explain.
Smells © UC Regents, LHS Living by Chemistry, 2003. 87
Lesson Guide: Investigation IV – Lesson 4 Polar Bears and Penguins
88 Smells © UC Regents, LHS Living by Chemistry, 2003.
Polar Bears and Penguins Lesson Guide: Investigation IV – Lesson 4
Smells © UC Regents, LHS Living by Chemistry, 2003. 89
Lesson Guide: Investigation IV – Lesson 4 Polar Bears and Penguins
90 Smells © UC Regents, LHS Living by Chemistry, 2003.
Polar Bears and Penguins Lesson Guide: Investigation IV – Lesson 4
Smells © UC Regents, LHS Living by Chemistry, 2003. 91
Lesson Guide: Investigation IV – Lesson 4 Polar Bears and Penguins
Complete the following for homework:
1. Using polar bears and penguins, create an illustration showing an ammonia
molecule, NH3. (Hint: You may wish to start with a Lewis dot structure.)
92 Smells © UC Regents, LHS Living by Chemistry, 2003.
Anda mungkin juga menyukai
- DLP For ObservationDokumen7 halamanDLP For ObservationElvie CristobalBelum ada peringkat
- A SIMPLE GUIDE TO POPULAR PHYSICS (COLOUR EDITION): AN INTRODUCTION TO PARTICLES, QUANTUM PHYSICS AND COSMOLOGY FOR ABSOLUTE BEGINNERSDari EverandA SIMPLE GUIDE TO POPULAR PHYSICS (COLOUR EDITION): AN INTRODUCTION TO PARTICLES, QUANTUM PHYSICS AND COSMOLOGY FOR ABSOLUTE BEGINNERSBelum ada peringkat
- Astm E1820Dokumen48 halamanAstm E1820dddBelum ada peringkat
- Chapter 2Dokumen10 halamanChapter 2AnonymousBelum ada peringkat
- Highlight Key ConceptsDokumen6 halamanHighlight Key ConceptsJojo The wonderful0% (1)
- Lesson Plan For LEWIS DOT STRUCTUREDokumen6 halamanLesson Plan For LEWIS DOT STRUCTUREHAROLD PAYUNAN100% (2)
- AP Bio Chapter 2 Active Reading GuideDokumen10 halamanAP Bio Chapter 2 Active Reading Guidesam quo yay100% (1)
- Abellanosa-Activity2 5Dokumen3 halamanAbellanosa-Activity2 5Venesse Aulga AbellanosaBelum ada peringkat
- G9 Q2 W1 Atomic ModelsDokumen20 halamanG9 Q2 W1 Atomic ModelsCherrilyn EnverzoBelum ada peringkat
- Covalentbondsse KeyDokumen6 halamanCovalentbondsse Keynona wayne dela peña59% (22)
- Physical Science - M3 - Polarity of MoleculesDokumen15 halamanPhysical Science - M3 - Polarity of MoleculesJodi RempilloBelum ada peringkat
- DLP On Covalent BondDokumen4 halamanDLP On Covalent BondHI M MABelum ada peringkat
- Jose P. Laurel Sr. High SchoolDokumen8 halamanJose P. Laurel Sr. High SchoolEricha SolomonBelum ada peringkat
- Physical Science Modules Week 2Dokumen6 halamanPhysical Science Modules Week 2RODJHEN ANNE P. BARQUILLABelum ada peringkat
- 12 Interview Question Related To Non - Destructive TestingDokumen4 halaman12 Interview Question Related To Non - Destructive TestingHary adiBelum ada peringkat
- Atoms: Inside OutDokumen17 halamanAtoms: Inside OutSanJoseHS67% (3)
- Quarter 1 - LM1Dokumen21 halamanQuarter 1 - LM1Wincy Rose PASQUILBelum ada peringkat
- London+Dumas+ +PolarBears+&Amp;+PenguinsDokumen7 halamanLondon+Dumas+ +PolarBears+&Amp;+Penguinsespikener26Belum ada peringkat
- 4.2.8 Polar Bears and Penguins WSDokumen2 halaman4.2.8 Polar Bears and Penguins WSLuna SantiagoBelum ada peringkat
- Chapter 2 Chemical Context of LifeDokumen8 halamanChapter 2 Chemical Context of LifeJADEN MANNBelum ada peringkat
- Molecular PolarityDokumen43 halamanMolecular Polaritychubbskie00Belum ada peringkat
- Physical ScienceDokumen22 halamanPhysical ScienceMary Joyce Nicolas ClementeBelum ada peringkat
- Genchem 1 DLPDokumen7 halamanGenchem 1 DLPDhevin VergaraBelum ada peringkat
- Home Test Game Contact: Join AUS-e-TUTEDokumen3 halamanHome Test Game Contact: Join AUS-e-TUTEmacgyverBelum ada peringkat
- Chapter 2 Active Reading GuideDokumen10 halamanChapter 2 Active Reading GuideAnonymous y0j9r8UBelum ada peringkat
- CH - 4 Carbon and Its CompoundsDokumen21 halamanCH - 4 Carbon and Its CompoundsClimaxBelum ada peringkat
- Corvalent Bonds LabDokumen7 halamanCorvalent Bonds LabAnthony GergesBelum ada peringkat
- Lesson Plan in Physical Science Molecular PolarityDokumen8 halamanLesson Plan in Physical Science Molecular Polarityartjill printingBelum ada peringkat
- Organic Chemistry Bonding and StructureDokumen46 halamanOrganic Chemistry Bonding and StructureCina YemataBelum ada peringkat
- CH - 4 Carbon and Its CompoundsDokumen21 halamanCH - 4 Carbon and Its CompoundsVensBelum ada peringkat
- Chemistry For Changing Times 14th Edition Hill Mccreary Solution ManualDokumen24 halamanChemistry For Changing Times 14th Edition Hill Mccreary Solution ManualElaineStewartieog100% (50)
- The Polarity of-WPS OfficeDokumen7 halamanThe Polarity of-WPS OfficeJoebelle GiananBelum ada peringkat
- Lewis Structure of C: O C ODokumen4 halamanLewis Structure of C: O C ODukeBelum ada peringkat
- Admmodule s1112ps Iiic 15Dokumen12 halamanAdmmodule s1112ps Iiic 15Lebz RicaramBelum ada peringkat
- Olivia Bennett - Gizmo-Covalent Bonds - 9835942Dokumen5 halamanOlivia Bennett - Gizmo-Covalent Bonds - 9835942Livi sBelum ada peringkat
- Fundamentals of General and Physical Chemistry Author J E Imanah, A O OladebeyeDokumen331 halamanFundamentals of General and Physical Chemistry Author J E Imanah, A O OladebeyeAbinow SBelum ada peringkat
- Dwnload Full Biology 10th Edition Solomon Solutions Manual PDFDokumen35 halamanDwnload Full Biology 10th Edition Solomon Solutions Manual PDFhudsonlosjames100% (13)
- Rizka Dwi Apriliani - 2313023054 - 2BDokumen5 halamanRizka Dwi Apriliani - 2313023054 - 2BRizka Dwi AprilianiBelum ada peringkat
- Chem Recovery Final Exam Review 2014Dokumen6 halamanChem Recovery Final Exam Review 2014api-33768097Belum ada peringkat
- Atomic Structure: Inside the AtomDokumen30 halamanAtomic Structure: Inside the Atommark08311980Belum ada peringkat
- UntitledDokumen18 halamanUntitledDorama AikaBelum ada peringkat
- DR ABRAHAM OLADEBEYE General Principles of ChemistryOER2031359Dokumen212 halamanDR ABRAHAM OLADEBEYE General Principles of ChemistryOER2031359KwongYew NguBelum ada peringkat
- Bonding 221217 205618Dokumen16 halamanBonding 221217 205618Ibse ussoBelum ada peringkat
- Self-Learning: Advanced Chemistry Antipolo City National Science and Technology High SchoolDokumen12 halamanSelf-Learning: Advanced Chemistry Antipolo City National Science and Technology High SchoolMikel SorianoBelum ada peringkat
- Chemical Bonding & Molecular Structure: Calculating Formal Charges and Understanding Bond ParametersDokumen3 halamanChemical Bonding & Molecular Structure: Calculating Formal Charges and Understanding Bond ParametersLakshaya SainiBelum ada peringkat
- Sch4uc Unit 1 Lesson 04Dokumen22 halamanSch4uc Unit 1 Lesson 04Luis David Lazo CondoriBelum ada peringkat
- Solution Manual For Chemistry An Atoms First Approach 2nd Edition by Zumdahl ISBN 1305079248 9781305079243Dokumen30 halamanSolution Manual For Chemistry An Atoms First Approach 2nd Edition by Zumdahl ISBN 1305079248 9781305079243henryarmstrongypajbizoqe100% (28)
- Module 3 and 4 Physical ScienceDokumen11 halamanModule 3 and 4 Physical ScienceBlake DoomedBelum ada peringkat
- Reflection 3 Chem LecDokumen3 halamanReflection 3 Chem LecPaul Winston RegaladoBelum ada peringkat
- PHY SCI DLP Q3 Week 2Dokumen15 halamanPHY SCI DLP Q3 Week 2Radish CucumberBelum ada peringkat
- Chapter 4 PDFDokumen138 halamanChapter 4 PDFRoopaBelum ada peringkat
- VSEPRDokumen14 halamanVSEPRKimsan Ong100% (1)
- 10th - SC - Ch04 - Carbon - and - Its - Compounds - EnglishDokumen107 halaman10th - SC - Ch04 - Carbon - and - Its - Compounds - Englishshivakumara k tBelum ada peringkat
- Solution Manual For Biology 13th Edition Sylvia Mader Michael WindelspechtDokumen38 halamanSolution Manual For Biology 13th Edition Sylvia Mader Michael Windelspechtoutbleatbesnoww4rg100% (18)
- M5A123Dokumen2 halamanM5A123valkyBelum ada peringkat
- November 8, 2022Dokumen4 halamanNovember 8, 2022Melanie CoronaBelum ada peringkat
- The Theory of The Chemical Bond: A Textbook For Chemistry 2160Dokumen38 halamanThe Theory of The Chemical Bond: A Textbook For Chemistry 2160Chris DuBelum ada peringkat
- C9e Answers Active Reading 02Dokumen6 halamanC9e Answers Active Reading 02Jaden VenturaBelum ada peringkat
- CHM 102 NotesDokumen38 halamanCHM 102 NotesagboanthonyokpeBelum ada peringkat
- Physical Science: Quarter 1 - Module 3: Polarity of MoleculesDokumen27 halamanPhysical Science: Quarter 1 - Module 3: Polarity of MoleculesArthur Laurel100% (1)
- Chemistry Fundamentals for ElectroplatersDokumen40 halamanChemistry Fundamentals for ElectroplatersAlejandro AvalosBelum ada peringkat
- AcceptanceDokumen5 halamanAcceptancePrueba3Belum ada peringkat
- Visiting Canada: Requirements, Activities, and RecommendationsDokumen5 halamanVisiting Canada: Requirements, Activities, and RecommendationsPrueba3Belum ada peringkat
- Attributed To Trying To Impress American Cultural Values On GermansDokumen3 halamanAttributed To Trying To Impress American Cultural Values On GermansPrueba3Belum ada peringkat
- What Is The Community That Surrounds The Narrator ("Bull Hands") in "The Moths"? What Is It Like?Dokumen1 halamanWhat Is The Community That Surrounds The Narrator ("Bull Hands") in "The Moths"? What Is It Like?Prueba3Belum ada peringkat
- YessicaDokumen1 halamanYessicaPrueba3Belum ada peringkat
- YessicaDokumen1 halamanYessicaPrueba3Belum ada peringkat
- Attributed To Trying To Impress American Cultural Values On GermansDokumen3 halamanAttributed To Trying To Impress American Cultural Values On GermansPrueba3Belum ada peringkat
- Visiting Canada: Requirements, Activities, and RecommendationsDokumen5 halamanVisiting Canada: Requirements, Activities, and RecommendationsPrueba3Belum ada peringkat
- What Makes A Great TED TalkDokumen4 halamanWhat Makes A Great TED TalkPrueba3Belum ada peringkat
- Monday Tuesday Wednesday Thursday FridayDokumen1 halamanMonday Tuesday Wednesday Thursday FridayPrueba3Belum ada peringkat
- The MPC and The MultiplierDokumen1 halamanThe MPC and The MultiplierPrueba3Belum ada peringkat
- EconomyDokumen1 halamanEconomyPrueba3Belum ada peringkat
- Cited: Arnold, Roger A. Macroeconomics. 12th Ed., Cengage Learning, 2016Dokumen1 halamanCited: Arnold, Roger A. Macroeconomics. 12th Ed., Cengage Learning, 2016Prueba3Belum ada peringkat
- FCJJ-16 Stoichiometry CH TeacherDokumen7 halamanFCJJ-16 Stoichiometry CH TeacherHermes Polanco J.Belum ada peringkat
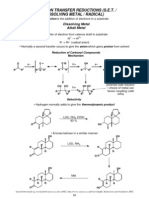
- Metal ReductionDokumen11 halamanMetal Reductiondeepthimahanthi sabithaBelum ada peringkat
- Examen IsothermDokumen9 halamanExamen IsothermAlonso FloresBelum ada peringkat
- FTL 51BDokumen52 halamanFTL 51Bradius SuharlinBelum ada peringkat
- Preventing Generator Damage from Hydrogen MoistureDokumen27 halamanPreventing Generator Damage from Hydrogen MoistureIskerBelum ada peringkat
- IJO Standard 98-01 (Revised 2005) Final Version PDFDokumen16 halamanIJO Standard 98-01 (Revised 2005) Final Version PDFWm Batz0% (1)
- NSK - 백색조직 개선 베어링Dokumen6 halamanNSK - 백색조직 개선 베어링gaus1685Belum ada peringkat
- 940 U S Face Permeability Monitoring For Gibe III RCC Dam and Resin Injection Works, Pittalis, Cagiano, Pietrangeli, BianDokumen8 halaman940 U S Face Permeability Monitoring For Gibe III RCC Dam and Resin Injection Works, Pittalis, Cagiano, Pietrangeli, BianDataniel RosarioBelum ada peringkat
- Lesson 1 Introduction To Organic Chemistry PDFDokumen4 halamanLesson 1 Introduction To Organic Chemistry PDFdela2Belum ada peringkat
- (G. - P. - Motulevich) Optical Properties of Metals and Intermolecular InteractionsDokumen234 halaman(G. - P. - Motulevich) Optical Properties of Metals and Intermolecular Interactions张尚宇Belum ada peringkat
- SN310 3 10 5BLDokumen9 halamanSN310 3 10 5BLvvvBelum ada peringkat
- Mucoadhesive DDS - 1749098145Dokumen14 halamanMucoadhesive DDS - 1749098145Vaibhav ThoratBelum ada peringkat
- Metco 54NS-1 (Aluminum Seal Coat) PDFDokumen3 halamanMetco 54NS-1 (Aluminum Seal Coat) PDFJ. BangjakBelum ada peringkat
- Module 3 Organic Reaction MechanismsDokumen7 halamanModule 3 Organic Reaction Mechanismsycca galianBelum ada peringkat
- TD RDMDokumen3 halamanTD RDMIbtissam LachguerBelum ada peringkat
- Simple Process Produces Boron Citrate PowderDokumen5 halamanSimple Process Produces Boron Citrate PowderAliBelum ada peringkat
- New Way of Hydroquinone and Catechol Synthesis Using NitrousDokumen10 halamanNew Way of Hydroquinone and Catechol Synthesis Using NitrousCintia Andrade MoóBelum ada peringkat
- Stability Indicating RP HPLC Method Development and Validation of Everolimus in Bulk and Pharmaceutical Dosage FormDokumen9 halamanStability Indicating RP HPLC Method Development and Validation of Everolimus in Bulk and Pharmaceutical Dosage FormEditor IJTSRDBelum ada peringkat
- Lecture 2Dokumen18 halamanLecture 2Henry biuwovwiBelum ada peringkat
- Fluid Mechanics Hydraulics by Gillesania Chapter 1Dokumen15 halamanFluid Mechanics Hydraulics by Gillesania Chapter 1Mary Grace BitantesBelum ada peringkat
- Inorganic ChemistryDokumen2 halamanInorganic ChemistryTaqeeb AbbasBelum ada peringkat
- Replacement of Gas Phase With Liquid HexamineDokumen6 halamanReplacement of Gas Phase With Liquid HexaminePradhita Ramdani HBelum ada peringkat
- Lab Report 3Dokumen8 halamanLab Report 3sagarchawlaBelum ada peringkat
- Tutorial 5Dokumen1 halamanTutorial 5SHOURYA SINGHBelum ada peringkat
- Presentation 2Dokumen9 halamanPresentation 2lucasBelum ada peringkat
- 2.02 Chemistry Intro Quiz (G9 Review) 2020-2021Dokumen3 halaman2.02 Chemistry Intro Quiz (G9 Review) 2020-2021ocBelum ada peringkat
- Kinetics OverviewDokumen168 halamanKinetics OverviewAlbert Daniel GarciaBelum ada peringkat