Photovoltaic Systems
Diunggah oleh
Nina AuslanderHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Photovoltaic Systems
Diunggah oleh
Nina AuslanderHak Cipta:
Format Tersedia
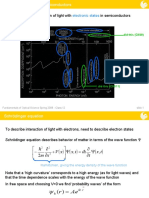
sunlight (photons) silicon’s valence electrons negative charge
phosphorus’s valence electrons positive charge
boron’s valence electrons
shared valence electrons
unbound/“extra” electron
“hole”—lack of electron
n-type semiconductor
p-n junction
p-type semiconductor
Si P
Si
In order to satisfy the octet rule, a phosphorus atom
Si
P Si needs three additional valence electrons. However,
silicon has four. As a result, when they bond, they
share three valence electrons. This leaves one
Si
unbound electron, facilitating a negative charge.
Si B
In order to satisfy the octet rule, a boron atom needs
Si
five additional valence electrons. However, silicon has
B Si four. As a result, when they bond, they share four
Si
valence electrons. This leaves one “hole” where the
Si
octet rule is not satisfied, facilitating a positive charge.
sunlight (photons)
unbound electrons in n-type layer
“holes” in p-type layer;
result in agitated
n-type semiconductor
particles
p-n junction
p-type semiconductor
-
When photons (light particles) hit the top layer of a solar panel (the n-
-
type semiconductor), the unbound electrons are knocked out of
place and begin to flow through the panel’s layers and into any
connected wires. These electrons facilitate an electric charge which
can be used to power a variety of objects.
DANI COOKE
Solar Group B
China & Energy: Spring 2018
Anda mungkin juga menyukai
- Fundamental Solid-State Principles: Pictures Are Redrawn (With Some Modifications) From by Robert T. PaynterDokumen31 halamanFundamental Solid-State Principles: Pictures Are Redrawn (With Some Modifications) From by Robert T. PaynterMujtabaRafique100% (1)
- HW03Dokumen3 halamanHW03何家銘Belum ada peringkat
- SolarDokumen1 halamanSolarapi-401892852Belum ada peringkat
- How Solar Cells Turn Sunlight Into ElectricityDokumen5 halamanHow Solar Cells Turn Sunlight Into Electricitysomeshshah23Belum ada peringkat
- In Schools: PV Lesson Plan 1 - Solar CellsDokumen5 halamanIn Schools: PV Lesson Plan 1 - Solar CellsJuan EsBelum ada peringkat
- How Solar Cells WorkDokumen12 halamanHow Solar Cells WorkmunirBelum ada peringkat
- Correlation Course in ElectronicsDokumen5 halamanCorrelation Course in ElectronicsCarmie BasilloteBelum ada peringkat
- Silicon Slides Topic 4Dokumen7 halamanSilicon Slides Topic 4AhmadSalimAlwohoushBelum ada peringkat
- Introduction To Bipolar Transistors: The Designer's Guide CommunityDokumen19 halamanIntroduction To Bipolar Transistors: The Designer's Guide CommunityQasim AliBelum ada peringkat
- U4Dokumen173 halamanU4Virthish DKBelum ada peringkat
- SEMICONDUCTORDokumen40 halamanSEMICONDUCTORfarhan jalaludinBelum ada peringkat
- Basic Electronics - Solid StatesDokumen25 halamanBasic Electronics - Solid Statesbangzai08Belum ada peringkat
- Ch1 EECE169 CSE16 Semiconductor DiodesDokumen45 halamanCh1 EECE169 CSE16 Semiconductor Diodessabbir hossainBelum ada peringkat
- Semiconductors and NanotechDokumen37 halamanSemiconductors and NanotechDavid JacquesBelum ada peringkat
- Solar at A Glance: Photovoltaic CellsDokumen1 halamanSolar at A Glance: Photovoltaic Cellsaktk1234Belum ada peringkat
- UNIT - IV - Semiconductor DevicesDokumen23 halamanUNIT - IV - Semiconductor DevicesGirish Shankar MishraBelum ada peringkat
- Barrier potential across semiconductor P-N junction and resting membrane potentialDokumen3 halamanBarrier potential across semiconductor P-N junction and resting membrane potentialkpilBelum ada peringkat
- PV SemiconductorsDokumen61 halamanPV SemiconductorsGaurav Sapkota0% (1)
- EC 104 Unit-1 1st JuneDokumen189 halamanEC 104 Unit-1 1st JuneHarsh KumarBelum ada peringkat
- EC 104 Unit 1 Part-1Dokumen64 halamanEC 104 Unit 1 Part-1Harsh KumarBelum ada peringkat
- Electronics DevicesDokumen44 halamanElectronics DevicesRhemjohn Dave PitongBelum ada peringkat
- PN Junction Lecture NotesDokumen10 halamanPN Junction Lecture NotessuperbangadakBelum ada peringkat
- Física dos Diodos - Sedra e SmithDokumen64 halamanFísica dos Diodos - Sedra e SmithWillian Cezar de Lima PintoBelum ada peringkat
- Elex 1Dokumen25 halamanElex 1cedricdimaligalig51Belum ada peringkat
- Lecture Note 1Dokumen15 halamanLecture Note 1nehal hasnain refathBelum ada peringkat
- 4330 Lecture1 Fall2022 StudentDokumen79 halaman4330 Lecture1 Fall2022 StudentchingkkBelum ada peringkat
- Elecctronics Unit 1Dokumen17 halamanElecctronics Unit 1Tangent ChauhanBelum ada peringkat
- chap16Dokumen35 halamanchap16jim.68.richtBelum ada peringkat
- 201806014043+HASAN MD TANVIR + Homework of Chapter 2Dokumen10 halaman201806014043+HASAN MD TANVIR + Homework of Chapter 2nahar elaBelum ada peringkat
- Lecture 1Dokumen28 halamanLecture 1abram samBelum ada peringkat
- Focus 3 Modelling Behaviour of SemiconductorsDokumen2 halamanFocus 3 Modelling Behaviour of SemiconductorsBilly BobBelum ada peringkat
- ELS 2202 Week 2 (1) .PPT - PpsDokumen61 halamanELS 2202 Week 2 (1) .PPT - PpsYohana Crisma LimbongBelum ada peringkat
- How Solar Cells WorkDokumen14 halamanHow Solar Cells WorkMonicaBelum ada peringkat
- Semiconductor DiodeDokumen48 halamanSemiconductor DiodeSubhash MurkuteBelum ada peringkat
- How Do Solar Panels WorkDokumen6 halamanHow Do Solar Panels WorkprabuparthibanBelum ada peringkat
- Understanding The P-N JunctionDokumen21 halamanUnderstanding The P-N JunctionwarrenronaldBelum ada peringkat
- Electronics 1 Lecture on Electronic Materials, Insulators, Conductors, and Semiconductors (ECMDokumen36 halamanElectronics 1 Lecture on Electronic Materials, Insulators, Conductors, and Semiconductors (ECMSyed AhsanBelum ada peringkat
- Module - 32: Semiconductor & Principle of CommunicationDokumen41 halamanModule - 32: Semiconductor & Principle of CommunicationTaksh GautamBelum ada peringkat
- 2 DiodespdfDokumen45 halaman2 DiodespdfUshan AdhikariBelum ada peringkat
- Unit 9 SemiconductorDokumen10 halamanUnit 9 SemiconductorSahil ChawlaBelum ada peringkat
- PN Junction DiodeDokumen44 halamanPN Junction DiodePrasanth. SBelum ada peringkat
- 2 Part B SIlicon Solar Cell Fabrication and Measurement V4 (1)Dokumen64 halaman2 Part B SIlicon Solar Cell Fabrication and Measurement V4 (1)Akash 21MAE0001Belum ada peringkat
- Semiconductors CH14 Part 2Dokumen17 halamanSemiconductors CH14 Part 2Rishab SharmaBelum ada peringkat
- Electrons and ''Holes'' - S.Dokumen5 halamanElectrons and ''Holes'' - S.Manikandan SundararajBelum ada peringkat
- Solar Panels: How Solar Cells Convert Sunlight into ElectricityDokumen4 halamanSolar Panels: How Solar Cells Convert Sunlight into ElectricitywalisyhBelum ada peringkat
- Presentation On Analog ElectronicsDokumen334 halamanPresentation On Analog ElectronicssamuelBelum ada peringkat
- Carrier Concentration in SemiconductorsDokumen36 halamanCarrier Concentration in SemiconductorsSapna NazirBelum ada peringkat
- AC-IV Solar PV SysstemsDokumen6 halamanAC-IV Solar PV SysstemsshreemantiBelum ada peringkat
- What Is Photovoltaic Effect?Dokumen2 halamanWhat Is Photovoltaic Effect?Anonymous xGKmBSrBelum ada peringkat
- Unit-Ii Junction Diode Characteristics and Special Semi Conductor DiodesDokumen118 halamanUnit-Ii Junction Diode Characteristics and Special Semi Conductor DiodesradsradBelum ada peringkat
- LECTURE - 5 MekatronikaDokumen12 halamanLECTURE - 5 MekatronikaKai Vector GalerryBelum ada peringkat
- Characteristics of PN Junction DiodeDokumen8 halamanCharacteristics of PN Junction DiodeMurali KrishnaBelum ada peringkat
- Semiconductor Electronics - Materials, Devices and Simple CircuitsDokumen25 halamanSemiconductor Electronics - Materials, Devices and Simple CircuitsAshish KumarBelum ada peringkat
- Semiconductor Electronics - Materials, Devices and Simple CircuitsDokumen25 halamanSemiconductor Electronics - Materials, Devices and Simple CircuitsVinay SinghBelum ada peringkat
- Topic 1:: Introduction To SemiconductorDokumen26 halamanTopic 1:: Introduction To SemiconductorfaizahBelum ada peringkat
- Introduction To SemiconductorsDokumen150 halamanIntroduction To SemiconductorsFira tubeBelum ada peringkat
- Lecture 10 (Introduction To Semiconductor)Dokumen31 halamanLecture 10 (Introduction To Semiconductor)samiullaharain636Belum ada peringkat
- Chapter 4-1 (II 2008-2009) (Compatibility Mode)Dokumen76 halamanChapter 4-1 (II 2008-2009) (Compatibility Mode)darrenneoyomanBelum ada peringkat
- Semiconductors and PN Junction Diode: Created and Forwarded By: Anjali R Asst. Prof AdhocDokumen35 halamanSemiconductors and PN Junction Diode: Created and Forwarded By: Anjali R Asst. Prof Adhocadkadrv4002Belum ada peringkat
- Elementary Particles: The Commonwealth and International LibraryDari EverandElementary Particles: The Commonwealth and International LibraryBelum ada peringkat
- Electronic SpectrosDokumen19 halamanElectronic Spectrosfathur fikranBelum ada peringkat
- Chapter 1 - Atomic Structure: Test Yourself (Page 3)Dokumen139 halamanChapter 1 - Atomic Structure: Test Yourself (Page 3)Aref DahabrahBelum ada peringkat
- 200 and More NMR Experiments A PracticalDokumen8 halaman200 and More NMR Experiments A Practicaldelfin000Belum ada peringkat
- Research Paper On Transmission Electron MicroscopeDokumen5 halamanResearch Paper On Transmission Electron MicroscopeaflbuagdwBelum ada peringkat
- Bragg's Law - Wikipedia, The Free EncyclopediaDokumen9 halamanBragg's Law - Wikipedia, The Free EncyclopediajosephBelum ada peringkat
- Grade 9 Academic Science (SNC 1D1) Unit 3: Chemistry: Atoms and The Periodic TableDokumen10 halamanGrade 9 Academic Science (SNC 1D1) Unit 3: Chemistry: Atoms and The Periodic Tablezia mooreBelum ada peringkat
- 2021 SEM 6 Physical Chemistry DSE 3Dokumen4 halaman2021 SEM 6 Physical Chemistry DSE 3Gaurav KumarBelum ada peringkat
- Photoelectron Spectroscopy Worksheet-KEYDokumen3 halamanPhotoelectron Spectroscopy Worksheet-KEYAriel NogalesBelum ada peringkat
- Characterization of the designer drug deschloroketamine (2-methylamino-2-phenylcyclohexanone) by gas chromatography/mass spectrometry, liquid chromatography/high-resolution mass spectrometry, multistage mass spectrometry, and nuclear magnetic resonanceDokumen10 halamanCharacterization of the designer drug deschloroketamine (2-methylamino-2-phenylcyclohexanone) by gas chromatography/mass spectrometry, liquid chromatography/high-resolution mass spectrometry, multistage mass spectrometry, and nuclear magnetic resonanceabazaba151Belum ada peringkat
- Student Exploration: Electron Configuration: NCVPS Chemistry Fall 2014Dokumen5 halamanStudent Exploration: Electron Configuration: NCVPS Chemistry Fall 2014Meghan ShankBelum ada peringkat
- Components of MatterDokumen60 halamanComponents of MatterIvy JoyceBelum ada peringkat
- MATTHEW NAZARRO - Chem 2208 Lab Experiment No. 8-Visible Spectrophotometry of Nickel (II) ChlorideDokumen7 halamanMATTHEW NAZARRO - Chem 2208 Lab Experiment No. 8-Visible Spectrophotometry of Nickel (II) ChlorideMATTHEW NAZARROBelum ada peringkat
- W.atoms and IonsDokumen2 halamanW.atoms and IonsMahmoud AladdasiBelum ada peringkat
- Ch-14 Semiconductor MCQDokumen10 halamanCh-14 Semiconductor MCQRaksha RajpurohitBelum ada peringkat
- Electron Diffraction - Electron Microscopy and DiffractionDokumen39 halamanElectron Diffraction - Electron Microscopy and DiffractionHoang Lam100% (1)
- VSS Lesson 1 Early Atomic Theories Chemistry 12Dokumen11 halamanVSS Lesson 1 Early Atomic Theories Chemistry 12Jaideep GillBelum ada peringkat
- The Deuteron Binding EnergyDokumen31 halamanThe Deuteron Binding Energymohammed1998Belum ada peringkat
- OSE5312 Slides Class 12 - Optical Properties of Semiconductors 1Dokumen22 halamanOSE5312 Slides Class 12 - Optical Properties of Semiconductors 1Mastering Zinc OxideBelum ada peringkat
- Understanding Wave-Particle DualityDokumen15 halamanUnderstanding Wave-Particle DualityomerpaBelum ada peringkat
- Its All About LG 102Dokumen105 halamanIts All About LG 102sossy046Belum ada peringkat
- MCQDokumen10 halamanMCQmiraupriyaBelum ada peringkat
- Core-Physical Science Q1 SLM - 3Dokumen30 halamanCore-Physical Science Q1 SLM - 3Christopher Agustin Tambogon Lpt100% (8)
- Adv Materials Inter - 2022 - Mu - in Situ Characterization Techniques Applied in Photocatalysis A ReviewDokumen23 halamanAdv Materials Inter - 2022 - Mu - in Situ Characterization Techniques Applied in Photocatalysis A ReviewPrasaanth RanuBelum ada peringkat
- Assignment Atomic Structure JH Sir-2611Dokumen30 halamanAssignment Atomic Structure JH Sir-2611Ghost Phyton RoorkeeBelum ada peringkat
- Periodic Table (Sample)Dokumen12 halamanPeriodic Table (Sample)Chick ChikBelum ada peringkat
- Atomic Polarizability: A Periodic Descriptor: Shalini Choudhary, Prabhat Ranjan and Tanmoy ChakrabortyDokumen8 halamanAtomic Polarizability: A Periodic Descriptor: Shalini Choudhary, Prabhat Ranjan and Tanmoy ChakrabortyZaara RyeenBelum ada peringkat
- Atoms: Neutrons ProtonsDokumen8 halamanAtoms: Neutrons ProtonsMelke JasinBelum ada peringkat
- VSEPRDokumen1 halamanVSEPRĐan KhanhBelum ada peringkat
- Electron Configuration Fall 2016Dokumen31 halamanElectron Configuration Fall 2016Aviral TiwariBelum ada peringkat