Step1.: Anta1 Antb1 Antc1 T
Diunggah oleh
Sonu PatelJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Step1.: Anta1 Antb1 Antc1 T
Diunggah oleh
Sonu PatelHak Cipta:
Format Tersedia
Step1. Does the system contain two phases?
Let's calculate the BULB P and DEW P of the
system at 80 C. Since the system follows Raoult's law, xi = zi and the BULB P can be estimated by
Eq. 23 of this notes. To estimate the vapor pressures in Eq. 23, the parameters in the Antoine
equation can be found from the book by Reid et al., The Properties of Gases and Liquids, 3th ed.
acetone = C3H6O; acetonitrile = C2H3N; nitromethane = CH3NO2
anta1 ≔ 16.6513 antb1 ≔ 2940.46 ⋅ K antc1 ≔ −35.93 ⋅ K T ≔ (80 + 273) ⋅ K kPa ≔ 10 3 ⋅ Pa
anta2 ≔ 16.2874 antb2 ≔ 2945.47 ⋅ K antc2 ≔ −49.15 ⋅ K
anta3 ≔ 16.2193 antb3 ≔ 2972.64 ⋅ K antc3 ≔ −64.15 ⋅ K
⎛ antb1 ⎞
Ps1 ≔ exp ⎜anta1 − ―――⎟ ⋅ 1.3158 ⋅ 10 −3 ⋅ atm Note that pressure is converted from mmHg to atm.
⎝ T + antc1 ⎠
⎛ antb2 ⎞
Ps2 ≔ exp ⎜anta2 − ―――⎟ ⋅ 1.3158 ⋅ 10 −3 ⋅ atm
⎝ T + antc2 ⎠
⎛ antb3 ⎞
Ps3 ≔ exp ⎜anta3 − ―――⎟ ⋅ 1.3158 ⋅ 10 −3 ⋅ atm
⎝ T + antc3 ⎠
Ps1 = 213.251 kPa Ps2 = 97.377 kPa Ps3 = 50.05 kPa
From the first part of Eq. 23, the BULB P can be estimated from Raoult's law. At BUBL P, xi=zi, thus,
x1 ≔ 0.45 x2 ≔ 0.35 x3 ≔ 0.20
Pb1 ≔ x1 ⋅ Ps1 + x2 ⋅ Ps2 + x3 ⋅ Ps3 Pb1 = 140.055 kPa
From the second part of Eq. 23, the DEW P can be estimated as following. At DEW P, yi = zi, thus,
y1 ≔ 0.45 y2 ≔ 0.35 y3 ≔ 0.20
1 Since Σxi=1, we have Σ(yi P/ Pisat) =1
Pb2 ≔ ――――――
⎛ y1 y2 y3 ⎞ Pb2 = 103.088 kPa (Raoult's) and P = 1 / (yi/Pisat).
⎜―― + ―― + ―― ⎟
⎝ Ps1 Ps2 Ps3 ⎠
The desired flash calculation is to be conducted at 110 kPa, a pressure between the bubble and
dew pressures; thus, a system of two phase exists.
{n}
Non-Commercial Use Only
Step 2. Flash calculation. Since the system follows the Raoult's law, eq. 28 in the notes gives Ki =
Psati / P. However, if the liquid phase is not a ideal solution or if the gas phase is not ideal, i or i
not equal to 1 and they will have to be estimated for the calculation of Ki, see Eq. 28 in the notes.
P ≔ 110 ⋅ kPa
Ps1 Ps2 Ps3
K1 ≔ ―― K2 ≔ ―― K3 ≔ ――
P P P
z1 ≔ 0.45 z2 ≔ 0.35 z3 ≔ 0.2
z1 ⋅ K1 z2 ⋅ K2 z3 ⋅ K3
y1 (V) ≔ ――――― y2 (V) ≔ ――――― y3 (V) ≔ ―――――
1 + V ⋅ (K1 − 1) 1 + V ⋅ (K2 − 1) 1 + V ⋅ (K3 − 1)
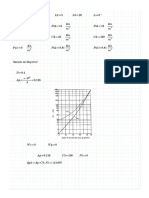
Trial value: V ≔ 0.5
Guess Values
Constraints
y1 (V) + y2 (V) + y3 (V) = 1
Solver
V ≔ Find (V) = 0.812
V = 0.812
L≔1−V
L = 0.188
y1 (V) = 0.495 y2 (V) = 0.342 y3 (V) = 0.163
y1 (V) y2 (V) y3 (V)
x1 ≔ ―― x2 ≔ ―― x3 ≔ ――
K1 K2 K3
x1 = 0.255 x2 = 0.386 x3 = 0.359
{n}
Non-Commercial Use Only
Anda mungkin juga menyukai
- Kuc: Modified Raoult's Law: ReviewDokumen20 halamanKuc: Modified Raoult's Law: ReviewAke TupeslaBelum ada peringkat
- Taller# 7 18-12Dokumen11 halamanTaller# 7 18-12Katty Chacón ZagalBelum ada peringkat
- Cargas puntuales y superficiales en terrenoDokumen2 halamanCargas puntuales y superficiales en terrenoArtudosky el ProBelum ada peringkat
- Análisis Modal Espectral E030Dokumen10 halamanAnálisis Modal Espectral E030Enrique DuvalBelum ada peringkat
- Mod RaoultDokumen20 halamanMod RaoultAraBelum ada peringkat
- Hints Assgn 2Dokumen3 halamanHints Assgn 2Eswaramoorthy SeenuBelum ada peringkat
- D 0.4 L1 5 L2 5 L3 20 ϕ 0 Ps1 15 ―― Kn m Ps2 15 ―― Kn m Ps3 18 ―― Kn m C1 25 Kn m C2 25 Kn m C3 100 ―― Kn m Pa1 0 Kn m Pa2 9.81 ―― Kn m Pa3 9.81 ―― Kn mDokumen5 halamanD 0.4 L1 5 L2 5 L3 20 ϕ 0 Ps1 15 ―― Kn m Ps2 15 ―― Kn m Ps3 18 ―― Kn m C1 25 Kn m C2 25 Kn m C3 100 ―― Kn m Pa1 0 Kn m Pa2 9.81 ―― Kn m Pa3 9.81 ―― Kn mJhazmani A. LlanosBelum ada peringkat
- A - 439180 - Luky Desyana Putri R - Tugas 2 Kesetimbangan KimiaDokumen6 halamanA - 439180 - Luky Desyana Putri R - Tugas 2 Kesetimbangan KimiaLuky DesyanaBelum ada peringkat
- VLE Problem PDFDokumen3 halamanVLE Problem PDFhappywhewmiBelum ada peringkat
- Para PonerDokumen5 halamanPara PonerLaloBelum ada peringkat
- Soil Bearing Capacity and Retaining Wall CalculationsDokumen10 halamanSoil Bearing Capacity and Retaining Wall CalculationsJuanKaBelum ada peringkat
- PERFIL W EN TENSION Y RESISTENCIA DE CONEXIONESDokumen2 halamanPERFIL W EN TENSION Y RESISTENCIA DE CONEXIONESEmel JimenezBelum ada peringkat
- Chapter - 6b Transmission Through A Layer Normal Incidence PDFDokumen14 halamanChapter - 6b Transmission Through A Layer Normal Incidence PDFAlinaBogoiBelum ada peringkat
- Steel Project Structural AnalysisDokumen12 halamanSteel Project Structural Analysisgio cumbicusBelum ada peringkat
- HW#1 Abdelrahman AwawdehDokumen6 halamanHW#1 Abdelrahman Awawdehabdelrahman awawdehBelum ada peringkat
- Term Odin A MicaDokumen10 halamanTerm Odin A MicaFelipe De Lima RomeroBelum ada peringkat
- Prova 01Dokumen9 halamanProva 01PedroH. R.SanchesBelum ada peringkat
- Problema TSFDokumen4 halamanProblema TSFJEYSON NILO HUARCAYA ARANDOBelum ada peringkat
- Gusset Plate Limit Checks and Force DistributionDokumen3 halamanGusset Plate Limit Checks and Force DistributionManuelGarciaBelum ada peringkat
- Chapter 12Dokumen12 halamanChapter 12StefanPerendijaBelum ada peringkat
- Problema 1 SustiDokumen3 halamanProblema 1 SustiJEYSON NILO HUARCAYA ARANDOBelum ada peringkat
- Prob 4.44 Table Lookups Given Values: R and V Are Constant So..Dokumen5 halamanProb 4.44 Table Lookups Given Values: R and V Are Constant So..tkshoeBelum ada peringkat
- Diseño de ZapatasDokumen4 halamanDiseño de ZapatasDaniel Garces OrregoBelum ada peringkat
- Memoria de Calculo Sesion 04Dokumen20 halamanMemoria de Calculo Sesion 04Victor OcampoBelum ada peringkat
- Chapter11 ADokumen33 halamanChapter11 ANic BlandoBelum ada peringkat
- Mathcad EjerDokumen18 halamanMathcad EjerVictor OcampoBelum ada peringkat
- Compressibility Factor Z Gas Calc 1Dokumen5 halamanCompressibility Factor Z Gas Calc 1xjaf010% (1)
- CH 10Dokumen34 halamanCH 10hirenpatel_universalBelum ada peringkat
- Activity 1 in PpeDokumen4 halamanActivity 1 in PpeJenny Mae Pomeda100% (1)
- Tarea 2 ConcretoDokumen9 halamanTarea 2 Concretomoy092Belum ada peringkat
- origin frez γ k ϕ μ μ1 μ t g A R0 h0 d0 dn dp bp drp i hp g iDokumen10 halamanorigin frez γ k ϕ μ μ1 μ t g A R0 h0 d0 dn dp bp drp i hp g iC Alexandru IulianBelum ada peringkat
- Light Scattering MITDokumen24 halamanLight Scattering MITYue LiuBelum ada peringkat
- Compressibility Factor Z CalcDokumen3 halamanCompressibility Factor Z Calcmohideenaliyarjafeer.shanawazBelum ada peringkat
- Compressibility of mercury and equations of state calculationsDokumen7 halamanCompressibility of mercury and equations of state calculationsAdhemazBelum ada peringkat
- Deber 3 Dem Gonzalez Loaiza RonaldDokumen14 halamanDeber 3 Dem Gonzalez Loaiza RonaldMiguel CasiqueBelum ada peringkat
- Chapter 14 - Section A - Mathcad SolutionsDokumen56 halamanChapter 14 - Section A - Mathcad SolutionsKhalid M MohammedBelum ada peringkat
- Termo 1' Modeli 3.doc AdmiriDokumen12 halamanTermo 1' Modeli 3.doc AdmiriFeritBelum ada peringkat
- Tutorial #2Dokumen5 halamanTutorial #2Hafizh Renanto AkhmadBelum ada peringkat
- Compressible Flow PDFDokumen90 halamanCompressible Flow PDFOmer TokhBelum ada peringkat
- 01.introduction Integrated CircuitDokumen34 halaman01.introduction Integrated CircuitMrinmoy DeyBelum ada peringkat
- Engineering Thermodynamics Entropy CalculationDokumen104 halamanEngineering Thermodynamics Entropy CalculationIsabelHutterBelum ada peringkat
- Chapter02 03 SJDokumen5 halamanChapter02 03 SJkaviBelum ada peringkat
- Fixed Bed Reactor, Modelling&Optimal DesignDokumen74 halamanFixed Bed Reactor, Modelling&Optimal DesignRana Uzair100% (1)
- Example 1Dokumen8 halamanExample 1jgolloberBelum ada peringkat
- CHE 3113 Spring 2022 - Rate Operations IIDokumen24 halamanCHE 3113 Spring 2022 - Rate Operations IIIqra SafdarBelum ada peringkat
- Assignment FINALDokumen67 halamanAssignment FINALlaila khanBelum ada peringkat
- Qdoc - Tips Theory of Elasticity by Sadhu SinghDokumen7 halamanQdoc - Tips Theory of Elasticity by Sadhu Singhking royalBelum ada peringkat
- Sheet (1&2) ThermoDokumen17 halamanSheet (1&2) ThermoAhmed A. TaimaBelum ada peringkat
- Vapor/Liquid Equilibrium: Vle by Modified Raoult'S LawDokumen16 halamanVapor/Liquid Equilibrium: Vle by Modified Raoult'S LawAby JatBelum ada peringkat
- Diseño Columna FinalDokumen7 halamanDiseño Columna Finalcarlosaperez4bwebgmfycBelum ada peringkat
- Shigley's tables and equations for bolt preload calculationsDokumen2 halamanShigley's tables and equations for bolt preload calculationsManuel MurilloBelum ada peringkat
- 4211 Sheet 3Dokumen2 halaman4211 Sheet 3Roy VeseyBelum ada peringkat
- Thermodynamic Processes and Energy Changes in Gas SystemsDokumen17 halamanThermodynamic Processes and Energy Changes in Gas SystemsOnur ÖZÇELİKBelum ada peringkat
- Quaternions and Rotations NotesDokumen12 halamanQuaternions and Rotations NotesBagat PlusBelum ada peringkat
- Iit Jee 2012 Paper2-Final SolnDokumen8 halamanIit Jee 2012 Paper2-Final Solnvarun303gr8Belum ada peringkat
- Self Assessment Solutions Tutorial 7 Self Assessment Exercise No. 1Dokumen5 halamanSelf Assessment Solutions Tutorial 7 Self Assessment Exercise No. 1Alexander MugabeBelum ada peringkat
- ACTUAL 4 CYCLE, 2 Revolutions/cycle: Compression 1-2 Combustion 2-3 Exhaust 4-1 Intake 0-1 Exhaust 1-0 Power 3-4Dokumen26 halamanACTUAL 4 CYCLE, 2 Revolutions/cycle: Compression 1-2 Combustion 2-3 Exhaust 4-1 Intake 0-1 Exhaust 1-0 Power 3-4Christed Aljo BarrogaBelum ada peringkat
- Desarrollo de EjerciciosDokumen11 halamanDesarrollo de EjerciciosMarino Pacheco DiazBelum ada peringkat
- Solution Manual for an Introduction to Equilibrium ThermodynamicsDari EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsBelum ada peringkat
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesDari EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesBelum ada peringkat
- PopulationSCST 2011census - PdfhimachalDokumen1 halamanPopulationSCST 2011census - Pdfhimachalsanjuair kumarBelum ada peringkat
- PDPU ACPC Vacant Seat Merit List 2016 PDFDokumen16 halamanPDPU ACPC Vacant Seat Merit List 2016 PDFSonu PatelBelum ada peringkat
- Project WorkDokumen1 halamanProject WorkSonu PatelBelum ada peringkat
- 3 PDFDokumen16 halaman3 PDFSonu PatelBelum ada peringkat
- Economic Decisions PDFDokumen30 halamanEconomic Decisions PDFSonu PatelBelum ada peringkat
- ONGC Recruitment for Graduate Trainees through GATE 2018Dokumen7 halamanONGC Recruitment for Graduate Trainees through GATE 2018wec33DFSBelum ada peringkat
- Questions Jeff and Mike PDFDokumen7 halamanQuestions Jeff and Mike PDFSonu PatelBelum ada peringkat
- Bussiness PlanDokumen15 halamanBussiness PlanmadhuriBelum ada peringkat
- CH General-Aptitude PDFDokumen15 halamanCH General-Aptitude PDFSonu PatelBelum ada peringkat
- Economic Decisions PDFDokumen30 halamanEconomic Decisions PDFSonu PatelBelum ada peringkat
- Bussiness PlanDokumen15 halamanBussiness PlanmadhuriBelum ada peringkat
- Petroleum Product Pricing MechanismDokumen20 halamanPetroleum Product Pricing MechanismSonu PatelBelum ada peringkat
- Week 8 BlogDokumen1 halamanWeek 8 BlogSonu PatelBelum ada peringkat
- Unit ConversionDokumen1 halamanUnit ConversionSonu PatelBelum ada peringkat
- 3 PDFDokumen16 halaman3 PDFSonu PatelBelum ada peringkat
- Reservoir Engineering StrategyDokumen2 halamanReservoir Engineering StrategyAshirbad Choudhury75% (4)
- Petroleum Product Pricing MechanismDokumen20 halamanPetroleum Product Pricing MechanismSonu PatelBelum ada peringkat
- REPORTDokumen23 halamanREPORTSonu PatelBelum ada peringkat
- Petroleum Natural Gas Exploration EngineeringDokumen2 halamanPetroleum Natural Gas Exploration EngineeringSonu PatelBelum ada peringkat
- Lect 4.2-Intro Rock CycleDokumen1 halamanLect 4.2-Intro Rock CycleSonu PatelBelum ada peringkat
- Current Affairs December 2015Dokumen14 halamanCurrent Affairs December 2015Alagu MurugesanBelum ada peringkat
- KPDokumen1 halamanKPSonu PatelBelum ada peringkat
- India: Alcohol Consumption: Levels and PatternsDokumen1 halamanIndia: Alcohol Consumption: Levels and PatternsSonu PatelBelum ada peringkat
- Well Testing ModuleDokumen3 halamanWell Testing ModuleSonu PatelBelum ada peringkat
- ONGC Recruitment for Graduate Trainees through GATE 2018Dokumen7 halamanONGC Recruitment for Graduate Trainees through GATE 2018wec33DFSBelum ada peringkat
- Electromagnetic Theory SummaryDokumen15 halamanElectromagnetic Theory SummarySonu Patel0% (1)
- 114106042Dokumen7 halaman114106042Sonu PatelBelum ada peringkat
- Final - Module 1-Origin EarthDokumen5 halamanFinal - Module 1-Origin EarthSonu PatelBelum ada peringkat
- Week 8 BlogDokumen1 halamanWeek 8 BlogSonu PatelBelum ada peringkat
- Lecture02 SKKC21333 1617-1Dokumen35 halamanLecture02 SKKC21333 1617-1Chai Hong LohBelum ada peringkat
- Kinetic Study of Hydrodeoxygenation of Stearic Acid As Model Compound For OilsDokumen14 halamanKinetic Study of Hydrodeoxygenation of Stearic Acid As Model Compound For OilsLaura RDBelum ada peringkat
- JOURNAL Analysis of Bentonite-LovibondDokumen9 halamanJOURNAL Analysis of Bentonite-LovibondDevie RasyidinBelum ada peringkat
- Elastic LiDAR, Theory, Practice, and Analysis MethodsDokumen572 halamanElastic LiDAR, Theory, Practice, and Analysis MethodsTumis BuduBelum ada peringkat
- Nuts and Bolts of First-Principles Simulation: Plane WavesDokumen33 halamanNuts and Bolts of First-Principles Simulation: Plane WavesFahd ElmourabitBelum ada peringkat
- Solar Energy: SciencedirectDokumen18 halamanSolar Energy: SciencedirectMohamed AliBelum ada peringkat
- 7 Transfer of Electrons at A DistanceDokumen15 halaman7 Transfer of Electrons at A DistancenamikBelum ada peringkat
- Why Is Moist Air Less Dense Than Dry AirDokumen2 halamanWhy Is Moist Air Less Dense Than Dry AirMehul ChaturvediBelum ada peringkat
- 操作规程 Operating Manual: Rev. Description Degn Chkd Revd Appd DateDokumen310 halaman操作规程 Operating Manual: Rev. Description Degn Chkd Revd Appd DateIffatBelum ada peringkat
- Chemical Reactions and Quantities: 5.5 The MoleDokumen15 halamanChemical Reactions and Quantities: 5.5 The Molejanaisha_bai7170Belum ada peringkat
- Ch-2 Acids Bases Salts Question BankDokumen2 halamanCh-2 Acids Bases Salts Question Bankvrat0% (2)
- Chapter 4 and 5Dokumen51 halamanChapter 4 and 5Kyla Gabrielle TutoBelum ada peringkat
- Stress Corrosion Cracking Behavior of MaterialsDokumen22 halamanStress Corrosion Cracking Behavior of MaterialsEduardo Pérez R.Belum ada peringkat
- Cita 55Dokumen6 halamanCita 55Karen Alejandra López CastañosBelum ada peringkat
- Let V Velocity of The Ith Species Relative To Stationary Coordinate AxisDokumen6 halamanLet V Velocity of The Ith Species Relative To Stationary Coordinate AxisDozdiBelum ada peringkat
- Electrochemistry and BatteriesDokumen15 halamanElectrochemistry and Batteriesvenugopal_aeroBelum ada peringkat
- 4CH0 1C Que 20180110januaryDokumen32 halaman4CH0 1C Que 20180110januaryMustufa KhalilBelum ada peringkat
- SHIP BOILERS WATERDokumen94 halamanSHIP BOILERS WATERalvarompsardinhaBelum ada peringkat
- Effect of Temperature To The Speed of SoundDokumen7 halamanEffect of Temperature To The Speed of Soundjanecil bonzaBelum ada peringkat
- Wastewater Treatment Design Aeration in PDFDokumen8 halamanWastewater Treatment Design Aeration in PDFHendraBelum ada peringkat
- Dehydrogenation Process Description المشروعDokumen5 halamanDehydrogenation Process Description المشروعsaeed909909Belum ada peringkat
- Mixing TankDokumen3 halamanMixing Tankleslie_adolfoBelum ada peringkat
- EIGA (2008) - Comparison of EP, USP & JP For Medicinal GasDokumen21 halamanEIGA (2008) - Comparison of EP, USP & JP For Medicinal GashuynhhaichauchauBelum ada peringkat
- Cape Unit 1 Past Papers Chem PDFDokumen15 halamanCape Unit 1 Past Papers Chem PDFSherlan TheodoreBelum ada peringkat
- MSC Physics Part-I - Part-IIDokumen16 halamanMSC Physics Part-I - Part-IIkumaradarshx321Belum ada peringkat
- Piping 30000Dokumen14 halamanPiping 30000Prasanta Kumar BeheraBelum ada peringkat
- Dew Point Calculation Chart: For Adhesive and Coating Applications Ambient Air Temperature in Degrees FahrenheitDokumen1 halamanDew Point Calculation Chart: For Adhesive and Coating Applications Ambient Air Temperature in Degrees FahrenheitBernathTurnipBelum ada peringkat
- Cbse Class XI Chemistry Sample Paper - 3 Solution Section ADokumen13 halamanCbse Class XI Chemistry Sample Paper - 3 Solution Section ABhabaniBelum ada peringkat
- Sonochemistry PDFDokumen46 halamanSonochemistry PDFLupita GarzaBelum ada peringkat
- 1 - 8 - Intercalation Compounds of Graphite PDFDokumen189 halaman1 - 8 - Intercalation Compounds of Graphite PDFAgtc TandayBelum ada peringkat