Laws of Corrresponding States - Nelson and Obert
Diunggah oleh
Ivan RodrigoHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Laws of Corrresponding States - Nelson and Obert
Diunggah oleh
Ivan RodrigoHak Cipta:
Format Tersedia
Laws of
Corresponding States
L. NELSON and E. F. OBERT
Northwestern Technological Institute, Evanston, Illinois
Various laws of corresponding states are examined briefly in this paper to show
the corrections that are in common lase to increase the accuracy of forecasting com-
pressibility factors. Two trends are noticeable: (1) the use of specific compressibility
charts with the results generalized for all gases either by corrections related to zo
(the compressibility factor at the critical point) or by the use of pseudocritical prop-
erties and (2) the use of % true generalized chart based upon averaged data. I t i s
shown that the selection of the plot parameters affects the accuracy of the generalized
chart. In recent years reduced parameters based upon kinetic theory have been pro-
posed but for a restricted class of gases (nonpolar gases with spherically symmetrical
molecules and negligible quantum effects). I t is shown that the kinetic parameters
are directly related to the critical constants and also that the kinetic parameter charts
can be used for all gases without serious loss of accuracy.
The compressibility factor z is two of the independent properties have different x , values, obviously
defined : p , v, and T . If p and T a r e selected from Equation (2) correlation a t
s o that f o r several gases T , , = T,, or near the critical point will be
Z =--PV
= T, and p , , = p , , = p,,, then by poor, but this is no assurance t h a t
RT Equation ( 2 ) , which is also an correlation will continue to be poor
exact equation, in regions other than t h e critical.
In other words a compensating ef-
In terms of reduced properties rela- fect for x , is the failure of Equa-
tive to the critical constants, Equa- tion (5) to hold exactly for all
tion (1) is equivalent to gases. Thus gases with the same
z, value usually correlate closely
Equation (4) shows t h a t the gen- on the chart, but this happens be-
eralization of Equation (3) is suc- cause the molecular structures a r e
cessful f o r gases having essentially closely related. When the struc-
the same x, values because the van tures or molecular laws of force
where x , is the compressibility f ac- der Waals law of corresponding are different, gases will not corre-
tor a t the critical point. A com- states, late well no matter whether the 2,
pressibility chart can be construct- values a r e equal o r not (and an
ed to correlate z with two selected additional parameter becomes
reduced properties, such as T, and necessary : a quantum parameter
p,, with acceptable accuracy for f o r t h e light gases such as helium
holds with adequate precision; the and hydrogen; a dipole-moment
most gases. As an approximation generalization is successful for
theref ore parameter f o r polar gases, such as
gases having different (or the the freons, water, and ammonia;
same) x, values because the modi- and size or shape parameters f o r
fied law of corresponding states the complex organic gases).
proposed by S u ( 3 ) ,
The implications of Equation (3) z, CORRECTION FACTORS
and other laws of corresponding Meissner and Seferian ( 2 ) have
states have been discussed many published correction charts to ac-
times [ ( I and 2 ) for examples], count f o r deviations in z, between
but several aspects of the general- holds with adequate precision. It gases. These charts a r e useful
ized laws are not usually recog- is emphasized t h a t where x, values when x values below 0.6 a r e indi-
nized. It may be considered t h a t a r e essentially constant, the regions cated and w h e n t h e molecular
for any one gas, as x is a state of the generalized chart where cor- s t r u c t u r e s a r e closely related. The
property, an exact functional rela- relation is exceptional can b e found law of corresponding states f o r
tionship exists between x and any only by trial. Similarly, when gases their work has the form
Page 74 A.1.Ch.E. Journal March, 1955
“laws” a r e exactly true. No one to ed group of fluids without undue
the authors’ knowledge has inves- labor is now available by a method
The corrections can be ignored tigated which of t h e three equa- derived from statistical mechanics
however for regions above T , = 1.3 tions would best serve for a gen- theory. Hirschfelder, Bird, and
and also for all similar gases with eralized compressibility chart, prob- Spotz ( 8 , 9 ) have developed a virial
values of x, between 0.25 and 0.28. ably because the critical volume has equation of state based upon the
In the latter case a difference of in the past been either unknown or Lennard-Jones force potential.
over 10% exists in x,; yet corre- else a variable of some disrepute. Their work is limited however to
lation on the compressibility chart F o r this reason a study was made of the semisymmetrical nonpolar
is well within 2 112% for most twelve gases (CH,, C2H4,N,,NH,, gases, and the analytical, results
gases. H,, C02, CO, N e , C,H,, X e , A , and include only t,he effects of the first
A fundamentally correct method a i r ) , which indicated t h a t a chart three virial coefficients. From the
f o r modifying the generalized x based upon one equation would underlying theory, reduced quanti-
values to account for t h e deviations yield regions of improved correla- ties, first proposed by deBoer ( l o ) ,
of polar gases has been recently tion relative to charts based upon can be defined:
proposed by Hall and Ibele(13). the other two equations, and of
Their work is based upon a law of course the opposite trend was also Reduced density
corresponding states of t h e form t r u e for other regions. For one
example the following data f o r PT’ = bop
water (x, = 0.232) and methane
(x, = 0.289) a t reduced pressures Reduced temperature
where pr is a reduced dipole mo- less than p , = 1.5 may be consid-
ment. The corrections of Hall and ered : 7 = T
Ibele enable x values for the polar e/k
gases to be obtained with surpris- MAXIMUMDEVIATIONS I N Xerp
ing accuracy from a generalized Chart of T , = 1.1 T , = 1.3 T , = 1.8 Reduced pressure
compressibility chart (such as Fig- z = f ( T,., p ? ) 0.05 0.038 0.015
ure 1). However, the important x = f ( Tr, p,,) 0.06 0.015 0.004
by-product of their work is the Thus for these two gases correla-
added reliance t h a t can now be tion has been improved in the high-
given to generalized property er T , regions with some loss of
charts when allowances a r e made The kinetic constants b, and E (and
accuracy near t h e critical. It is the gas constants k and R ) will not
for the type of molecular structure. suggested that further work along be discussed here since several pa-
this line may prove profitable. pers on them a r e available in the
CHANGE I N INDEPENDENT
VARIABLE literature (10, 1 1 , 1 2 ) . The impor-
The inconsistency between Equa- EMPIRICAL PSEUDOCRITICAL tant point is that this analytical
CONSTANTS work rests upon a law of corre-
tions ( 2 ) and ( 5 ) was first recog-
nized by Onnes ( 4 ), who proposed To improve the correlation of sponding states of the form
combining x, and v, because in data, corrections can be applied t o
those days critical volumes were the critical constants. Such correc-
open to serious question. The Onnes tions can b e found by either graph-
transformation yields the S u law ical or analytical methods but in
of corresponding states, Equation both methods a solution by trial is which is equivalent, for one ex-
( 6 ) , which is equivalent to Equa- required. Empirical corrections ample, to
tion ( 3 ) , as have been proposed by Morgan and
Childs ( 5 ) and by Maslan and Litt-
m a n ( 6 ) , among others. A study of
certain of these corrections shows
that the correction does no more The Hirschfelder-Bird-Spotz tables
than to shift t h e region of devia- ( 9 ) are limited to the low-density
For that matter, the variable x , tion, and unsuspected errors may
could have been combined with region (up to 40% of t h e critical
arise; for example, the so-called density) since evaluations of t h e
either T , or p,, rather than v,, t o
Newton corrections f o r H 2 and H e fourth and higher virials were not
yield a pseudoreduced temperature
and a pseudoreduced pressure :
+ +
(T, 8, p , 8) when used in re- made. But if values of the reduced
gions below the Boyle-point iso- parameters are plotted as in Fig-
therm have been reported by sev- ure 1 from experimental data [de-
eral investigators (2,7) to cause tails of construction in (791, t h e
errors as much as 40% in z al- resulting plot represents a virial
though improving considerably t h e equation with an infinite number
The laws of corresponding states correlation a t higher temperatures. of terms*. I n other words, Figure
for these two cases are of t h e form It seems reasonable to conclude 1 extends by graphical means the
that the selection of correction Hirschfelder-Bird-Spotz tables to
factors by trial, because of the regions of high density.
labor involved, is practically an im- Once the kinetic parameters are
possibility. determined, pseudocritical con-
The correlations implied by Equa-
tions (3), ( l o ) , and (11) a r e not stants can be directly calculated
equivalent because none of the THEORETICAL PSEUDOCRITICAL from the following equations and
CONSTANTS Equations ( 1 2 ) , ( 1 3 ) , and (14) :
A means f o r obtaining the vari- __
*A series of various compressibility charts can
able correction factors for a select- he obtained by writing to the authore.
Uol. 1, No. 1 A.1.Ch.E. Journal Page 75
m Table 2 has been constructed to
show the agreement of t h e chart
with experimental data and with
values obtained from existing
charts. The data were selected not
p; = ___
P (18) f o r high z values, but to agree es-
C2pc
sentially with Table 2 of Maslan
and Littman (6). Figure 1 does not
include the critical region [where p,L _----
33*5 (64'1)] = 22.52 p , (21)
the method and corrections of 95.4
Meissner and Seferian(2) may be In the same manner, pseudoforce
with values for various gases as preferable]. constants can be determined :
shown in Table 1. As an aside comment on t h e use
of Figure 1, it should be noted t h a t t'lk = 0.756 T , \22)
Example I. Find the compressibili- the isotherms f o r nitrogen corre-
I
t y factor for methane a t 251.5"K. late other gases either on a chart TC
and 131.3 atm. based on critical constants or on a b, = 17- (23)
Pc
I10
dk
N
I00
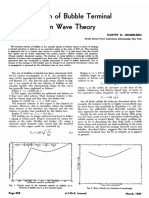
R!.
FIG. 1. GENERALIZEDCOMPRESSIBILITY CHART.
Solution chart such as Figure 1. This fact Example 2 . Find the compressibili-
From Table 1, allows the Bird-Spotz tables and t y factor for ethylene a t 425°K.
Figure 1 to be extended to include and 126 atm.
251.5
_-__- all gases with a f a i r degree of
= 1.70 accuracy ( a t x values above 3 ) . Solution
0.775 (191)
Equations ( 1 2 ) , ( 1 3 ) , and (14)
Rp,, = Rp
__ 82.06 (131.3) -62.1
_____-
= - can be generalized by substituting
Czp, 3.79 (45.8) reduced and critical values of nitro-
gen f o r ?, T and p , for example, p 126
cc. atm Pr - = 2.5
p, 50.5
(Note R = 82.06 Z K )
From Figure 1, x = 0.66 T
= 0.659)
(zeGp
T -__=__ _=
425 1.5
'- T, 283.1
Page 76 A.1.Ch.E. Journal March, 1955
With Equations ( 2 0 ) and 2 1 ) , C, = kinetic critical-temperature E' = pseudoforce constant = 0.756
z = 1.325 T , = 1.325 (1.5) = 1.99 Elk TCk
constant = p T , = kinetic reduced density = gbo
Rp,' = 2 2 . 5 2 ~=~22.52 (2.5) = 56.3 1,
C, = kinetic critical-pressure con- T = kinetic reduced temperature
Figure 1 f o r these values yields R (Elk) = T klE = TICiT,
x = 0.822 , stant= ~
p. = molecular dipole moment
bop,
which is also t h e experimental C3 = kinetic critical-density con- pv = reduced
.. . dipole moment =
value. stant = bo/pc p2/ ( R T , ) ( v , / N )
TABLEI.-A COMPARISON OF T H E CRITICAL CONSTANTSAND T H E KINETICCONSTANTS
FOR SOME SEMISPHERICAL NONPOLAR GASES
Substance Tc"K. pc atm. vc cc./mole E/k OK. bo cc./mole Ct cz c
3
NZ 126.3 33.3 79.8 95.4 64.1 0.752 3.65 1.24
0, 154.8 50.1 74.4 117.0 54.4 0.758 3.53 1.36
Air 132.5 37.2 82.9 102.0 60.3 0.769 3.73 1.33
Ne 44.4 26.9 41.7 34.9 27.1 0.788 3.94 1.54
co 132.9 34.5 90.0 100.2 67.2 0.752 3.55 1.34
CHn 191.0 45.8 98.7 148.2 70.2 0.775 3.79 1.41
A 150.7 48.0 75.2 119.8 49.8 0.794 4.29 1.51
Xe 289.8 57.9 113.7 22 1 86.9 0.764 3.60 1.31
Kr 209.4 54.2 107.3 117 58.9 0.820 4.38 1.83
H* 33.2 12.8 65.0 29.2 29.8 0.877 6.25 2.18
Average (except H2) 0.770 3.82 1.43
TABLE2.-cOMPARISON DATA FOR SOME NONPOLARGASES W I T H
O F EXPERIMENTAL
T H E GENERALIZED
COMPRESSIBILITY CHARTS
T:ry~. Pressure ExD..
_ . - & Childs
Maslan & Littman Mornan Nelson & Obert
11. atm. z Chart z Chart z Chart z
Neon 60.04 59.77 0.712 0.730 0.680 0.715
90.5 41.37 0.964 0.950 0.950 0.967
293.1 61.7 1.040 1.030 1.020 1.043
Argon 157.26 41.9 0.673 0.700 0.675 0.705
215.4 35.1 0.925 0.917 0.920 0.921
293.5 61.7 0.970 0.956 0.962 0.964
373.2 188.0 1.015 0.980 1.020 1.006
co 273.2 150 0.989 ..... 0.962 0.987
323.2 200 1.061 ..... 1.054 1.064
473.2 200 1.101 ..... 1.095 1.103
CHI 248.2 200 0.697 ...... 0.710 0.700
323.2 200 0.881 ...... 0.900 0.874
473.2 300 1.070 ...... 1.085 1.073
If the Bird-Spotz tables a r e to k = Boltzmann constant LITERATURE CITED
be used, Equations ( 2 2 ) and ( 2 3 ) N IAvogadro's number 1. Joffe, J., C h e m . E n g . Progr., 45,
will yield the correct pseudocon- p = pressure, atm. 160 (1949).
stants for the Bird-Spotz equa- p , = critical pressure 2. Meissner, H. P., and R. Seferian,
tions. If the real-force constants 11,. = reduced pressure = plp, Chenz. Eng. Progr., 47, 579
listed by Bird-Spotz f o r ethylene p,.' = pseudoreduced pressure = (1951).
were to be used, t h e x value would p v,i R T , also, kinetic reduced 3. Su. G. J.. Znd. Ena. Chem.. 38. . -
be greatly in error as ethylene does pressure = p k b,/RE = p/c,p, 803 (1946).
4. Kammerlingh Onnes, H., Com-
not have the spherical symmetry R = universal gas constant = 82.06 mun. Phys. Lab. Univ. Leiden, 11,
of the gases listed in Table 1. cc.atm.1mole OK. SuppZ. 23, 115 (1912).
It should be emphasized however yo = collision diameter between 5. Morgan, R. A., and J. H. Childs,
that the method shown in Example two molecules with negligible Znd. E n g . Chem., 37, 669 (1945).
2 can yield no better results than a kinetic energy 6. Maslan, F. D., and T. M. Littman,
good compressibility chart based T = absolute temperature, OK. Znd. E n g . Chem., 45, 1566 (1953).
upon critical constants. The ad- T , = critical temperature 7. Nelson, L. C., and E. F. Obert,
vantage of the proposed method T , = reduced temperature = T I T , Trans. Am. Soc. Mech. Engrs., 76,
lies in the derivatives of t h e Bird- ;'2 = pseudoreduced temperature = 1057 (October, 1954).
Spotz tables, which allow thermo- RT/P,V, 8. Hirschfelder, J. O., R. B. Bird,
dynamic properties to be readily v = specific volume = cc./mole and E. L. Spotz, Trans. Am. SOC.
calculated (rather than t o find v, = critical volume Mech. Engrs., 71, 921 (1949).
compressibility .factors). Since v,. = reduced volume = v l v , 9. Bird, R. B., and E. L. Spotz, U n i v .
the computations for property v,.' = pseudoreduced volume = p,v/ W i s c o n s i n , CM-599 (May, 1950).
values are tedious, no example is .&T, 10. DeBoer, J., and A. Michaels,
given here; the foregoing discus- z = compressibility factor = pvl Physica, 6, 97 (1939).
sion and Equations ( 2 0 ) to ( 2 3 ) RT 11. Hirschfelder, J. O., R. B. Bird,
outline sufficiently the proposed x,, = compressibility factor a t the and E. L. Spotz, C h e m . Revs., 44,
method. critical point = p,vc/ RT, 205 (1949).
12. Lennard-Jones, J. E., Proc. Roy.
NOTATION Greek Letters SOC.( L o n d o n ) , A106, 463 (1924).
b, = kinetic constant = 2/3d?r,3 B = kinetic force constant or maxi- 13. Hall, N. A., and W. Ibele, Pre-
b,' = pseudokinetic constant = 17 mum energy of attraction be- print Paper 54-A-140, Am. SOC.
TCIPG tween two molecules Mech. Engrs. (Dec. 3, 1954).
Vol. 1, No. 1 A.1.Ch.E. Journal Page 77
Anda mungkin juga menyukai
- Mendelson 1967Dokumen4 halamanMendelson 1967maxi roaBelum ada peringkat
- Análisis de Las Estructuras Del Español Irene GartzDokumen265 halamanAnálisis de Las Estructuras Del Español Irene Gartzrerini3720Belum ada peringkat
- Differential Equations and Dinamic SystemsDokumen571 halamanDifferential Equations and Dinamic SystemsBlink SupadreBelum ada peringkat
- Lebon G Et Al - Understanding Non-Equilibrium ThermodynamicsDokumen331 halamanLebon G Et Al - Understanding Non-Equilibrium ThermodynamicsMichele MatteiBelum ada peringkat
- Libro Metodos Cuantitativos I, UnahDokumen286 halamanLibro Metodos Cuantitativos I, UnahdafcoBelum ada peringkat
- BOOK ThermodynamicsDokumen705 halamanBOOK Thermodynamicselnmanea100% (1)
- (Iain G. Currie) Fundamental Mechanics of Fluids PDFDokumen545 halaman(Iain G. Currie) Fundamental Mechanics of Fluids PDFreimoroBelum ada peringkat
- Heat and Mass Transfer PDFDokumen267 halamanHeat and Mass Transfer PDFAngel RoyBelum ada peringkat
- Hydrodynamics Horace LambDokumen728 halamanHydrodynamics Horace LambNishant PandaBelum ada peringkat
- Surface Tension and The Principle of Corresponding StatesDokumen4 halamanSurface Tension and The Principle of Corresponding StatesJose Francisco Olivares QuevedoBelum ada peringkat
- Entanglement Theory and The Second Law of ThermoDokumen5 halamanEntanglement Theory and The Second Law of ThermoAnuj singhBelum ada peringkat
- Mixing RulesDokumen15 halamanMixing RulesCremorlabBelum ada peringkat
- Improved Discretization of The Kardar-Parisi-Zhang Equation: N N N N N NDokumen4 halamanImproved Discretization of The Kardar-Parisi-Zhang Equation: N N N N N NNicolas de la RosaBelum ada peringkat
- A New Cubic Equation of State For Fluids and Fluid MixturesDokumen11 halamanA New Cubic Equation of State For Fluids and Fluid MixturesBriam TicaBelum ada peringkat
- Enkog Castillo1990Dokumen12 halamanEnkog Castillo1990Mikhail TarabrinBelum ada peringkat
- Fluids With Highly Directional Attractive Forces. II. Thermodynamic Perturbation Theory and Integral EquationsDokumen13 halamanFluids With Highly Directional Attractive Forces. II. Thermodynamic Perturbation Theory and Integral EquationsNatalia StefaniaBelum ada peringkat
- Vapor-Liquid Equilibria of Nonideal Solutions: Harrison C. Carlson, and Allan P. ColburnDokumen10 halamanVapor-Liquid Equilibria of Nonideal Solutions: Harrison C. Carlson, and Allan P. ColburnAlfonso Dominguez GonzalezBelum ada peringkat
- Akarsu 2011Dokumen10 halamanAkarsu 2011Rakesh DabgarBelum ada peringkat
- Thermodynamics 2Dokumen77 halamanThermodynamics 213670319Belum ada peringkat
- AgghDokumen4 halamanAgghJoão IderBelum ada peringkat
- Area Laws and Efficient Descriptions of Quantum Many-Body StatesDokumen10 halamanArea Laws and Efficient Descriptions of Quantum Many-Body StatesToon PillaertBelum ada peringkat
- Tables of Hertzian Contact-Stress Coefficients: Ncoordinated Scien Ce LaboratoryDokumen46 halamanTables of Hertzian Contact-Stress Coefficients: Ncoordinated Scien Ce LaboratoryCorneBelum ada peringkat
- Cmes 2006 012 121Dokumen16 halamanCmes 2006 012 121tijsnoyonBelum ada peringkat
- Braak RabiDokumen4 halamanBraak RabiMaryam SalehiBelum ada peringkat
- The Ground State Oi The Bose Gas By: AbstractDokumen16 halamanThe Ground State Oi The Bose Gas By: AbstractAnonymous FigYuONxuuBelum ada peringkat
- Churchill 1977Dokumen7 halamanChurchill 1977LaviejafcBelum ada peringkat
- Tutorial Virial ExpansionDokumen16 halamanTutorial Virial Expansion87871547Belum ada peringkat
- Dirac 1959Dokumen7 halamanDirac 1959Louis DeprezBelum ada peringkat
- Joljrnal of Computational PhysicsDokumen17 halamanJoljrnal of Computational PhysicsKaustubhBelum ada peringkat
- PhysRevA.31.2520 Gas IdealDokumen5 halamanPhysRevA.31.2520 Gas Idealmuhammad fadliBelum ada peringkat
- Materi 3bDokumen6 halamanMateri 3bWit TiaBelum ada peringkat
- Experiment 4Dokumen21 halamanExperiment 4Krizz AstorgaBelum ada peringkat
- 0378 38122987010 7Dokumen15 halaman0378 38122987010 7Tiên PhạmBelum ada peringkat
- Cosmologia Con Energia Oscura Tipo RicciDokumen6 halamanCosmologia Con Energia Oscura Tipo RicciWilliam AlgonerBelum ada peringkat
- Chapter 1. Review of Thermodynamics: Essential Graduate Physics SM: Statistical MechanicsDokumen24 halamanChapter 1. Review of Thermodynamics: Essential Graduate Physics SM: Statistical MechanicsRauni MarquesBelum ada peringkat
- Nasa TN D-2502Dokumen20 halamanNasa TN D-2502danishmoin1991100% (1)
- The Behaviour of Porous Catalyst Particles in View of Internal Mass and Heat Diffusion EffectsDokumen8 halamanThe Behaviour of Porous Catalyst Particles in View of Internal Mass and Heat Diffusion EffectsJosé Manuel CarreónBelum ada peringkat
- A Comparison of Equations of StateDokumen8 halamanA Comparison of Equations of StateDarren Sean HoBelum ada peringkat
- Householder 1939Dokumen13 halamanHouseholder 1939manuel.araya.floresBelum ada peringkat
- Agmon 1964Dokumen58 halamanAgmon 1964CoBelum ada peringkat
- Entropy and The Uncertainty PrincipleDokumen6 halamanEntropy and The Uncertainty Principlebmalki68Belum ada peringkat
- Liquid-Liquid: and Heat Transfer From in ExtractionDokumen10 halamanLiquid-Liquid: and Heat Transfer From in ExtractionGustavo Gabriel JimenezBelum ada peringkat
- A Liquid-State Theory That Remains Successful in The Critical RegionDokumen28 halamanA Liquid-State Theory That Remains Successful in The Critical Regionadam_k113Belum ada peringkat
- Bruno Nachtergaele - Bounds On The Mass Gap of The Ferromagnetic XXZ ChainDokumen18 halamanBruno Nachtergaele - Bounds On The Mass Gap of The Ferromagnetic XXZ ChainPo48HSDBelum ada peringkat
- Steady-State Measurements and Analytical Correlations of Axial Effective Thermal Conductivities in Packed Beds at Low Gas Flow RatesDokumen8 halamanSteady-State Measurements and Analytical Correlations of Axial Effective Thermal Conductivities in Packed Beds at Low Gas Flow Ratescpgcha57Belum ada peringkat
- Kubo Formulae For Second-Order Hydrodynamic CoefficientsDokumen4 halamanKubo Formulae For Second-Order Hydrodynamic CoefficientskayBelum ada peringkat
- 15938.45 252 260 355 24 BoettcherDokumen9 halaman15938.45 252 260 355 24 BoettcherF SedighiBelum ada peringkat
- Virial Equation of StateDokumen9 halamanVirial Equation of StateSaba ArifBelum ada peringkat
- Cherman Et Al. - 2019 - Bose-Fermi Cancellations Without SupersymmetryDokumen21 halamanCherman Et Al. - 2019 - Bose-Fermi Cancellations Without SupersymmetryvdbBelum ada peringkat
- Hayden 1975Dokumen8 halamanHayden 1975SandraColoradoBelum ada peringkat
- Ash Kin 1943Dokumen7 halamanAsh Kin 1943Boris Atenas NuñezBelum ada peringkat
- W e A K Commutativity (Ao B B o A) (X,, yDokumen16 halamanW e A K Commutativity (Ao B B o A) (X,, yPress OfficeBelum ada peringkat
- Phase Transition of Real Gases. Journal 1Dokumen15 halamanPhase Transition of Real Gases. Journal 1Neil BrionesBelum ada peringkat
- RK Mixing RulesDokumen7 halamanRK Mixing RulesadrianrrccBelum ada peringkat
- Modification of Hall-Yarborough Equation of State For Predicting Gas Compressibility FactorsDokumen11 halamanModification of Hall-Yarborough Equation of State For Predicting Gas Compressibility FactorsNicolas CastañoBelum ada peringkat
- 49 Wertheim1984 PDFDokumen16 halaman49 Wertheim1984 PDFNatalia StefaniaBelum ada peringkat
- Significance of Ehrenfest Theorem, D Sen, 2001, (6p)Dokumen6 halamanSignificance of Ehrenfest Theorem, D Sen, 2001, (6p)Elcan DiogenesBelum ada peringkat
- Conformal Field Theory Interpretation of Black Hole Quasi-Normal ModesDokumen4 halamanConformal Field Theory Interpretation of Black Hole Quasi-Normal ModesLeonardo BossiBelum ada peringkat
- Francisco C. Alcaraz Et Al - Three-Dimensional Spin Systems Without Long-Range OrderDokumen26 halamanFrancisco C. Alcaraz Et Al - Three-Dimensional Spin Systems Without Long-Range OrderImaxSWBelum ada peringkat
- Momentum Absorption by Vegetation: Quart. 97Dokumen16 halamanMomentum Absorption by Vegetation: Quart. 97Ivan RodrigoBelum ada peringkat
- Momentum Transfer at The Boundary Between A Porus Medium and A Homogeneous FluidDokumen12 halamanMomentum Transfer at The Boundary Between A Porus Medium and A Homogeneous FluidIvan RodrigoBelum ada peringkat
- H - III: United States Patent (19) 11 Patent Number: 5,153,131Dokumen10 halamanH - III: United States Patent (19) 11 Patent Number: 5,153,131Ivan RodrigoBelum ada peringkat
- Orbital Angular Momentum of Light and The Transformation of Laguerre-Gaussian Laser ModesDokumen6 halamanOrbital Angular Momentum of Light and The Transformation of Laguerre-Gaussian Laser ModesIvan RodrigoBelum ada peringkat
- Duction Coll: Kaon ONS (1), C. atDokumen7 halamanDuction Coll: Kaon ONS (1), C. atIvan RodrigoBelum ada peringkat
- On The Dynamic Behavior of Continuous Stirred Tank ReactorsDokumen19 halamanOn The Dynamic Behavior of Continuous Stirred Tank ReactorsIvan RodrigoBelum ada peringkat
- Zawodzinski1991 PDFDokumen5 halamanZawodzinski1991 PDFIvan RodrigoBelum ada peringkat
- Efective Mass Transfer Area - de BritoDokumen10 halamanEfective Mass Transfer Area - de BritoIvan RodrigoBelum ada peringkat
- Factsheet C UnderstandingJobHazards ESDokumen1 halamanFactsheet C UnderstandingJobHazards ESIvan RodrigoBelum ada peringkat
- ANSI Tabla 4.1Dokumen1 halamanANSI Tabla 4.1Ivan RodrigoBelum ada peringkat
- Catálogo de Bombas de Diafragma - ARODokumen52 halamanCatálogo de Bombas de Diafragma - AROIvan RodrigoBelum ada peringkat
- Liquid Surge Capacity - Mehra PDFDokumen2 halamanLiquid Surge Capacity - Mehra PDFIvan RodrigoBelum ada peringkat
- Diseño de Reactor de BurbujeoDokumen23 halamanDiseño de Reactor de BurbujeoIvan RodrigoBelum ada peringkat
- Performance of Packed Columns - Shulman 1955Dokumen7 halamanPerformance of Packed Columns - Shulman 1955Ivan RodrigoBelum ada peringkat
- Ceramic Surface TensionDokumen104 halamanCeramic Surface TensionIvan RodrigoBelum ada peringkat
- Effect of Producer Gas Addition and Air Excess RatioDokumen12 halamanEffect of Producer Gas Addition and Air Excess RatiodfcortesvBelum ada peringkat
- Super-Laminiir Flow Beaills I I S SeilsDokumen510 halamanSuper-Laminiir Flow Beaills I I S SeilsJuan Luis Campos CiezaBelum ada peringkat
- Activity sHEETS Q4Dokumen5 halamanActivity sHEETS Q4Jim Alvarez RomeroBelum ada peringkat
- XII Notes WajahatDokumen237 halamanXII Notes Wajahatsaim.akhtar.4.5.6Belum ada peringkat
- Thermodynamics Sample ProblemsDokumen42 halamanThermodynamics Sample ProblemsCarlo Quinsayas SablanBelum ada peringkat
- 11 ChapterDokumen24 halaman11 ChapterShashwat SahayBelum ada peringkat
- Tutorial Materials SelectionDokumen2 halamanTutorial Materials SelectionSyahmiBelum ada peringkat
- Oil MistDokumen14 halamanOil MistazisyuswandiBelum ada peringkat
- HRSG SimulationDokumen7 halamanHRSG Simulationkaruna346Belum ada peringkat
- Erosion in TurbomachinaryDokumen11 halamanErosion in TurbomachinaryJulio Herrera Venegas100% (1)
- Final Exam Fall 2013Dokumen10 halamanFinal Exam Fall 2013JOSHUA WHEDEGARBelum ada peringkat
- Chemistry 9 Class Paper JalalDokumen1 halamanChemistry 9 Class Paper JalalCRO TJSSBelum ada peringkat
- Aeronautical Engineering 2nd Year SyllabusDokumen39 halamanAeronautical Engineering 2nd Year SyllabusAditya ThakurBelum ada peringkat
- Chemistry 101A General College Chemistry PDFDokumen603 halamanChemistry 101A General College Chemistry PDFMark ChangBelum ada peringkat
- Basic Pecvd Plasma Processes (Sih Based) : Pecvd Sinx: Sih + NH (+H) or Sih + N (+H)Dokumen36 halamanBasic Pecvd Plasma Processes (Sih Based) : Pecvd Sinx: Sih + NH (+H) or Sih + N (+H)Anonymous lidok7lDiBelum ada peringkat
- Module The MoleDokumen44 halamanModule The MoleChin Chin YipBelum ada peringkat
- Science KS3 Handbook PDFDokumen8 halamanScience KS3 Handbook PDFLê Quốc Nhật VinhBelum ada peringkat
- Ch.4 Cementing AdditivesDokumen10 halamanCh.4 Cementing AdditivesHoan HoanBelum ada peringkat
- FIORENTINI Gas MeteringDokumen4 halamanFIORENTINI Gas MeteringggrapsasBelum ada peringkat
- Lecture 1 - Matter and Its Properties (Corrected)Dokumen5 halamanLecture 1 - Matter and Its Properties (Corrected)katey perryBelum ada peringkat
- Cangas Manual - enDokumen41 halamanCangas Manual - enDaniel BarbuBelum ada peringkat
- Emx20Clc: General DataDokumen3 halamanEmx20Clc: General Dataأبو زينب المهندسBelum ada peringkat
- Chapter 9:hydraulics and PneumaticsDokumen14 halamanChapter 9:hydraulics and PneumaticsGovind RajBelum ada peringkat
- Underbalance Drilling Manual PDFDokumen36 halamanUnderbalance Drilling Manual PDFCami CorderoBelum ada peringkat
- Properties of AirDokumen4 halamanProperties of AirNachiketBelum ada peringkat
- Redox ReactionDokumen52 halamanRedox ReactionChauhan DharmendraBelum ada peringkat
- Orifice Sizing Instructions TablesDokumen8 halamanOrifice Sizing Instructions TablesMatt AndersonBelum ada peringkat
- Exc 7 Gas SM 09Dokumen3 halamanExc 7 Gas SM 09Tushar AgrawalBelum ada peringkat
- Flow Measurement PDFDokumen81 halamanFlow Measurement PDFCarlos PalmaBelum ada peringkat