Experiment 6.2
Diunggah oleh
jueliiyaHak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Experiment 6.2
Diunggah oleh
jueliiyaHak Cipta:
Format Tersedia
Name : TP 4 DAN TP 5 Class :
Experiment 6.2
Problem statement : What are the differences between metals and non-metals?
Hyphothesis : Metal and non-metals are different in the aspects of appearance, ductility,
malleability, electrical conductivity, heat conductivity and melting point.
Aim : To distinguish between the physical properties of metals and non-metals
Manipulated variable : Type of material
Responding variable : Physical properties of the material
Constant variable : Type of sandpaper, strength used to bend and hammer the material, length of
material and size of material
Materials and apparatus : Carbon rod, copper rod, glass rod, sandpaper, wooden block, hammer,
dry cells, switch, crocodile clips, ammeter, Bunsen burner, tripod stand, thumbtacks, wire gauze,
copper foil, sulphur foil and crucible
Procedure Results Discussion
A – Appearances a- Copper rod=
1. The surface of a b- Carbon rod=
copper rod and carbon
rod are rubbed with a
sand paper
2. The appearance of the
copper and carbon rods
are observed and
recorded
B- Ductility a- Copper wire
1. A copper rod and b- Pencil lead
carbon rod are bent
2. The changes in the
copper and carbon rods
are observed and
recorded
C- Malleability a- Iron piece-
1. A IRON PIECE and b- Copper piece-
CARBON PIECE are
hammered
2. The change in the
shape of the copper
and carbon rods are
observed and recorded
D – Electrical conductivity a- Copper -
1. An electrical circuit is b- Iron –
set up. c- Carbon -
2. Both ends of the
copper rod are
connected to crocodile
clips
3. Deflection of the
ammeters needle is
observed and recorded
4. The steps above are
repeated using a glass
rod
E – Heat conductivity a- Copper –
1. Two thumbtacks are b- Iron –
attached to one ends of c- Carbon -
the copper rod and
glass rod with wax
2. The other ends of the
copper and carbon rods
are heated
simultaneously
3. The order of the
thumbtacks falling off
is observed and
recorded
F – Melting point 1. Copper foil does not 1. Copper has a high
1. A copper foil is placed melts melting point
into a crucible and 2. Sulphur foil melts 2. Sulphur has a low
heated easily melting point
2. The time taken for
copper foil to melt is
recorded
3. The steps above are
repeated using a
sulphur foil
Conclusion :
QUESTION :
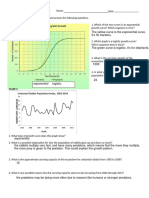
1. What is the difference between the appearance of copper and carbon rod surface?
2. Which one can conduct electricity, iron, carbon or sulphur?
3. Between iron, and carbon rod, which one can conduct heat?
4. Between tin and sulphur powder, which one has a higher melting point?
Anda mungkin juga menyukai
- Long QuizDokumen2 halamanLong QuizvilmarBelum ada peringkat
- Climate Change Lesson PlanDokumen3 halamanClimate Change Lesson PlanApple ArellanoBelum ada peringkat
- Environmental Security in The Philippines - POWERPOINTDokumen23 halamanEnvironmental Security in The Philippines - POWERPOINTDerrick de los ReyesBelum ada peringkat
- 1.2 - HydrocarbonsDokumen3 halaman1.2 - HydrocarbonskiranBelum ada peringkat
- Lewis Dot Structure Mini Lessonand WorksheetDokumen3 halamanLewis Dot Structure Mini Lessonand WorksheetElmaBelum ada peringkat
- Evidence Submission FormDokumen1 halamanEvidence Submission FormDimasalang PerezBelum ada peringkat
- Brgy NedDokumen2 halamanBrgy Nedjerickhu100% (1)
- Biotechnology 8 Worksheet 1 Topic: Introduction To BiotechologyDokumen3 halamanBiotechnology 8 Worksheet 1 Topic: Introduction To BiotechologyElcyn Andrew BoocBelum ada peringkat
- Measuring The Level of Awareness of Cabadbaranon in Solid Waste ManagementDokumen7 halamanMeasuring The Level of Awareness of Cabadbaranon in Solid Waste ManagementJeczayde Veign ReyesBelum ada peringkat
- 79 1 ET V1 S1 - Unit - 1 PDFDokumen14 halaman79 1 ET V1 S1 - Unit - 1 PDFvandana upadhyayBelum ada peringkat
- Art 27 30 RPCDokumen18 halamanArt 27 30 RPCKimmy Domingo0% (1)
- Application Form: Professional Regulation CommissionDokumen1 halamanApplication Form: Professional Regulation CommissionMercia AlidoBelum ada peringkat
- Earth's Atmosphere: Culture SocietyDokumen9 halamanEarth's Atmosphere: Culture SocietyGavin FabeliñaBelum ada peringkat
- Las Science 9 Melc 1 q4 Week-1Dokumen4 halamanLas Science 9 Melc 1 q4 Week-1ROVELYN FURATEROBelum ada peringkat
- Quiz 4 Agriculture and Industrial Revolutions (Open Note Quiz)Dokumen5 halamanQuiz 4 Agriculture and Industrial Revolutions (Open Note Quiz)jessicaraealt0% (1)
- Chapter Two Review of Related Literature and Studies 2.1 EarthquakesDokumen8 halamanChapter Two Review of Related Literature and Studies 2.1 EarthquakesJholo BuctonBelum ada peringkat
- 2022-23 Hornby Trust Teacher Association Projects-Application - Guidance - E8q4s6CIS5Wd0LYT6FMFDokumen4 halaman2022-23 Hornby Trust Teacher Association Projects-Application - Guidance - E8q4s6CIS5Wd0LYT6FMFCaroline MiBelum ada peringkat
- Climate Grade 9 ActivityDokumen5 halamanClimate Grade 9 ActivityAlexes Alyza CañeteBelum ada peringkat
- Rolling ToyDokumen2 halamanRolling Toywendell wendellBelum ada peringkat
- Movie Critique, NEO, RALPH LOWIE L.Dokumen3 halamanMovie Critique, NEO, RALPH LOWIE L.Ralph Lowie NeoBelum ada peringkat
- Chemical BondingDokumen27 halamanChemical BondingLovejoy TiñaBelum ada peringkat
- P.D. 984 Pollution Control LawDokumen12 halamanP.D. 984 Pollution Control Lawwinard2175% (4)
- Module 2 Lesson 5 Weather Disturbances and VariabilityDokumen41 halamanModule 2 Lesson 5 Weather Disturbances and VariabilityClarisse DiconBelum ada peringkat
- Newtons Laws PowerpointDokumen29 halamanNewtons Laws PowerpointAriel KedemBelum ada peringkat
- Wave Mechanics 1Dokumen8 halamanWave Mechanics 1DeanielBelum ada peringkat
- PDF 4th Quarter Examination Science 7 - CompressDokumen3 halamanPDF 4th Quarter Examination Science 7 - CompressRhean Mae AddatuBelum ada peringkat
- SCIENCE LAS-week1&2Dokumen4 halamanSCIENCE LAS-week1&2Cas AnnBelum ada peringkat
- Gay LussacDokumen2 halamanGay LussacKelvin BarceLonBelum ada peringkat
- Lesson 3 Phy 1Dokumen20 halamanLesson 3 Phy 1Gen Z LearnersBelum ada peringkat
- Takdang Aralin Sa Araling Panlipunan Rizzan Murillo 1Dokumen5 halamanTakdang Aralin Sa Araling Panlipunan Rizzan Murillo 1Haydee DionaldoBelum ada peringkat
- Module 4-The Reproductive Health LawDokumen3 halamanModule 4-The Reproductive Health LawjessafesalazarBelum ada peringkat
- Performance Task 2 (Term3-FINALS)Dokumen2 halamanPerformance Task 2 (Term3-FINALS)Emmanuel Mascuñana BacayBelum ada peringkat
- The Relationship Between The Visible Constellations in The Sky and Earth's Position Along Its OrbitDokumen4 halamanThe Relationship Between The Visible Constellations in The Sky and Earth's Position Along Its OrbitMelbert John Gregorio GarciaBelum ada peringkat
- Research Chapter 1Dokumen16 halamanResearch Chapter 1Leonie Cruz ReyesBelum ada peringkat
- Reflection and RefractionDokumen27 halamanReflection and RefractionEstelaBenegildoBelum ada peringkat
- Education and Law Related Studies Final Examination PDFDokumen1 halamanEducation and Law Related Studies Final Examination PDFTan-Uy Jefferson Jay100% (1)
- Energy Flow Through The EcosystemDokumen5 halamanEnergy Flow Through The EcosystemCiprian BitcaBelum ada peringkat
- Structure of Organic CompoundsDokumen27 halamanStructure of Organic CompoundsBetty Weiss100% (1)
- Applications of Isotopes C11!3!01&C11!3!02Dokumen12 halamanApplications of Isotopes C11!3!01&C11!3!02Olivia M OliverBelum ada peringkat
- Q4, Peformance Task #2 - Ener-Vention!Dokumen2 halamanQ4, Peformance Task #2 - Ener-Vention!Ericha Solomon100% (1)
- Family-Planning NSTPDokumen2 halamanFamily-Planning NSTPMary Grace Marquez ReanoBelum ada peringkat
- Sponsorship Letter SKATE and MUSIC FEST DAY 2019Dokumen4 halamanSponsorship Letter SKATE and MUSIC FEST DAY 2019MaraBelum ada peringkat
- Curriculum-Mapping PHYSICS 9Dokumen8 halamanCurriculum-Mapping PHYSICS 9kristinebarredoBelum ada peringkat
- Laboratory Activity NO. 2: Structure of HydrocarbonsDokumen14 halamanLaboratory Activity NO. 2: Structure of HydrocarbonsLyra Ane IlaganBelum ada peringkat
- 9 PPT Images Formed in LensesDokumen43 halaman9 PPT Images Formed in LensesBianca Manimtim100% (1)
- Bautista vs. ApareceDokumen7 halamanBautista vs. Aparecejoselle gaviolaBelum ada peringkat
- An Overview: Comprehensive Dangerous Drugs Act OF 2002Dokumen37 halamanAn Overview: Comprehensive Dangerous Drugs Act OF 2002aldin100% (1)
- The Law On Natural Resources Bit College of LawDokumen3 halamanThe Law On Natural Resources Bit College of LawdanBelum ada peringkat
- Q1-Week 5-Convergent Plate BoundaryDokumen3 halamanQ1-Week 5-Convergent Plate BoundarykumiBelum ada peringkat
- GEWE Plan 2019 2025 Results MatricesDokumen106 halamanGEWE Plan 2019 2025 Results Matricesruby casasBelum ada peringkat
- GR 105 Scra 359Dokumen2 halamanGR 105 Scra 359icBelum ada peringkat
- Third Written Test in Science 8 QUARTER 4, SY 2021-2022 Instructions: Read Each Question Carefully and Write The Correct Answer in ADokumen5 halamanThird Written Test in Science 8 QUARTER 4, SY 2021-2022 Instructions: Read Each Question Carefully and Write The Correct Answer in Ajoan marie PeliasBelum ada peringkat
- 7 E Model Lesson PlanDokumen7 halaman7 E Model Lesson Plangloria tolentinoBelum ada peringkat
- Lesson Plan Gas Laws, Laws of Chemical Combination, Carbon and Its CompoundsDokumen8 halamanLesson Plan Gas Laws, Laws of Chemical Combination, Carbon and Its CompoundsGBENGA50% (2)
- Improvised PeriscopeDokumen1 halamanImprovised PeriscopeNovy-Ann SanchoBelum ada peringkat
- L - Logistic and Exponential Graphs WorksheetDokumen2 halamanL - Logistic and Exponential Graphs WorksheetHexagon LyricsBelum ada peringkat
- Matter Classification WorksheetDokumen7 halamanMatter Classification WorksheetAsru Rojam100% (1)
- Achieving Economy of ExpressionsDokumen2 halamanAchieving Economy of ExpressionsAllen Leyola JaroBelum ada peringkat
- Experiment SadurDokumen4 halamanExperiment SadurApik Suhimi100% (1)
- Draw Figure 4.5, Figure 4.6, Figure 4.7 and Figure 4.8Dokumen5 halamanDraw Figure 4.5, Figure 4.6, Figure 4.7 and Figure 4.8cyberbat2008Belum ada peringkat
- Recommended Daily Caloric Intake: ChartDokumen1 halamanRecommended Daily Caloric Intake: ChartMohd Fakrul Zaman OthmanBelum ada peringkat
- Water Treatment PlantDokumen3 halamanWater Treatment PlantjueliiyaBelum ada peringkat
- Activity 1.11Dokumen1 halamanActivity 1.11jueliiya0% (1)
- Criteria Aspects of Being AssessedDokumen6 halamanCriteria Aspects of Being AssessedjueliiyaBelum ada peringkat
- Form 2 Science Chapter 7Dokumen32 halamanForm 2 Science Chapter 7qq23582% (11)
- Activity 6.2 Aim: To Determine Acidic and Alkaline Substances in Daily Life Using Various Indicators. Observation Substance IndicatorDokumen1 halamanActivity 6.2 Aim: To Determine Acidic and Alkaline Substances in Daily Life Using Various Indicators. Observation Substance IndicatorjueliiyaBelum ada peringkat
- Composting ProcessDokumen5 halamanComposting ProcessjueliiyaBelum ada peringkat
- Chapter 1 Introduction To Science PDFDokumen8 halamanChapter 1 Introduction To Science PDFAnonymous 7pbegA100% (2)
- Learning Objective: 1.3 Understanding That Cells Form Tissues, Organs and Systems in HumanDokumen2 halamanLearning Objective: 1.3 Understanding That Cells Form Tissues, Organs and Systems in HumanjueliiyaBelum ada peringkat
- Learning Objective: 1.1 Understanding CellsDokumen2 halamanLearning Objective: 1.1 Understanding CellsNhs NhsBelum ada peringkat
- 1 Monday 1.1.2018 5SV 8.00 AM-9.10 AM Science Microorganisms and Their Effects On Living ThingsDokumen4 halaman1 Monday 1.1.2018 5SV 8.00 AM-9.10 AM Science Microorganisms and Their Effects On Living ThingsjueliiyaBelum ada peringkat
- L Obj 1.2Dokumen2 halamanL Obj 1.2jueliiyaBelum ada peringkat
- Learning Objective: 1.1 Understanding That Matter Has Mass and Occupies SpaceDokumen2 halamanLearning Objective: 1.1 Understanding That Matter Has Mass and Occupies SpacejueliiyaBelum ada peringkat
- Learning Objective: 1.1 Understanding CellsDokumen2 halamanLearning Objective: 1.1 Understanding CellsjueliiyaBelum ada peringkat
- Differences Between Compound and MixturesDokumen2 halamanDifferences Between Compound and MixturesjueliiyaBelum ada peringkat
- b2 Sel Haiwan Dan TumbuhanDokumen2 halamanb2 Sel Haiwan Dan TumbuhanNhs NhsBelum ada peringkat
- L Obj 1.3Dokumen2 halamanL Obj 1.3jueliiyaBelum ada peringkat
- State The Steps in Scientific Investigation. 1 Phrases BelowDokumen2 halamanState The Steps in Scientific Investigation. 1 Phrases Belowazamainz123Belum ada peringkat
- Part of Microscope FunctionDokumen2 halamanPart of Microscope FunctionjueliiyaBelum ada peringkat
- Learning Objective: 1.4 Understanding The Use of Measuring ToolsDokumen2 halamanLearning Objective: 1.4 Understanding The Use of Measuring Toolsapit9Belum ada peringkat
- L Obj 1.1Dokumen2 halamanL Obj 1.1Norddin Bin AhmadBelum ada peringkat
- 4.3 The Importance of The Variety of Earth's Resources To ManDokumen1 halaman4.3 The Importance of The Variety of Earth's Resources To ManjueliiyaBelum ada peringkat
- ReviewDokumen2 halamanReviewjueliiyaBelum ada peringkat
- Brownian MotionDokumen2 halamanBrownian MotionjueliiyaBelum ada peringkat
- Part of Microscope FunctionDokumen2 halamanPart of Microscope FunctionjueliiyaBelum ada peringkat
- 6.2 Renewable and Non-RenewableDokumen2 halaman6.2 Renewable and Non-RenewablejueliiyaBelum ada peringkat
- 4.3 The Importance of The Variety of Earth's Resources To ManDokumen1 halaman4.3 The Importance of The Variety of Earth's Resources To ManjueliiyaBelum ada peringkat
- 7.2 Heat Flow and Its EffectDokumen2 halaman7.2 Heat Flow and Its EffectjueliiyaBelum ada peringkat
- 6.1 Various Forms and Sources of EnergyDokumen3 halaman6.1 Various Forms and Sources of EnergyjueliiyaBelum ada peringkat
- Trueline Reference Catalog KGVDokumen20 halamanTrueline Reference Catalog KGVtoto-gmbhBelum ada peringkat
- Chem-CGS P1Dokumen17 halamanChem-CGS P1dimpledblissBelum ada peringkat
- Application of Carbon Nanotubes in Solar EnergyDokumen30 halamanApplication of Carbon Nanotubes in Solar EnergyZohaib NarejoBelum ada peringkat
- Kinetic Modeling PFR - FluidizedDokumen8 halamanKinetic Modeling PFR - FluidizedTeo Han ChuinBelum ada peringkat
- Carbon-14 in Diamonds: The UCI DataDokumen14 halamanCarbon-14 in Diamonds: The UCI DataSean Pitman, MDBelum ada peringkat
- CBSE-X Chapterwise (Previous Years) Qs - Science - SOL-min PDFDokumen60 halamanCBSE-X Chapterwise (Previous Years) Qs - Science - SOL-min PDFmathanagopal balasundram100% (1)
- Latihan Soal Difusi PadatanDokumen3 halamanLatihan Soal Difusi Padatanyosephine92100% (1)
- AITS 1819 FT X ADV PAPER 1 SolDokumen10 halamanAITS 1819 FT X ADV PAPER 1 SolDEBJIT SHARMABelum ada peringkat
- AOC Corrosion GuideDokumen36 halamanAOC Corrosion Guidetarun.imdrBelum ada peringkat
- Articol Privind Diferenta Data de Negru FumDokumen10 halamanArticol Privind Diferenta Data de Negru FumcronoromBelum ada peringkat
- What Is Organic ChemistryDokumen3 halamanWhat Is Organic ChemistryERICKA DIANNE ABELLABelum ada peringkat
- ICAR JRF Question Paper With Answers 2009 Free Download General Agriculture Paper 2009 ICAR JRF General Agriculture For ICAR All India Entrance Examination For Admission To Master Degree Programmer 20Dokumen5 halamanICAR JRF Question Paper With Answers 2009 Free Download General Agriculture Paper 2009 ICAR JRF General Agriculture For ICAR All India Entrance Examination For Admission To Master Degree Programmer 20Rama Krishnan Subbiah75% (4)
- An Automated Media Fill Microbial InspectionDokumen4 halamanAn Automated Media Fill Microbial InspectionTorres XiaBelum ada peringkat
- Homework Booklet (4, S)Dokumen55 halamanHomework Booklet (4, S)VarshLokBelum ada peringkat
- Kuiz BombaDokumen2 halamanKuiz Bombakaren limBelum ada peringkat
- IBIA Glossary of TerminologyDokumen65 halamanIBIA Glossary of TerminologyJohn GreenBelum ada peringkat
- Organic Compounds and The Atomic Properties of CarbonDokumen110 halamanOrganic Compounds and The Atomic Properties of Carbonmahbobullah rahmaniBelum ada peringkat
- Galata CFA Properties GuideDokumen12 halamanGalata CFA Properties GuideToniHospitalerBelum ada peringkat
- Mat Paper: Space For Rough WorkDokumen24 halamanMat Paper: Space For Rough WorkDatta NawadikarBelum ada peringkat
- Pure Metals: 3.2 Bonding: The Structure of MatterDokumen5 halamanPure Metals: 3.2 Bonding: The Structure of MatterVickneswary MuniyanBelum ada peringkat
- Everyday Science MCQsDokumen469 halamanEveryday Science MCQsFarhan AbbasBelum ada peringkat
- Tugas Kimia Kelas A - RevisiDokumen2 halamanTugas Kimia Kelas A - RevisiYudha Pratama SitumorangBelum ada peringkat
- Chem 1 Quiz 3 StoichiometryDokumen2 halamanChem 1 Quiz 3 StoichiometryHailey Zane IgarashiBelum ada peringkat
- Carbon and Water Cycle Work SheetDokumen4 halamanCarbon and Water Cycle Work SheetRasha GhabbounBelum ada peringkat
- Thermophysical Properties of Carbon DioxideDokumen4 halamanThermophysical Properties of Carbon DioxideAnonymous 6cQRWqBelum ada peringkat
- Perkin and Kipping's Organic Chemistry, Part IIDokumen362 halamanPerkin and Kipping's Organic Chemistry, Part IIPetrovic NenadBelum ada peringkat
- Why Is Carbon So Important in Spite of It Being Present in A Very Small QuantityDokumen2 halamanWhy Is Carbon So Important in Spite of It Being Present in A Very Small QuantitySuman DasBelum ada peringkat
- Carbon Nanotube From CementDokumen17 halamanCarbon Nanotube From CementazizhamdiBelum ada peringkat
- Basic Concepts of ChemistryDokumen2 halamanBasic Concepts of ChemistryVenkitaraj K PBelum ada peringkat
- Cbse Class XI Chemistry Sample Paper - 2 Solution Section ADokumen12 halamanCbse Class XI Chemistry Sample Paper - 2 Solution Section ABhabaniBelum ada peringkat