Tid/g: P-D Hybridization Gives Rise To An Antiferromagnetic Cu2+-O-exchange
Diunggah oleh
KetanJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Tid/g: P-D Hybridization Gives Rise To An Antiferromagnetic Cu2+-O-exchange
Diunggah oleh
KetanHak Cipta:
Format Tersedia
5.
5 Correlated Insulators 249
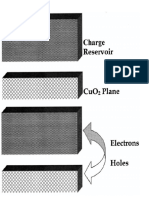
non-conductor, but also an extra spin into an ordered magnet. Namely,
a phole amounts to leaving one of the plobes of an oxygen ion with
aa uncompensated spin. This spin is sorrounded by Cu-site spins, and
p-d hybridization gives rise to an antiferromagnetic Cu2+-O- exchange
interaction which consists of contributions t i d / g and
N t:d/(Ud -
N
ep). Actually, it is best to start by considering the four oxygen sites
surrounding a central copper site, and let the phole spread out to the
four sites occupying an orbital which has the same symmetry as the spin-
carrying d-orbital at the central Cu site. This pstate binds optimally
to the central d-spin, forming a Bang-Rice singlet [468]. Low-level Sr
doping of La2CuO4 can be envisaged as giving rise to a dilute gas of
Zhang-Rice singlets which, though they are fairly complex, spread-out
objectsao, still can propagate21.
The cwe of electron doping is different in the sense that it does
not distinguish between the two classes of insulators: in either case,
added electrons go into the upper Hubbard subband". This means that
electron doping and hole doping play asymmetric roles in charge transfer
insulators: for instance, electron-type LazCuO4-like superconductors (of
which Nd2-,CeZCu04 provides an example) we very different from the
hole-type ones. In contrast, for a Mott insulator, the effects of electron-
doping and holedoping can be interpreted in a unified scheme.
Doping is often not intentional at all. Apart from the unavoidable
impurities, transition metal oxides just do not want to grow stoichiomet-
rically: it is not unusual to find several per cent deviation of the oxygen
content from its nominal integer value23. This is a serious problem:
20Lookingfrom far away, we see three charges missing from the immediate envi-
ronment of a Cu atom, so we may think it is the low-spin state of Cu3+. At a closer
look, into resolves into an ordinary CuZf bound to a surrounding phole complex. It
is a not unfrequent experience that unwmmonly looking higher oxidation states of
metal ions had better be envisaged as the ion in a lower oxidation state, bound by
exchange interaction to a surrounding ligand hole.
21Hawever,consideration of the long-range Coulomb force may lead to the conclu-
sion that they localize into thin domain walls. See p. 568.
22Wemight have thought that sometimes they go into the 43 band. However, this
case does not seem to be realized.
231n spite of this fact, COO or magnetite are not metallic. The reason is that,
because of the narrowness of the 3d-band, polaronic effects come into play. Small
polaton8 are heavy quasi-particles which are readiiy localized by the random potentid
Anda mungkin juga menyukai
- Electronic Layers in Copper Oxide SuperconductorsDokumen1 halamanElectronic Layers in Copper Oxide SuperconductorsSJBelum ada peringkat
- Metallic Character: Transition-ElementsDokumen10 halamanMetallic Character: Transition-ElementsRadamael MaembongBelum ada peringkat
- Why Mercury Liquid?: Or, Why Do Relativistic Effects Not Get Into Chemistry Textbooks?Dokumen4 halamanWhy Mercury Liquid?: Or, Why Do Relativistic Effects Not Get Into Chemistry Textbooks?leonardo_strajaneliBelum ada peringkat
- Chem 3621 Assigment Memo-1Dokumen6 halamanChem 3621 Assigment Memo-1MULAMULELI RAMURUNZIBelum ada peringkat
- Physics of Copper in SiliconDokumen11 halamanPhysics of Copper in SiliconAce CerBelum ada peringkat
- Tpss 435: Chapter 6: Cation Exchange ReactionsDokumen11 halamanTpss 435: Chapter 6: Cation Exchange ReactionsRonny NguyenBelum ada peringkat
- Copper ActivationDokumen17 halamanCopper ActivationJoseph RamirezBelum ada peringkat
- Metallic Character: Transition-ElementsDokumen9 halamanMetallic Character: Transition-ElementsaimanhazimBelum ada peringkat
- ATOICV1 7 0 Metal Ligand BondingDokumen42 halamanATOICV1 7 0 Metal Ligand BondingGote KhusiBelum ada peringkat
- Theory and Importance of Oxygen Bridge BondingDokumen23 halamanTheory and Importance of Oxygen Bridge BondingHarryBelum ada peringkat
- Why Is Mercury A LiquidDokumen2 halamanWhy Is Mercury A LiquidImodoye AbioroBelum ada peringkat
- Gap, A A: Is An Interesting Little Subclass Represented by Y2Bani05. ItDokumen1 halamanGap, A A: Is An Interesting Little Subclass Represented by Y2Bani05. ItKetanBelum ada peringkat
- Metal-Thiolate Bonds in Bioinorganic ChemistryDokumen14 halamanMetal-Thiolate Bonds in Bioinorganic ChemistrykawtherahmedBelum ada peringkat
- Electric Properties: 5.1 Conductivity of PolymersDokumen29 halamanElectric Properties: 5.1 Conductivity of PolymersMichael ChuBelum ada peringkat
- Crystal Field Theory - NURDokumen5 halamanCrystal Field Theory - NURNurhajrahBelum ada peringkat
- D & F Block Best NotesDokumen29 halamanD & F Block Best Noteshtis4363hBelum ada peringkat
- Transition Metal 4Dokumen4 halamanTransition Metal 4Sushant ShahBelum ada peringkat
- MetalsDokumen39 halamanMetalsAditya ChudasamaBelum ada peringkat
- Selected Topics in Chemistry For Non-Major 1: Dr. Adebisi, A. A. Department of Chemistry, Covenant University, OtaDokumen25 halamanSelected Topics in Chemistry For Non-Major 1: Dr. Adebisi, A. A. Department of Chemistry, Covenant University, OtaIfiok UsoroBelum ada peringkat
- A Mathematical Model For The Porous Lead Dioxide ElectrodeDokumen10 halamanA Mathematical Model For The Porous Lead Dioxide Electrodesumit singhBelum ada peringkat
- Chapter 5Dokumen23 halamanChapter 5eimaiokanenasBelum ada peringkat
- Ligand Field StrengthDokumen24 halamanLigand Field StrengthIrvandar NurviandyBelum ada peringkat
- 3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1Dokumen1 halaman3.1) Is of The Cu Ions Is Approximately Octahedral As in Fig. 3.1Kamleshkekane1Belum ada peringkat
- 0953-4075 33 2 310Dokumen15 halaman0953-4075 33 2 310beaveacedemiaBelum ada peringkat
- CFT 1Dokumen19 halamanCFT 1Muhammad Umair IqbalBelum ada peringkat
- Superconductivity, A Physical Chemical Perspective: Ralph C. DoughertyDokumen36 halamanSuperconductivity, A Physical Chemical Perspective: Ralph C. Doughertyjuan lopezBelum ada peringkat
- Galvanic CellsDokumen4 halamanGalvanic CellsshariziBelum ada peringkat
- J Inoche 2003 12 027Dokumen2 halamanJ Inoche 2003 12 027гогавагановBelum ada peringkat
- Some Basic Considerations: 5.1.3 Doping of Compound SemiconductorsDokumen4 halamanSome Basic Considerations: 5.1.3 Doping of Compound SemiconductorsprashantsheetalBelum ada peringkat
- Insulators: Ch. 5 MottDokumen1 halamanInsulators: Ch. 5 MottKetanBelum ada peringkat
- ElectrochemistryDokumen58 halamanElectrochemistryWatan SahuBelum ada peringkat
- Photocatalysis Challenges and PotentialsDokumen65 halamanPhotocatalysis Challenges and PotentialssridharbkpBelum ada peringkat
- Activity 4Dokumen7 halamanActivity 4Jherby TeodoroBelum ada peringkat
- 1 s2.0 S0360319901001598 Main PDFDokumen6 halaman1 s2.0 S0360319901001598 Main PDFDr ChBelum ada peringkat
- HybridizationDokumen9 halamanHybridizationSatyaki MajumdarBelum ada peringkat
- A - Lehmann-Szweykowska - 2006 - J. - Phys.A Microscopic Model of Oxygen Vacancies in Cadoped YIGDokumen9 halamanA - Lehmann-Szweykowska - 2006 - J. - Phys.A Microscopic Model of Oxygen Vacancies in Cadoped YIGDuong Nguyen PhucBelum ada peringkat
- ExceptionsDokumen6 halamanExceptionsmifal0102Belum ada peringkat
- Mechanism of Atmospheric RustingDokumen20 halamanMechanism of Atmospheric Rustingarman mohammadiBelum ada peringkat
- Si:P As A Laboratory Analogue For Hydrogen On High Magnetic Field White Dwarf StarsDokumen8 halamanSi:P As A Laboratory Analogue For Hydrogen On High Magnetic Field White Dwarf StarsAlex FlemingBelum ada peringkat
- D and F Block Elements With AnswersDokumen5 halamanD and F Block Elements With AnswersFool TheBelum ada peringkat
- Molecular Orbital of Chemisorbed Carbon Monoxide: GeohgeDokumen6 halamanMolecular Orbital of Chemisorbed Carbon Monoxide: GeohgeLuis M. MolinaBelum ada peringkat
- Flipped: Born-Haber Cycles: Chemsheets Definitions TaskDokumen3 halamanFlipped: Born-Haber Cycles: Chemsheets Definitions TaskShahnaz AhmedBelum ada peringkat
- Org A No Met PDFDokumen9 halamanOrg A No Met PDFSandipan SahaBelum ada peringkat
- L0 - Bonding NotesDokumen23 halamanL0 - Bonding NotesRuha VBelum ada peringkat
- Electronic Ferroelectricity in II-VI Semiconductor ZnODokumen26 halamanElectronic Ferroelectricity in II-VI Semiconductor ZnOArghyadeep Das ph19c005Belum ada peringkat
- Conceptualized Transmutation Reactor Based On Cold Fusion Mechanism (Possibility of Transmutation To Superheavy Element by Cold Fusion Mechanism)Dokumen10 halamanConceptualized Transmutation Reactor Based On Cold Fusion Mechanism (Possibility of Transmutation To Superheavy Element by Cold Fusion Mechanism)International Journal of Innovative Science and Research TechnologyBelum ada peringkat
- 10 Chapter Electrochemistry Text Book ExerciseDokumen31 halaman10 Chapter Electrochemistry Text Book ExerciseSajid AzeemBelum ada peringkat
- Chapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismDokumen13 halamanChapter 11 - Coordination Chemistry: Bonding, Spectra, and MagnetismAlia AliaBelum ada peringkat
- 12 SACE Start of Year Revision SOLUTIONSDokumen6 halaman12 SACE Start of Year Revision SOLUTIONSLydia LamBelum ada peringkat
- T. J. Greytak Et Al - Bose-Einstein Condensation in Atomic HydrogenDokumen9 halamanT. J. Greytak Et Al - Bose-Einstein Condensation in Atomic HydrogenJuaxmawBelum ada peringkat
- Initial Oxidation Kinetics of Copper (1 1 0) Film Investigated by in Situ UHV-TEMDokumen9 halamanInitial Oxidation Kinetics of Copper (1 1 0) Film Investigated by in Situ UHV-TEMheat_wikBelum ada peringkat
- CFTDokumen15 halamanCFTGaurav BothraBelum ada peringkat
- Crystal Field TheoryDokumen26 halamanCrystal Field TheorySahil Qaiser100% (1)
- Chemistry 251A - Problem Set 2 KeyDokumen4 halamanChemistry 251A - Problem Set 2 KeyThảo HàBelum ada peringkat
- 1/2 Spins of The Ti3+ Ions (See, However,: Ch. 10 The Correlated Metallic StateDokumen1 halaman1/2 Spins of The Ti3+ Ions (See, However,: Ch. 10 The Correlated Metallic StateSupriyaBelum ada peringkat
- Chap 7Dokumen6 halamanChap 7api-3704690Belum ada peringkat
- Self Limiting PT EALDDokumen22 halamanSelf Limiting PT EALDnm53fBelum ada peringkat
- Progress in the Science and Technology of the Rare Earths: Volume 2Dari EverandProgress in the Science and Technology of the Rare Earths: Volume 2Belum ada peringkat
- 31 JLSL: 5 Mott InsulatorsDokumen1 halaman31 JLSL: 5 Mott InsulatorsKetanBelum ada peringkat
- Ch. 5 Mott Insulators: (Cfatcfalcf6tcfblDokumen1 halamanCh. 5 Mott Insulators: (Cfatcfalcf6tcfblKetanBelum ada peringkat
- Digression: Symmetry Breaking (Or The Lack It) : (17, 3821: As We 1,2,. ADokumen1 halamanDigression: Symmetry Breaking (Or The Lack It) : (17, 3821: As We 1,2,. AKetanBelum ada peringkat
- '&xisition Metals: and AlloysDokumen1 halaman'&xisition Metals: and AlloysKetanBelum ada peringkat
- Itinerant Electron Magnetism: 1 of Is A A AsDokumen1 halamanItinerant Electron Magnetism: 1 of Is A A AsKetanBelum ada peringkat
- G1, Glj2 Where ?-Spins Igl,. G L / 2) : 6.3 For EvenDokumen1 halamanG1, Glj2 Where ?-Spins Igl,. G L / 2) : 6.3 For EvenKetanBelum ada peringkat
- The Problems: SolutionsDokumen1 halamanThe Problems: SolutionsKetanBelum ada peringkat
- I To A: Zlansition Metals and AlloysDokumen1 halamanI To A: Zlansition Metals and AlloysKetanBelum ada peringkat
- Solutions: ProblemsDokumen1 halamanSolutions: ProblemsKetanBelum ada peringkat
- So We: ShouldDokumen1 halamanSo We: ShouldKetanBelum ada peringkat
- So Go: Itinerant Electron MagnetismDokumen1 halamanSo Go: Itinerant Electron MagnetismKetanBelum ada peringkat
- 7.7 Zhnsition Metals and Alloys 1 Also: So The Stoner Theory Is Not Valid in Its Original NaiveDokumen1 halaman7.7 Zhnsition Metals and Alloys 1 Also: So The Stoner Theory Is Not Valid in Its Original NaiveKetanBelum ada peringkat
- Ofa As: !hasition andDokumen1 halamanOfa As: !hasition andKetanBelum ada peringkat
- Psat (442) (290) 1751: I Cannot Be The BareDokumen1 halamanPsat (442) (290) 1751: I Cannot Be The BareKetanBelum ada peringkat
- 7.7 Transition Metals and AlloysDokumen1 halaman7.7 Transition Metals and AlloysKetanBelum ada peringkat
- Tiansition Metals and AlloysDokumen1 halamanTiansition Metals and AlloysKetanBelum ada peringkat
- 7.6 Spin: Problem 7.6Dokumen1 halaman7.6 Spin: Problem 7.6KetanBelum ada peringkat
- 396 7 Itinerant Electron Magnetism: A So Pro-IsDokumen1 halaman396 7 Itinerant Electron Magnetism: A So Pro-IsKetanBelum ada peringkat
- 33meaning The Nearest-Neighbour Hopping Model On A Hypercubic LatticeDokumen1 halaman33meaning The Nearest-Neighbour Hopping Model On A Hypercubic LatticeKetanBelum ada peringkat
- 7.7.2 LSDA Versus Lattice Fermion Models: Is ofDokumen1 halaman7.7.2 LSDA Versus Lattice Fermion Models: Is ofKetanBelum ada peringkat
- Ch. 7 Itinerant Electron Magnetism: T,, U, U. TDokumen1 halamanCh. 7 Itinerant Electron Magnetism: T,, U, U. TKetanBelum ada peringkat
- 7.7 Tkansition Metals and AlloysDokumen1 halaman7.7 Tkansition Metals and AlloysKetanBelum ada peringkat
- 7.6 Spin Density: SDW MottDokumen1 halaman7.6 Spin Density: SDW MottKetanBelum ada peringkat
- 00396Dokumen1 halaman00396KetanBelum ada peringkat
- Pm-Pi: Away From Half-FillingDokumen1 halamanPm-Pi: Away From Half-FillingKetanBelum ada peringkat
- 382 Ch. 7 Itinerant Electron Magnetism: RNQ (A) CosDokumen1 halaman382 Ch. 7 Itinerant Electron Magnetism: RNQ (A) CosKetanBelum ada peringkat
- 7 Itinerant Electron Magnetism: (166) Felt Compelled To Conjecture That Any Ordering Away FromDokumen1 halaman7 Itinerant Electron Magnetism: (166) Felt Compelled To Conjecture That Any Ordering Away FromKetanBelum ada peringkat
- L'Kansition Metals Alloys: &F-PKJDokumen1 halamanL'Kansition Metals Alloys: &F-PKJKetanBelum ada peringkat
- 7.6 Spin Density Waves: (SDWI)Dokumen1 halaman7.6 Spin Density Waves: (SDWI)KetanBelum ada peringkat
- 7.6.3 Strong Coupling LimitDokumen1 halaman7.6.3 Strong Coupling LimitKetanBelum ada peringkat
- Cloud Physics Review - Includes Cloud Physics and Cloud DynamicsDokumen5 halamanCloud Physics Review - Includes Cloud Physics and Cloud DynamicsJames WallaceBelum ada peringkat
- 3 EC4-2 Background Gerhard HanswilleDokumen67 halaman3 EC4-2 Background Gerhard Hanswilleantonio111aBelum ada peringkat
- Continuity EquationDokumen5 halamanContinuity EquationCh ArsalanBelum ada peringkat
- WEEK 7 Center of MassDokumen10 halamanWEEK 7 Center of Massmaria1345Belum ada peringkat
- GRB Physical Chemistry IIT JEE PDFDokumen995 halamanGRB Physical Chemistry IIT JEE PDFsachin gupta75% (12)
- Chemistry James Bond LabDokumen2 halamanChemistry James Bond Labapi-273494632Belum ada peringkat
- SpaceCAD Model Rocket SoftwareDokumen7 halamanSpaceCAD Model Rocket Softwareheric19886445Belum ada peringkat
- Understanding API SIRE Reading-1 Part 2 of 2Dokumen358 halamanUnderstanding API SIRE Reading-1 Part 2 of 2Charlie Chong83% (6)
- Angle Brackets For Buildings: Complete RangeDokumen6 halamanAngle Brackets For Buildings: Complete RangeKenan AvdusinovicBelum ada peringkat
- Air Fillter - Ly Thuyet Loc Cho CleanroomDokumen101 halamanAir Fillter - Ly Thuyet Loc Cho CleanroomMai Phuong Phan TranBelum ada peringkat
- Effectiveness of Different Concentrations of Ethanol On Beetroot MembraneDokumen7 halamanEffectiveness of Different Concentrations of Ethanol On Beetroot MembraneellaloftiBelum ada peringkat
- Project For Carbon Fiber Composite MaterialDokumen15 halamanProject For Carbon Fiber Composite MaterialAbdallah M Al-MajaliBelum ada peringkat
- Ceiling PHR PHS Suspension IsolatorsDokumen14 halamanCeiling PHR PHS Suspension IsolatorsKunal GujarathiBelum ada peringkat
- Data SHEET SEPARADOR 3Dokumen1 halamanData SHEET SEPARADOR 3Diego RuanoBelum ada peringkat
- Vapo Hybrid ECO: Textile Steaming SystemDokumen6 halamanVapo Hybrid ECO: Textile Steaming SystemSajjad AhmedBelum ada peringkat
- BNWQ 3 Ak TZ3 RNG Z4 QGW WaDokumen14 halamanBNWQ 3 Ak TZ3 RNG Z4 QGW Wathealtamash21Belum ada peringkat
- CHF101 CHW S16 PDFDokumen1 halamanCHF101 CHW S16 PDFImmalatulhusnaBelum ada peringkat
- Physical Sciences Paper 1 (Physics) Grade 12 Terms & Definitions, Questions & Answers Per Topic 2019Dokumen124 halamanPhysical Sciences Paper 1 (Physics) Grade 12 Terms & Definitions, Questions & Answers Per Topic 2019BONGUMENZI MTSHALI88% (8)
- Hybridization: DR Karimah Kassim CHM 475Dokumen34 halamanHybridization: DR Karimah Kassim CHM 475api-386303659Belum ada peringkat
- Utkrisht Test-6Dokumen5 halamanUtkrisht Test-6Mayank GoelBelum ada peringkat
- Reductor SHB50-FDokumen28 halamanReductor SHB50-FJaime Casas-corderoBelum ada peringkat
- A Comparative Study Between A Rubberized, Cotton and Braided Nylon Shoelaces With Regards To Its Tensile StrengthDokumen38 halamanA Comparative Study Between A Rubberized, Cotton and Braided Nylon Shoelaces With Regards To Its Tensile StrengthAlan Je MusketerBelum ada peringkat
- How To Inspect A GearboxDokumen13 halamanHow To Inspect A Gearboxkamal arabBelum ada peringkat
- ConclusionDokumen3 halamanConclusionnehal vaghelaBelum ada peringkat
- Chemistry Test 2 Study GuideDokumen3 halamanChemistry Test 2 Study GuideI-Reen FrancisquiteBelum ada peringkat
- Nozzle Thrust and Efficiency MeasurementDokumen12 halamanNozzle Thrust and Efficiency MeasurementDhruvNagpalBelum ada peringkat
- TraversingDokumen14 halamanTraversingZaff CarrickBelum ada peringkat
- Geothermal Well CapacityDokumen7 halamanGeothermal Well Capacityhendratondang5294Belum ada peringkat
- Chapter - 1: Matter in Our SurroundingsDokumen27 halamanChapter - 1: Matter in Our Surroundingsuma mishra0% (1)
- Negative Poisson's RatioDokumen9 halamanNegative Poisson's RatioCivilEngClubBelum ada peringkat