E XMXV: Gravitational Potential Energy Mass X Gravitational Field Strength X Height
Diunggah oleh
KryptosDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
E XMXV: Gravitational Potential Energy Mass X Gravitational Field Strength X Height
Diunggah oleh
KryptosHak Cipta:
Format Tersedia
P1 P1
Energy can be kept in a number of different stores. The equation for kinetic energy is:
EK = 0.5 x m x v2
Chemical energy store: Different chemical bonds store different amounts of energy. Kinetic energy = 0.5 x mass x velocity2 EK
Kinetic energy store: Anything which is moving.
Gravitational potential energy store: Anything above the surface of a planet. EK = Kinetic energy (J)
Thermal energy store: Anything which is above -273oC m = mass (kg)
Elastic potential energy store: Anything which is stretched out of its resting shape. v = velocity (m/s) ½ x m x v2
Vibrational energy store: Anything moves to and fro.
Nuclear energy store: Atoms being split apart of fused together.
Magnetostatic/electrostatic energy store: When magnets and electric charges are
The equation for elastic potential energy is:
attracting or repelling.
Ee = 0.5 x k x e2
Elastic potential energy = 0.5 x spring constant x extension2
Ee
A system is an object or group of objects. Ee = Elastic potential energy (J)
Energy can move between stores when a system changes. k = Spring constant (N/m)
2
For example: e = extension (m) ½xkxe
An object projected upwards: (e.g. ball thrown upwards)
Kinetic energy store of ball → Gravitational potential energy store of ball
A moving object hitting an obstacle: (e.g. car hitting a traffic cone)
Kinetic energy store of moving object → Kinetic energy store of obstacle The equation for gravitational potential energy is:
An object accelerated by a constant force: (e.g. skydiver accelerated by their EP = m x g x h
weight) Gravitational potential energy = mass x gravitational field strength x height EP
Gravitational potential energy of skydiver → Kinetic energy of skydiver
A vehicle slowing down (e.g. car applying brakes) EP = Gravitational potential energy (J)
Kinetic energy store of car → Thermal energy store of brake pads m = Mass (kg)
Bringing water to boil in an electrical kettle g = gravitational field strength (N/kg) mxgxh
Thermal energy store of element → Thermal energy store of water in kettle h = Height (m)
The equation for change in thermal energy is:
Changes in the amount of energy stored in a system can be caused by:
ΔE = m x c x Δθ
heating Change in thermal energy = mass x specific heat capacity x change in temperature
(ΔE = mcΔθ) ΔE
Change in thermal energy = mass x specific heat capacity x change in temperature ΔE = Change in thermal energy (J)
work done by forces m = Mass (kg)
(W = Fd) c = Specific heat capacity (J/kgoC)
Work done = Force x distance Δθ = Change in temperature (oC) m x c x Δθ
work done when a current flows
(W = IVt) The specific heat capacity of a substance is the amount of energy required to raise
Work done = Current x potential difference x time the temperature of one kilogram of the substance by one degree Celsius.
P1 P1
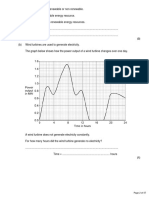
Efficiency is a measure of how much something does what we want it to do.
Power is defined as the rate at which energy is
The energy efficiency for any energy transfer can be calculated using the equation:
transferred or the rate at which work is done.
efficiency = useful output energy transfer ÷ total input energy transfer
The equation for power is: E Efficiency may also be calculated using the equation:
efficiency = useful power output ÷ total power input
P=E÷t
Power = Energy transferred ÷ time
P = Power (W) P x t A renewable energy resource is one that is being (or can be) replenished as it is used.
E = Energy transferred (J)
t = time (s) The uses of energy resources include: transport, electricity generation and heating.
The equation for power can also be written:
P=W÷t W Energy
Renewable/
Non- Description
Environmental
Uses of
energy Reliability
Power = Energy transferred ÷ time Resource impact
renewable resource
Coal, oil and gas can be burned to Electricity
P = Power (W) Fossil Non- Greenhouse
P x t heat water, to make stream, to generation, Reliable
fuels renewable Gases
W = Work done (J) turn a turbine. transport
t = time (s)
Non- Nuclear fission heats water, to Radioactive Electricity
Nuclear Reliable
renewable make steam, to turn a turbine. waste generation
Electricity
An energy transfer of 1 joule per second is equal to a power of 1 watt. Biofuel Renewable
Biofuel is burnt to heat water, to Carbon - generation,
Reliable
make steam, to turn a turbine. neutral heating,
transport
Electricity
Wind Renewable Wind turns a turbine. Noise Unreliable
Energy can be transferred usefully, stored or dissipated, but energy cannot be generation
created or destroyed.
Hydro- Water through a dam turns a Flooding of Electricity
Renewable Reliable
electric turbine. habitats generation
Sometimes energy is dissipated, so that it is stored in less useful ways. This energy
Heat from underground heats Electricity
is often described as being ‘wasted’. Geother
Renewable water, to make steam, to turn a None generation, Reliable
mal
Because energy cannot be lost: Total energy = useful energy + wasted energy turbine. heating
Water is trapped behind a
Flooding of Electricity
Unwanted energy transfers can be reduced by a range of methods, for example Tides Renewable barrage at high tide and released
habitats generation
Reliable
turning a turbine.
through lubrication and the use of thermal insulation.
Photovoltaic cells turn light into Electricity
The higher the thermal conductivity of a material the higher the rate of energy Sun Renewable electricity. Solar cells heat None generation, Unreliable
water for heating. heating
transfer by conduction across the material.
Water The motion of a wave turns a Electricity
Renewable None Reliable
waves turbine. generation
The rate of cooling of a building is affected by the thickness and thermal
conductivity of its walls.
P1 P1
Required Practical: Thermal Insulators Required Practical: Specific Heat Capacity
1. Get a set of 5 boiling tubes and wrap one in each of the insulating 1. Measure and record the mass of the copper
materials (leave one beaker without any insulation.) block in kg.
2. Use the kettle to boil water. 2. Wrap the insulation around the block.
3. Measure 50ml of hot water into each container. 3. Place the heater in the larger hole in the block.
4. Insert the thermometer so that its bulb is in the hot water. 4. Connect the ammeter, power pack and heater
5. Record the temperature of the water and start the stopwatch. in series.
6. Record the temperature of the water every 3 minutes for 18 minutes 5. Connect the voltmeter across the heater.
7. Add your results to the results table. 6. Use the pipette to put a small amount of water
in the other hole.
7. Put the thermometer in this hole.
8. Set the power pack to 12 V. Switch on the
power pack to turn on the heater.
9. Record the ammeter and voltmeter readings. These shouldn’t change
during the experiment.
10. Measure the temperature and start the stopwatch.
11. Record the temperature every minute for 10 minutes.
12. Calculate the power of the heater in watts.
Power in watts = potential difference in volts x current in amps
13. Calculate the energy transferred (work done) by the heater. To do this,
multiply the time in seconds by the power of the heater.
8. Plot cooling curve graphs for each material with: 14. Plot a graph of the temperature in oC against work done in J.
‘Temperature in °C’ on the y-axis 15. Draw a line of best fit. Take care as the beginning of the graph may be
‘Time in minutes’ on the x-axis. curved.
9. Use your graphs to determine which material is the best insulator. 16. Calculate the gradient of the straight part of your graph.
(The gradient is Δθ ÷ ΔE)
90
17. Rearrange the equation for Change in thermal energy to get:
85 Δθ = 1
80 ΔE mc
Temperature (oC)
75 18. We therefore know that the gradient
70 is equal to:
gradient = 1
65
mc
60
19. We can calculate c (specific heat
55 capacity)
50 c= 1
0 3 6 9 12 15 m x gradient
Time (minutes)
No insulation Bubble wrap Newspaper Tin foil
Anda mungkin juga menyukai
- PHYSICS - Science Notes For End of Year 9 AssessmentDokumen6 halamanPHYSICS - Science Notes For End of Year 9 AssessmentJenny DavidsonBelum ada peringkat
- Physics Revision Booklet (Higher) PDFDokumen30 halamanPhysics Revision Booklet (Higher) PDFChandrasekaran SubramaniamBelum ada peringkat
- GCSE Comb Higher Physics 2023Dokumen72 halamanGCSE Comb Higher Physics 2023Ramy MohamedBelum ada peringkat
- Work and Energy Summary SheetDokumen1 halamanWork and Energy Summary SheetJeremy Neile CruzBelum ada peringkat
- Energy Work and PowerDokumen35 halamanEnergy Work and PowerFabian ClarkeBelum ada peringkat
- Work Power and EnergyDokumen45 halamanWork Power and EnergyrebugiocosmeBelum ada peringkat
- Mind MapDokumen1 halamanMind Mapمروان ابراهيم حمد عبدBelum ada peringkat
- Physic WorkPowerEnergy Layaalin.M 5301421020Dokumen16 halamanPhysic WorkPowerEnergy Layaalin.M 5301421020Layaalin MutmainahBelum ada peringkat
- Worksheet Ms. Enorio EDITEDDokumen13 halamanWorksheet Ms. Enorio EDITEDDarryl Hannah Amado SilvaBelum ada peringkat
- 11 - EnergyDokumen51 halaman11 - EnergyRija FatimaBelum ada peringkat
- 2.10 Work, Power, and EfficiencyDokumen20 halaman2.10 Work, Power, and EfficiencyShidevBelum ada peringkat
- Energy, Work and Power Energy: The Capacity To Do "Work".Dokumen1 halamanEnergy, Work and Power Energy: The Capacity To Do "Work".jahajaha_svensson609Belum ada peringkat
- AQA GCSE Physics - Equations & Formulae: Higher Tier Only Separate Physics OnlyDokumen2 halamanAQA GCSE Physics - Equations & Formulae: Higher Tier Only Separate Physics OnlyLuisBelum ada peringkat
- Dynamics Energy MomentumDokumen28 halamanDynamics Energy MomentumCharlotte Frias BenitoBelum ada peringkat
- 2018 Edexcel IGCSE Work Energy and Power Mark SchemeDokumen3 halaman2018 Edexcel IGCSE Work Energy and Power Mark SchemeGovind ShankarBelum ada peringkat
- Workenergyandpowerppt 131208202046 Phpapp02Dokumen26 halamanWorkenergyandpowerppt 131208202046 Phpapp02joyBelum ada peringkat
- Physics Important ConceptsDokumen2 halamanPhysics Important Conceptsazhar aliBelum ada peringkat
- Physics Important ConceptsDokumen2 halamanPhysics Important Conceptsazhar aliBelum ada peringkat
- Physics Important ConceptsDokumen2 halamanPhysics Important Conceptsazhar aliBelum ada peringkat
- General Physics 1 Fluid MechanicsDokumen1 halamanGeneral Physics 1 Fluid MechanicsJam JungBelum ada peringkat
- YEAR 7 PHY Transfer of Energy LN 3Dokumen2 halamanYEAR 7 PHY Transfer of Energy LN 3ResianBelum ada peringkat
- Force, Energy, and Work ConceptsDokumen13 halamanForce, Energy, and Work ConceptsErrol WaltersBelum ada peringkat
- O' Level Physics Formula Sheet: MeasurementsDokumen2 halamanO' Level Physics Formula Sheet: MeasurementsXOXOuser39938Belum ada peringkat
- Physics Formula Sheet For OlevelsDokumen2 halamanPhysics Formula Sheet For OlevelsMomin BabarBelum ada peringkat
- O Level Physics Formula SheetDokumen2 halamanO Level Physics Formula SheeteltytanBelum ada peringkat
- O Level Physics Formula SheetDokumen2 halamanO Level Physics Formula SheetJereme Cheong93% (56)
- O LEVEL FORMULADokumen2 halamanO LEVEL FORMULAPREM OfFiCiAlBelum ada peringkat
- O Level Physics FormulaDokumen2 halamanO Level Physics FormulaAukasha AhmedBelum ada peringkat
- O Level Physics Formula SheetDokumen2 halamanO Level Physics Formula SheetMomin BabarBelum ada peringkat
- 3E Sci Phy - Chap 7 - TRDokumen40 halaman3E Sci Phy - Chap 7 - TRXu JianhangBelum ada peringkat
- P1 Revision OverviewDokumen3 halamanP1 Revision Overviewian McMillanBelum ada peringkat
- CHAPTER 5 PhysicDokumen4 halamanCHAPTER 5 PhysicWaleska MendezBelum ada peringkat
- Work. Energy and Power - Theory - As - PDF FormDokumen8 halamanWork. Energy and Power - Theory - As - PDF FormShayani PereraBelum ada peringkat
- IGCSE Physics-Formula SheetDokumen2 halamanIGCSE Physics-Formula SheetPraveen PeterBelum ada peringkat
- Thermodynamic EnergyDokumen9 halamanThermodynamic EnergyJohn Lorenz Delos SantosBelum ada peringkat
- Work and Energy: W F × S (Unit - Joule, 1 J 1 N-M)Dokumen3 halamanWork and Energy: W F × S (Unit - Joule, 1 J 1 N-M)Avinash MansukBelum ada peringkat
- Energy, Work and Power SC 2023Dokumen60 halamanEnergy, Work and Power SC 2023shanilboodhooa47Belum ada peringkat
- LESSON 2.10 Understanding Work, Energy, Power and EfficiencyDokumen12 halamanLESSON 2.10 Understanding Work, Energy, Power and Efficiencychekgu_2007Belum ada peringkat
- Kinetic and potential energy in simple harmonic motionDokumen3 halamanKinetic and potential energy in simple harmonic motionAriyan Abrar SaifBelum ada peringkat
- Work - Energy & Impulse - MomentumDokumen90 halamanWork - Energy & Impulse - Momentumlil KamalBelum ada peringkat
- Work Energy and PowerDokumen10 halamanWork Energy and PowerCake RuskBelum ada peringkat
- Energy Transfers and StoresDokumen13 halamanEnergy Transfers and StoresKudakwasheBelum ada peringkat
- Energy, Work and PowerDokumen5 halamanEnergy, Work and PowerSolaarBelum ada peringkat
- AQA GCSE Combined Science Equation SheetDokumen5 halamanAQA GCSE Combined Science Equation SheetJoel OkohBelum ada peringkat
- Food - HEAT TRANSFERDokumen17 halamanFood - HEAT TRANSFERSubhankar MaityBelum ada peringkat
- Work Energy and PowerDokumen35 halamanWork Energy and PowerManas ThakurBelum ada peringkat
- ENERGY TRANSFORMATION & CONSERVATIONDokumen12 halamanENERGY TRANSFORMATION & CONSERVATIONSumeet KumarBelum ada peringkat
- Energy and MomentumDokumen20 halamanEnergy and MomentumAndrei Alido100% (1)
- METD0213 Thermodynamics 1 Lecture 2: Units, Forms, and Transfers of EnergyDokumen12 halamanMETD0213 Thermodynamics 1 Lecture 2: Units, Forms, and Transfers of EnergyShaira MedinaBelum ada peringkat
- Work & Energy FormulaeDokumen13 halamanWork & Energy FormulaeAsterisk KhantBelum ada peringkat
- Chapter 8 Conservation of Energy: Energy, Which Is Energy That A System Possesses by VirtueDokumen15 halamanChapter 8 Conservation of Energy: Energy, Which Is Energy That A System Possesses by VirtueLilrozayBelum ada peringkat
- Work, Energy and Energy Conservation: Learning CompetenciesDokumen4 halamanWork, Energy and Energy Conservation: Learning CompetenciesNathalie SerafinBelum ada peringkat
- The First Law of Thermodynamics1Dokumen50 halamanThe First Law of Thermodynamics1Beshir Heyru MohammedBelum ada peringkat
- Problems in Quantum Mechanics: Third EditionDari EverandProblems in Quantum Mechanics: Third EditionPenilaian: 3 dari 5 bintang3/5 (2)
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsDari EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsBelum ada peringkat
- Feynman Lectures Simplified 2B: Magnetism & ElectrodynamicsDari EverandFeynman Lectures Simplified 2B: Magnetism & ElectrodynamicsBelum ada peringkat
- Strength of Materials and Structures: An Introduction to the Mechanics of Solids and StructuresDari EverandStrength of Materials and Structures: An Introduction to the Mechanics of Solids and StructuresPenilaian: 4 dari 5 bintang4/5 (1)
- Atoms and Radiation 2Dokumen78 halamanAtoms and Radiation 2KryptosBelum ada peringkat
- Page 2 of 57Dokumen56 halamanPage 2 of 57KryptosBelum ada peringkat
- Year 13. Student PackDokumen60 halamanYear 13. Student PackKryptosBelum ada peringkat
- AtomsandRadiation2 AnswersDokumen39 halamanAtomsandRadiation2 AnswersKryptosBelum ada peringkat
- Number H Multiples in Context v2Dokumen2 halamanNumber H Multiples in Context v2KryptosBelum ada peringkat
- Box Plots (H) : Name: Total MarksDokumen4 halamanBox Plots (H) : Name: Total MarksKryptosBelum ada peringkat
- GCSE Maths Number Problems Sample QuestionsDokumen4 halamanGCSE Maths Number Problems Sample QuestionsKryptosBelum ada peringkat
- Geometry H Rotations v2Dokumen3 halamanGeometry H Rotations v2KryptosBelum ada peringkat
- Atoms and IsotopesDokumen80 halamanAtoms and IsotopesKryptosBelum ada peringkat
- Atoms and Nuclear Radiation 1Dokumen43 halamanAtoms and Nuclear Radiation 1KryptosBelum ada peringkat
- Atoms and Radiation 2Dokumen78 halamanAtoms and Radiation 2KryptosBelum ada peringkat
- AtomsandIsotopes AnswersDokumen38 halamanAtomsandIsotopes AnswersKryptosBelum ada peringkat
- AtomsandNuclearRadiation1 AnswersDokumen20 halamanAtomsandNuclearRadiation1 AnswersKryptosBelum ada peringkat
- Black Body RadiationDokumen46 halamanBlack Body RadiationKryptosBelum ada peringkat
- Number H Percentages v3Dokumen6 halamanNumber H Percentages v3KryptosBelum ada peringkat
- Number H Factors Multiples Primes Number Properties v2Dokumen3 halamanNumber H Factors Multiples Primes Number Properties v2KryptosBelum ada peringkat
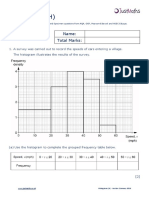
- Histograms (H) : Name: Total MarksDokumen12 halamanHistograms (H) : Name: Total MarksKryptosBelum ada peringkat
- GCSE Maths Number Problems Sample QuestionsDokumen4 halamanGCSE Maths Number Problems Sample QuestionsKryptosBelum ada peringkat
- GCSE Maths Number Problems Sample QuestionsDokumen4 halamanGCSE Maths Number Problems Sample QuestionsKryptosBelum ada peringkat
- Statistics H Scatter Graphs SolutionsDokumen5 halamanStatistics H Scatter Graphs SolutionsKryptosBelum ada peringkat
- Number H Multiples in Context v2Dokumen2 halamanNumber H Multiples in Context v2KryptosBelum ada peringkat
- Number H Fractions v2 1Dokumen5 halamanNumber H Fractions v2 1KryptosBelum ada peringkat
- Number H Product of Prime Factors HCF LCM v2Dokumen2 halamanNumber H Product of Prime Factors HCF LCM v2KryptosBelum ada peringkat
- Multiples in Context GCSE Maths Sample QuestionsDokumen2 halamanMultiples in Context GCSE Maths Sample QuestionsKryptosBelum ada peringkat
- Sampling GCSE Maths Questions on Estimation and StatisticsDokumen4 halamanSampling GCSE Maths Questions on Estimation and StatisticsKryptosBelum ada peringkat
- Sampling GCSE Maths Questions on Estimation and StatisticsDokumen4 halamanSampling GCSE Maths Questions on Estimation and StatisticsKryptosBelum ada peringkat
- Number H Fractions v2 1Dokumen5 halamanNumber H Fractions v2 1KryptosBelum ada peringkat
- Number H Estimation v2Dokumen3 halamanNumber H Estimation v2KryptosBelum ada peringkat
- Chiral Symmetry Breaking by A Magnetic Field in GrapheneDokumen38 halamanChiral Symmetry Breaking by A Magnetic Field in GrapheneKadhim JabbarBelum ada peringkat
- 1-Shear Strength of SoilDokumen20 halaman1-Shear Strength of Soilbekayazo6248Belum ada peringkat
- Preview: Objectives Defining Temperature Thermal Equilibrium Thermal Expansion Measuring TemperatureDokumen33 halamanPreview: Objectives Defining Temperature Thermal Equilibrium Thermal Expansion Measuring TemperatureMega DarkBelum ada peringkat
- Design ConsiderationsDokumen18 halamanDesign ConsiderationsCherry ObiasBelum ada peringkat
- Analogue Gravity PhenomenologyDokumen452 halamanAnalogue Gravity PhenomenologyJuan Felipe BravoBelum ada peringkat
- Science 8 First Quarter ExamDokumen3 halamanScience 8 First Quarter ExamAvril Jade EsperanzaBelum ada peringkat
- SolutionDokumen3 halamanSolutionbahast faiqBelum ada peringkat
- Comparative Study of Seismic Performance of High Rise 30 Storey Building With Beam Slab, Flat Slab and Alternate Flat - Beam Slab Systems in Zone VDokumen5 halamanComparative Study of Seismic Performance of High Rise 30 Storey Building With Beam Slab, Flat Slab and Alternate Flat - Beam Slab Systems in Zone VGunderao V NandiBelum ada peringkat
- Understanding Airfoil Characteristics at Low Reynolds NumbersDokumen19 halamanUnderstanding Airfoil Characteristics at Low Reynolds NumbersSiddharthBelum ada peringkat
- FIITJEE AITS 2015 ScheduleDokumen4 halamanFIITJEE AITS 2015 ScheduleAlkaChoudharyBelum ada peringkat
- c2Dokumen39 halamanc2Patrik GăiceanBelum ada peringkat
- Pipeline Hydro Test Pressure Determination - Pipeline & Gas JournalDokumen7 halamanPipeline Hydro Test Pressure Determination - Pipeline & Gas Journalmailmaverick8167100% (1)
- CFD Manipal NotesDokumen15 halamanCFD Manipal NotesVishal ThakurBelum ada peringkat
- Chapter 3 Gas Well TestingDokumen93 halamanChapter 3 Gas Well TestingIrawan RianBelum ada peringkat
- Thermal Properties of MaterialsDokumen21 halamanThermal Properties of MaterialsBhaagi SirdBelum ada peringkat
- BachoduDokumen175 halamanBachoduShubham KothariBelum ada peringkat
- Lessons From Crane RunwaysDokumen5 halamanLessons From Crane Runwaysdicktracy11Belum ada peringkat
- Modeling Fracture and Failure With AbaqusDokumen24 halamanModeling Fracture and Failure With AbaqusFaizan Rashid100% (1)
- A Review of Residual Stresses and TensioningDokumen14 halamanA Review of Residual Stresses and TensioningFernando Spanholi TelesBelum ada peringkat
- Saavedra 2001Dokumen9 halamanSaavedra 2001raifelmbBelum ada peringkat
- Penetration of Microwaves Into AluminumDokumen5 halamanPenetration of Microwaves Into AluminumJohn Michael WilliamsBelum ada peringkat
- Avo Modelling PDFDokumen14 halamanAvo Modelling PDFZahra AnnaqiyahBelum ada peringkat
- Pressure Distribution and Choking in Convergent Divergent NozzleDokumen6 halamanPressure Distribution and Choking in Convergent Divergent NozzleS R Akhil KrishnanBelum ada peringkat
- Tugas Praktikum 4 MEH PDFDokumen9 halamanTugas Praktikum 4 MEH PDFArdian RizaldiBelum ada peringkat
- HDRB 2nd Penang BridgeDokumen44 halamanHDRB 2nd Penang BridgebriankimbjBelum ada peringkat
- Simulation of Piezoelectric Devices by Two - and Three-Dimensional Finite ElementsDokumen15 halamanSimulation of Piezoelectric Devices by Two - and Three-Dimensional Finite Elementszhangcwy1225Belum ada peringkat
- Column Design - Centric and Eccentric LoadingDokumen5 halamanColumn Design - Centric and Eccentric Loadingozlem ozdemirBelum ada peringkat
- Behaviour of Aluminium Structures in Fire - A ReviDokumen7 halamanBehaviour of Aluminium Structures in Fire - A RevitamaranmBelum ada peringkat
- Statics Dynamics Assignment 3 SolutionDokumen9 halamanStatics Dynamics Assignment 3 SolutionGanga DahalBelum ada peringkat
- Recommendations For SC PDFDokumen15 halamanRecommendations For SC PDFPau VallsBelum ada peringkat