Cacl2 Concntrating - 1 PDF
Diunggah oleh
vahid0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
21 tayangan2 halamanJudul Asli
cacl2 concntrating_1.pdf
Hak Cipta
© © All Rights Reserved
Format Tersedia
PDF, TXT atau baca online dari Scribd
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
0 penilaian0% menganggap dokumen ini bermanfaat (0 suara)
21 tayangan2 halamanCacl2 Concntrating - 1 PDF
Diunggah oleh
vahidHak Cipta:
© All Rights Reserved
Format Tersedia
Unduh sebagai PDF, TXT atau baca online dari Scribd
Anda di halaman 1dari 2
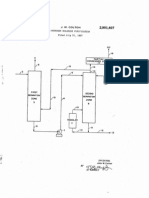
Patented June 12, 1951 2,556,184
UNITED STATES PATENT OFFICE
2,556,184
ANHYDROUS CALCIUM CHLORIDE PROCESS
Arthur George Matthew Hedley, Northwich, Eng
land, assignor to Imperial Chemical Industries
Limited, a corporation of Great Britain
No Drawing. Application March 21, 1947, Serial
No. 736,423. In Great Britain March 25, 1946
5 Clains. (C. 159-4)
2
This invention relates to a new process for the oration of liquor to give 70 to 72% CaCl2, as al
manufacture of solid calcium chloride, and more though the evaporation up to about 65% can be
particularly to a new and improved method for effected easily and efficiently, it is the further
the dehydration of relatively concentrated Solu concentration which involves the inefficiencies of
: tions of calcium chloride. pot working. Moreover it is clear that the conver
The process in commercial operation for the Sion of 70% CaCl2 liquor to a Solid containing
manufacture of calcium chloride employs a dilute 75% CaCl2 or higher has hitherto been a diffi
liquor as starting point, which is available as a cult operation. It involves the preparation and
by-product of the ammonia-soda, process. This handling of the intermediate solid, and the trans
liquor is evaporated in a steam heated or other O fer thereto of at least 65 ton calories per ton 75%
type of evaporator until a concentration of about CaCl2 in order to effect the dehydration. Such
40% to 50% CaCl2 is reached, if desired under heat can only be transferred through the walls
vacuum, and any deposited Solids are removed. With difficulty because of the deplorably poor
From this stage onwards it is not convenient to heat transfer to a Solid in this Way. It is there
use a reduced pressure as at high concentrations 15 fore usually Supplied from the current of hot
this results in deposition of Solid in the evapo air, which has to be bled off to remove the evap
rator and generally blockage thereof. It is there Orated water at a low partial Water Vapour preS
'', fore customary in commercial practice to carry Sure, and therefore far more heat is in fact used
out the further evaporation by direct heating in up in this process than would appear from this
pots at atmospheric pressure, during which proc 20 theoretical figure.
ess the concentration and temperature rise pro One object of the present invention is to pro
gressively to 70 to 72% CaCl2 and 170° C. At wide an in proved proceSS for the manufacture of
this point the solution is cooled, conveniently as 75 to 77% CaCl2. Another object is to decrease
a thin surface on a cooled rotating drum, which the amount of heat which must be Supplied in the
dips into the liquor, to yield flakes, or in a cooled 25 manufacture of commercial calcium chloride, and
granulating machine to yield granules, and the to provide an improved evaporation process. Yet
solid so obtained contains 70 to 72% CaCl2. In form another object is to provide a new and useful
another method, the Solution is cooled to 160° C. of connecial calcium chloride.
with agitation while preventing any further evap I have found that there are certain conditions
oration, to give a mush of crystals and liquor, 30 of concentration and temperature which, if prop
which is then cooled further to give unpS. erly chosen, can be employed for the conversion
In Some commercial practice this Solid is fur Of concentrated calcium-chloride solution to Solid
ther dehydrated by putting it in an oven or by calcium chloride and Water vapour with a con
passing it through a rotating drier which is heat Siderable Saving of heat over the customary proc
ed either through the walls or by a current of hot 35 eSS. Solutions of calcium chloride which are
air, to give a product containing 76 to 78% CaCl2. nearly Saturated at their boiling point at or near
This product requires less packing and transport atmospheric preSSure, When subjected to reduc
than 70 to 72% CaCl2 and is also leSS liable to tion of partial water vapour pressure, can be
cake on storage. Another method of making made to undergo Separation into water vapour
a substantially anhydrous product is to Spread 40 and Solid 70 to 76% calcium chloride by a process
the 70%. CaCl2 solution on a conveyor which which continues to completion a diabatically.
takes it through a furnace at 350° to 450° C. to In one method of carrying out this invention,
raise it to 85 to 90% CaCl2, and then through a I take a solution containing about 70 to 72%
furnace at 500 to 600° C. to give substantially CaCl2 and the rest Water except for Small amounts
anhydrous calcium chloride. 45 of inapurities which may amount to 1 to 2% taken
Evaporation in direct-fired pots is a relatively together. The temperature of this solution is
inefficient way of employing heat, compared for adjusted to about 170° to 75° C. The Solution
example with multiple effect evaporation Which is then Subjected to conditions in which the par
can be employed for Solutions of lower concen tial Water vapour pressure above the solution is
trations. In addition, the heat efficiency of Such 50 kept below atmospheric, e.g. is kept at 100 to 400
a pot is low, being only of the order of 40 to 50%. mn. Of mercury, and a large surface area of so
Furthermore, the large floor space occupied by lution is provided e. g. by spraying the solution
Such an installation and the relatively large down an empty tower. This permits autoevap
amount of repairs makes pots unattractive. Most Oration of the water, and calcium chloride is thus
Of this difficulty is due to the last stages of evap 55 deposited as a granular, dry solid containing 75%
2,556,184
3 4
to 76% CaCl2 by these operations alone. The low as 150° to 160° C., which will autoevaporate
above description represents the optimum Case a diabatically to give solid 70% calcium chloride by
where it is unnecessary to introduce any ileat reducing the partial vapour pressure of Water
whatsoever, and the only heat precautions which down to about 50 mm. of mercury. Alternatively,
must be taken are that undue losses to the at the same effect can be achieved by starting with
mosphere must be avoided. 65% calcium chloride solution at a higher tem
Another method of operation is to Spray an peratures and subjecting it to the Sane proceSS,
appropriate Solution down a tube in which thei'e but this does not give the same thermal ad
is an ascending current of air to cally off the vantage. Thus there is a comparatively restricted
water vapour and thus maintain a low water 10 range of concentrations in which my proceSS Op
vapour partial pressure. This method also gives erates without the introduction of any heat.
dry granular calcium chloride. The air Should Within this general range of 65% to 75% CaCl2
be warn to avoid undue heat loSSes fron the Solu the preferred concentrations lie between 68%
tion, and it may if desired be hot enough to Sup and 72% CaCl2, and for the 68% liquor it has
ply some heat to the process. 5 been found preferable to start with a temperature
The method of providing a large Surface airea between 150° and 170° C., and for the 72% liquor
may be by any of the standard methods normally it has been found preferable to start at a tempera
adopted for this purpose, e. g. Spraying, eXpos ture between 170° and 180° C.
ing as a film, agitating a Solution of distributing Whilst this invention has hitherto been de
it on previously produced granular calcium chlo scribed as an adiabatic process which in the ideal
ride as carrier for the solution in a creeper mixer. case is also an isothermal process, I include with
In any of these processes the time involved in in the present invention the substantially adia
autoevaporation is much less than is normaliy batic process where some heat is added but only
taken in evaporating calcium chloride Solutions or a small amount relative to that which has hither
in further dehydrating solid 70% CaCl2. In ad to been thought necessary. The known conver
dition, we avoid the consumption of much of the sion of solid 70% CaCl2 to solid 76% CaCl2 and
heat hitherto required, more especially the trans water vapour by the conventional hot air drying
mission of heat to a Solid Which is a difficult cp process involves the transfer to it of about 65
eration, and also avoid the need for Superheating ton calories per ton of product, and a wastage
or superSaturating at raised pressure as a means : of several times this amount in the discharged
of reaching higher concentrations. air. I have shown that i can make this 76%
Thus it will be seen that my invention com product from the 70% liquor without the intro
prises adiabatic or substantially adiabatic con duction of any heat and without having to handle
version of an appropriately chosen solution into the intermediate product, by Working under Speci
vapour and Solid merely by subjecting it to the 35 fied conditions, but for simplification in operation
neceSSary reduction of partial Water vapour preS it is sometimes desirable to use a weaker solution
sure. In the ideal case this a diabatic process is and to add an amount not exceeding 30 and gen
also an isothermal one. erally not exceeding 10 ton calories per ton of
The success of my invention is due in a large product. This is conveniently applicable when
extent to the discovery that the heat required 40 the process is carried out in a creeper mixer. I
for vaporising the water from a calcium chloride also include the case where a small amount of
Solution of certain specific concentrations can heat is lost, for example by radiation from the
be provided by the heat liberated from crystallisa substance or from the walls of the vessel, or by
tion of calcium chloride dihydrate from such so transmission to cool air used to effect autoevapo
lutions. Thus, I have found that the crystallisa 45 ration.
tion of calcium chloride dihydrate from highly In one method of carrying out the process as
concentrated solutions liberates considerable heat a continuous proceSS in a creeper mixer I employ
notwithstanding the Well known fact that the dis a paddle creeper which is open at the top for
olution of calcium chloride dihydrate in water acceSS of air. The paddle creeper contains granu
liberates a very large amount of heat. Moreover, lar calcium chloride, and a 68% to 72%, calcium
I have discovered that With specific conditions this chioride liquor at a temperature of 150° to 180° C.
crystallisation liberates enough heat to vaporise is fed in at One end of this creeper. A pool of
all of the water from these concentrated Solutions. liquor thus forms at the feed end, and further
By operating in this fashion, calcium chloride along the creeper the contents become mushy
dihydrate can easily be obtained even though it is 55 and SubSequently become dry, and eventually
extremely deliqueScent. In other words, this dis free-flowing granular calcium chloride runs off
covery makes possible production of calcium chlo at the far end. Although theoretically there is
ride by overcoming many of the difficulties and no need to introduce heat into this creeper proc
peculiar problems associated with the dehydra eSS, yet We find it convenient to heat the walls of
tion of calcium chloride, e. g. the complicated 60 the creeper, and/or to see that the air which
passes freely over the surface of the solid in the
problems of heat transfer are dispensed with since Creeper and thus removes the Water vapour is
by operating under my conditions the heat re hot, e. g. at 100 to 200° C. In practice, of course,
quired is actually generated internally as required. the amount of heat which can be transferred
The ideal case is represented by the use of about 65 through the metal Surface to a mushy solid or
72% calcium chloride solution at about 170° to to a Substantially dry or free-flowing solid as is
175 C., but there are other conditions within the present in the creeper is extremely small because
immediate vicinity of this point where the oper of the resistance to heat flow from the creeper
ation though a diabatic is not isothermal in that walls to the Solid. In the case where I do not
Some change of the temperature occurs during use Strictly adiabatic operation but provide some
the autoevaporation. As the higher limit, solu heat either through the walls or by contact with
tions can be used containing as much as 75% hot air, I can With a rather longer time of
CaCl2 at a temperature not exceeding 200° C. As Operation manage to use a feed liquor contain
a lower limit I may use liquor containing 67% ing as little as 60% CaCl2 at a temperature of
calcium chloride with an initial temperature as 75 120° to 150° C.
Anda mungkin juga menyukai
- Pole Shift 2012Dokumen432 halamanPole Shift 2012akergerhetBelum ada peringkat
- The Preparation of Methylamine Hydrochloride From Acetamide by Means of Calcium HypochloriteDokumen3 halamanThe Preparation of Methylamine Hydrochloride From Acetamide by Means of Calcium Hypochloritegeovani2100% (1)
- Sodium Hydroxide Production With A Calcium CarbonaDokumen7 halamanSodium Hydroxide Production With A Calcium CarbonaFebri SandiBelum ada peringkat
- Radiant Cooling in Humid Climates - Uponor - Radiant - Vs - AllAir - eBOOKDokumen43 halamanRadiant Cooling in Humid Climates - Uponor - Radiant - Vs - AllAir - eBOOKsmautifBelum ada peringkat
- Geothermal Power Plant Problem SetDokumen8 halamanGeothermal Power Plant Problem SetAriel GamboaBelum ada peringkat
- Thermal TestingDokumen11 halamanThermal TestingEngr Arfan Ali DhamrahoBelum ada peringkat
- Effect of Sodium Salts On Demercaptanization ProcessDokumen4 halamanEffect of Sodium Salts On Demercaptanization ProcessInternational Journal of Science and Engineering InvestigationsBelum ada peringkat
- Sure. in The Ideal Case This A Diabatic Process IsDokumen2 halamanSure. in The Ideal Case This A Diabatic Process IsvahidBelum ada peringkat
- Vapor-liquid phase separation of hydroconversion process effluentDokumen6 halamanVapor-liquid phase separation of hydroconversion process effluentGalang Hanif AbdulahBelum ada peringkat
- United States Patent (191: Lietard Et A1. (45) Jan. 3, 1978Dokumen7 halamanUnited States Patent (191: Lietard Et A1. (45) Jan. 3, 1978syafiq izzuddin bin sapriBelum ada peringkat
- EP0884278A1Dokumen7 halamanEP0884278A184105851Belum ada peringkat
- United States Patent 0: '3, l50, l74 ICCDokumen2 halamanUnited States Patent 0: '3, l50, l74 ICCMuhammadAmdadulHoqueBelum ada peringkat
- ,425,500. Patented Aug. 8, 1922.: H. W., Matheson and G, E, GrattanDokumen3 halaman,425,500. Patented Aug. 8, 1922.: H. W., Matheson and G, E, GrattanrzgarBelum ada peringkat
- United States Patent 1191: Tu (45) Sep. 7, 1982Dokumen4 halamanUnited States Patent 1191: Tu (45) Sep. 7, 1982AdyBelum ada peringkat
- C H CL C H CL + HCL: Cracking EdcDokumen5 halamanC H CL C H CL + HCL: Cracking Edczein2000Belum ada peringkat
- US ButyraldehydeDokumen2 halamanUS ButyraldehydeAstriany AnyBelum ada peringkat
- United States Patent Office: Patented June 29, 1948Dokumen3 halamanUnited States Patent Office: Patented June 29, 1948jhartmann8Belum ada peringkat
- United States Patent Office: Patented Sept. 5, 1950Dokumen2 halamanUnited States Patent Office: Patented Sept. 5, 1950ari factoryBelum ada peringkat
- Us 4396789Dokumen7 halamanUs 4396789Elias JúniorBelum ada peringkat
- Sulfuric Acid TreatmentDokumen9 halamanSulfuric Acid TreatmentShankar AcharBelum ada peringkat
- Translate Paten US5976485Dokumen24 halamanTranslate Paten US5976485Lenywulandari AyundaBelum ada peringkat
- Chlorination of cyanuric acid without salt co-productsDokumen2 halamanChlorination of cyanuric acid without salt co-productsAhmad DawamBelum ada peringkat
- Sodium CasienatDokumen9 halamanSodium Casienatbuana semestaBelum ada peringkat
- Us 1045139Dokumen1 halamanUs 1045139mahmoud IbrahimBelum ada peringkat
- F. J. Klem 3,218,128 : Filed Sept. l0, 1962Dokumen10 halamanF. J. Klem 3,218,128 : Filed Sept. l0, 1962Yourdan WijayaBelum ada peringkat
- Us 20120046495Dokumen10 halamanUs 20120046495AllajayprakashBelum ada peringkat
- Processes and Apparatus For Producing Acetic Acid From. Ace Aldehyde.Dokumen4 halamanProcesses and Apparatus For Producing Acetic Acid From. Ace Aldehyde.juanBelum ada peringkat
- United States Patent 0 ": Main, Germany Claims Priority, Application Germany, Aug. 10, 1963Dokumen1 halamanUnited States Patent 0 ": Main, Germany Claims Priority, Application Germany, Aug. 10, 1963هیمن مBelum ada peringkat
- ' United States Patent Office : Ljatented Nov. 7, 195.0Dokumen2 halaman' United States Patent Office : Ljatented Nov. 7, 195.0Agape Ruth BaliloBelum ada peringkat
- Patent 01Dokumen3 halamanPatent 01fatemeh afariBelum ada peringkat
- Continuous Process for High-Strength Bleach SolutionsDokumen5 halamanContinuous Process for High-Strength Bleach SolutionsEby OkvaleyBelum ada peringkat
- United States Patent Office: Patented Aug. 17, 1965Dokumen2 halamanUnited States Patent Office: Patented Aug. 17, 1965Vinod AvBelum ada peringkat
- Indigo Prodn. From Phenyl-Glycine Carboxylic Acid Salt - by Fusion in Mixed Potassium Hydroxide and Sodium Hydroxide Melt, Then OxidnDokumen4 halamanIndigo Prodn. From Phenyl-Glycine Carboxylic Acid Salt - by Fusion in Mixed Potassium Hydroxide and Sodium Hydroxide Melt, Then OxidnCillian CreedonBelum ada peringkat
- US4780224Dokumen4 halamanUS4780224Mohamad Reza JahanbakhshBelum ada peringkat
- Us2901407 PDFDokumen4 halamanUs2901407 PDFMufita RamadhinaBelum ada peringkat
- Us4018815 PDFDokumen4 halamanUs4018815 PDFFatih AkmanBelum ada peringkat
- Us3048465 PDFDokumen8 halamanUs3048465 PDFrevider451Belum ada peringkat
- US3218127Dokumen6 halamanUS3218127PABLO URIZ CEREZOBelum ada peringkat
- 1 ProcessDokumen2 halaman1 ProcessUmar DrazBelum ada peringkat
- US3507886 Continuous Pretreatment BASF 1970Dokumen2 halamanUS3507886 Continuous Pretreatment BASF 1970daraj darajBelum ada peringkat
- United States Patent Office: Patented Nov. 6, 1956Dokumen2 halamanUnited States Patent Office: Patented Nov. 6, 1956Syahrul SandreaBelum ada peringkat
- #US2703316Dokumen3 halaman#US2703316Citra Adelina SitorusBelum ada peringkat
- TCCA - US3898222 - P - Process For Preparing Trichloroisocyanuric AcidDokumen3 halamanTCCA - US3898222 - P - Process For Preparing Trichloroisocyanuric AcidListya Eka AnggrainiBelum ada peringkat
- Us 2498806Dokumen7 halamanUs 2498806regina pramuditaBelum ada peringkat
- Aiiii: July 7, 1942. E. Mazabraud 2,289,286Dokumen3 halamanAiiii: July 7, 1942. E. Mazabraud 2,289,286Özlem YılmazBelum ada peringkat
- Patented Nov. 7, 1950 Method for Preparing CinnamaldehydeDokumen2 halamanPatented Nov. 7, 1950 Method for Preparing CinnamaldehydebayuminecraftBelum ada peringkat
- United States PatentDokumen4 halamanUnited States Patentwiam wiamBelum ada peringkat
- Acrylic 2520acid Methods 2520of 2520 ProductionDokumen8 halamanAcrylic 2520acid Methods 2520of 2520 Productionapi-3714811Belum ada peringkat
- Production of Sodium DithioniteDokumen10 halamanProduction of Sodium DithioniteDhaval PadaliaBelum ada peringkat
- 1940 Patent for Hydrochloric Acid Absorption Process Using Air CoolingDokumen7 halaman1940 Patent for Hydrochloric Acid Absorption Process Using Air CoolingCésarSánchezRosasBelum ada peringkat
- Removing H2S from CO2-rich gas with CO2-containing solventDokumen4 halamanRemoving H2S from CO2-rich gas with CO2-containing solventIveth RomeroBelum ada peringkat
- Calcining CopperasDokumen3 halamanCalcining Copperasanaaziz100% (1)
- Material ScienceDokumen33 halamanMaterial ScienceLyvea PagaduanBelum ada peringkat
- Anhydrous Hydrogen Chloride Gas From Hydrochloric Acid and CDokumen3 halamanAnhydrous Hydrogen Chloride Gas From Hydrochloric Acid and CbigriverflowsBelum ada peringkat
- United States Patent (191 - (11) 4,052,458: Klein Et Al. (45) Oct. 4, 1977Dokumen3 halamanUnited States Patent (191 - (11) 4,052,458: Klein Et Al. (45) Oct. 4, 1977bvritBelum ada peringkat
- Us 4377495Dokumen5 halamanUs 4377495DWNLD USRMLBelum ada peringkat
- United States: Patent OfficeDokumen3 halamanUnited States: Patent OfficeIRIENE DELFITA TKIMBelum ada peringkat
- United States Patent Office: Patented Mar. 21, 1950Dokumen2 halamanUnited States Patent Office: Patented Mar. 21, 1950alexBelum ada peringkat
- IPT HCLDokumen35 halamanIPT HCLParv pandyaBelum ada peringkat
- KOH From K2SO4 and NaOHDokumen3 halamanKOH From K2SO4 and NaOHamirBelum ada peringkat
- Visbreaking, Thermal Cracking, and Coking: Mercaptan ExtractionDokumen4 halamanVisbreaking, Thermal Cracking, and Coking: Mercaptan ExtractionChetan CherryBelum ada peringkat
- Product Description (KNO3)Dokumen2 halamanProduct Description (KNO3)Vanjared OcampoBelum ada peringkat
- Mechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesDari EverandMechanics of the Household: A Course of Study Devoted to Domestic Machinery and Household Mechanical AppliancesBelum ada peringkat
- Inso نﺎﻣزﺎﺳ ﻲﻠﻣ ناﺮﻳا دراﺪﻧﺎﺘﺳا: ناﺮﻳا ﻲﻠﻣ دراﺪﻧﺎﺘﺳا 14607 14607 لوا پﺎﭼ 1st. EditionDokumen8 halamanInso نﺎﻣزﺎﺳ ﻲﻠﻣ ناﺮﻳا دراﺪﻧﺎﺘﺳا: ناﺮﻳا ﻲﻠﻣ دراﺪﻧﺎﺘﺳا 14607 14607 لوا پﺎﭼ 1st. EditionvahidBelum ada peringkat
- Orsi 2005Dokumen10 halamanOrsi 2005vahidBelum ada peringkat
- Us5834394 2Dokumen2 halamanUs5834394 2vahidBelum ada peringkat
- Heater Specification Table Plastic Extrusion SystemDokumen2 halamanHeater Specification Table Plastic Extrusion SystemvahidBelum ada peringkat
- Us20170291826a1 2Dokumen2 halamanUs20170291826a1 2vahidBelum ada peringkat
- 08 - Chapter 3 - 5 PDFDokumen2 halaman08 - Chapter 3 - 5 PDFvahidBelum ada peringkat
- Us5834394 5Dokumen2 halamanUs5834394 5vahidBelum ada peringkat
- Us20170291826a1 4Dokumen2 halamanUs20170291826a1 4vahidBelum ada peringkat
- Us20170291826a1 1Dokumen2 halamanUs20170291826a1 1vahidBelum ada peringkat
- Us5834394 3Dokumen2 halamanUs5834394 3vahidBelum ada peringkat
- Us5834394 1Dokumen2 halamanUs5834394 1vahidBelum ada peringkat
- Cassava Master PlanDokumen9 halamanCassava Master PlanendosporaBelum ada peringkat
- 08 - Chapter 3Dokumen2 halaman08 - Chapter 3vahidBelum ada peringkat
- Equipment Description: 4.1 CasingDokumen2 halamanEquipment Description: 4.1 CasingvahidBelum ada peringkat
- 4.1.2 Steel Casing - LQ DesignDokumen2 halaman4.1.2 Steel Casing - LQ DesignvahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 5 PDFDokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 5 PDFvahidBelum ada peringkat
- H2so4 3 48x72x59 DDFLHDokumen8 halamanH2so4 3 48x72x59 DDFLHvahidBelum ada peringkat
- Pyrite roasting plant comparisonDokumen2 halamanPyrite roasting plant comparisonvahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 7Dokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 7vahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 3Dokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 3vahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 8Dokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 8vahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 1Dokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 1vahidBelum ada peringkat
- Fluidized Bed Systems for Pyrite RoastingDokumen2 halamanFluidized Bed Systems for Pyrite RoastingvahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 2Dokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 2vahidBelum ada peringkat
- TST Company Profile 2013 - 1Dokumen2 halamanTST Company Profile 2013 - 1vahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 4Dokumen2 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JH - 4vahidBelum ada peringkat
- 10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JHDokumen26 halaman10 Runkel - Sulphuric 2009 - Pyrite Roasting - Runkel Sturm - OUTOTEC JHvahidBelum ada peringkat
- Methylated Mesoporous SnsiDokumen2 halamanMethylated Mesoporous SnsivahidBelum ada peringkat
- Impact of climate change on soilDokumen11 halamanImpact of climate change on soilasdfgBelum ada peringkat
- GTU BE Semester VI Exam on Wind and Solar EnergyDokumen1 halamanGTU BE Semester VI Exam on Wind and Solar Energyfeyayel990Belum ada peringkat
- Chapter - 17 Static ElectricityDokumen54 halamanChapter - 17 Static ElectricityNayeem HakimBelum ada peringkat
- Activity 9.1: OIL Eeming With IFEDokumen16 halamanActivity 9.1: OIL Eeming With IFEAlisha ChopraBelum ada peringkat
- CHEM 101 LECTURE EXCERCISE 2 On Mass Spectrometer and Quantum Theory - E. N DIM-1-1Dokumen4 halamanCHEM 101 LECTURE EXCERCISE 2 On Mass Spectrometer and Quantum Theory - E. N DIM-1-1Ekene Agwu100% (1)
- LTV - Thi Thu Vao 10 Lan 1 - 2019Dokumen5 halamanLTV - Thi Thu Vao 10 Lan 1 - 2019Thùy Linh NguyễnBelum ada peringkat
- Unit 1 Scientific Investigation - Chapter 1 Thinking Like A ScientistDokumen2 halamanUnit 1 Scientific Investigation - Chapter 1 Thinking Like A ScientistKayla OsbornBelum ada peringkat
- Assessment of Erocivity of Geological Formations and Transferred Materials Production of The Drainage of Thriassion Plain, GreeceDokumen9 halamanAssessment of Erocivity of Geological Formations and Transferred Materials Production of The Drainage of Thriassion Plain, GreeceStanley StreinBelum ada peringkat
- M3 - Refrigeration CycleDokumen133 halamanM3 - Refrigeration CycleKrishna KumarBelum ada peringkat
- Allotropes of IronDokumen3 halamanAllotropes of Ironravi2007Belum ada peringkat
- Answer Key: PHYSICS 1050 Mid-Term Test 2Dokumen8 halamanAnswer Key: PHYSICS 1050 Mid-Term Test 2hey_hopBelum ada peringkat
- Physics CapsuleDokumen33 halamanPhysics CapsulepranodanBelum ada peringkat
- Indian Energy ScenarioDokumen19 halamanIndian Energy ScenarioVishal RamakrishnanBelum ada peringkat
- Wqi Report Group 1Dokumen10 halamanWqi Report Group 1Kimaii ZakiBelum ada peringkat
- How Is The Philippines Affected by Climate ChangeDokumen2 halamanHow Is The Philippines Affected by Climate ChangeQueenie SantosBelum ada peringkat
- 2 7Dokumen9 halaman2 7Rifat032017Belum ada peringkat
- Prabharani Public SchoolDokumen30 halamanPrabharani Public SchoolMainak RayBelum ada peringkat
- ESSA - Ammonia Low Carbon EnergyDokumen2 halamanESSA - Ammonia Low Carbon EnergyFauzi BahananBelum ada peringkat
- The Impact of Chemical Fertilizers On Our Environment and EcosystemDokumen19 halamanThe Impact of Chemical Fertilizers On Our Environment and EcosystemDiana DainaBelum ada peringkat
- Delphi AB Wind Supply Chain Study Final ReportDokumen89 halamanDelphi AB Wind Supply Chain Study Final Reportayhan mentesBelum ada peringkat
- Photosynthesis Lesson at Fisher Valley CollegeDokumen5 halamanPhotosynthesis Lesson at Fisher Valley CollegeRomielyn MenguezBelum ada peringkat
- CO2 Conversion and Utilization-American Chemical Society (2002)Dokumen427 halamanCO2 Conversion and Utilization-American Chemical Society (2002)Nguyen TrangBelum ada peringkat
- HKUST CIVIL ENGINEERING FOUNDATION PROJECT REPORTDokumen12 halamanHKUST CIVIL ENGINEERING FOUNDATION PROJECT REPORTJun KangBelum ada peringkat
- Al11 Weekend Worksheet 16Dokumen4 halamanAl11 Weekend Worksheet 16ulusoy2005Belum ada peringkat
- Ped QuestionsDokumen11 halamanPed QuestionsYashPatel100% (1)
- ThermodynamicsDokumen6 halamanThermodynamicsabdulhafizmy87100% (1)