Vapor Pressure of Ammonium Nitrate Je60013a020
Diunggah oleh
jerryDeskripsi Asli:
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Vapor Pressure of Ammonium Nitrate Je60013a020
Diunggah oleh
jerryHak Cipta:
Format Tersedia
unit area (-S*/ia). Those investigators point out that No. 2, Chap.
3, Butterworths Scientific Publications, London,
surface heat content per unit area is a constant and inde- 1959.
pendent of temperature, if the temperature coefficient of Bockris, J., O’M., White, J.L., Mackenzie, J.D., eds.,
surface tension is constant for the molten salts. The present “Physico-Chemical Measurements at High Temperatures,”
Buttenvorths Scientific Publications, London, 1959.
authors hoped t o test the applicability of this concept to the Janz, G.J., Lorenz, M.R., Reu. Sci. Instr. 31, 18-22 (1960).
lead molybdate-bismuth molybdate system. However, they Janz, G.J., Solomons, C., Gardner, H.J., Chem. Reus. 58,
are unable to use the concept because the temperature 461-505 (1958).
coefficient of surface tension is not invariant for some of Jones, G., Bradshaw, B.C., J . A m . Chem. SOC.55, 1780 (1933).
the mixtures of their system. Morris, K.B., Cook, M.I., Sykes, C.Z., Templeman, M.B.,
Ibid., 77, 851 (1955).
Parsons, R., “Handbook of Electrochemical Constants,”
ACKNOWLEDGMENT Academic Press, New York, 1959.
We thank Marlene C. Morris, Constitution and Micro- Stern, K.H., Amis, E.S., Chem. Reus. 59, 30 (1959).
structure Laboratory, National Bureau of Standards, for Van Artsdalen, E.R., Yaffe, I.S., J . Phys. Chem. 59, 118
(1955).
supplying spectroscopic information of the purity of Yaffe, I.S., Van Artsdalen, E.R., Ibid., 60, 1125 (1956).
our salts. Zambonini, F., Gazz. chim. ital. 50 (11), 128-46 (1920).
LITERATURE CITED RECEIVED for review August 28, 1961. Accepted October 20, 1961.
Based in part on dissertations submitted by N. McNair (Major,
(1) Bloom, H., Davis, F.G., James, D.W., Trans. Faraday SOC. USAF) and Gordon Koops (Captain, USAF) to the School of
56,1179 (1960). Engineering at Air Force Institute of Technology in partial
(2) Bloom, H., Heymann, E., Proc. Roy. SOC.(London) A188, 392 fulfillment of the requirements for the degree of master of science
(1947). in aeronautical engineering, August 1961. Work performed under
(3) Bockris, J., O’M., ed., “Modern Aspects of Electrochemistry,” Research Project AFIT 59-6.
Vapor Pressure of Ammonium Nitrate
J. D. BRANDNER, N O R M A N M. JUNK, J. W. LAWRENCE, a n d JACK ROBINS
Atlas Chemical Industries, Inc., Reynolds Experimental Laboratory, Tamaqua, Pa., a n d
Technical Center, Wilmington 99,Del.
FEICK(~) determined the vapor pressure of liquid
ammonium nitrate from 190” to 270” C. using a modified
Chromel-Alumel thermocouple placed directly over the
sample.
boiling point apparatus. He showed that, a t these tempera- Repeated runs a t different flow rates produced only
tures, ammonium nitrate vaporizes by dissociation: small differences in vapor pressure and no consistent
pattern in these differences other than that of experimental
NH4NOs(l) 2 NHs(g) + HNOi(g) error. Calculations indicated that the carrier gas became
saturated with ammonium nitrate in a fraction of a second.
The object of this investigation was to extend the work For solid ammonium nitrate, the gain in weight of the
of Feick on liquid ammonium nitrate to lower temperatures condensation trap (after the contents had been dried in
and to determine the vapor pressure of the solid. vacuo at room temperature) was essentially equal to the
weight loss of the sample, which was taken as the weight
EXPERIMENTAL of vaporized ammonium nitrate. For liquid ammonium
nitrate the weight gain in the condensation trap was less
The vapor pressure was measured by a transpiration than the sample weight loss, this difference in weight being
method based on the relationship first derived by taken as the amount decomposed. T h e loss due to decom-
Regnault ( 5 ) . position ranged from 0.12% of a 15-gram sample in 6 hours
p=Pu/V a t 170” C. to 15% of the sample in 40 minutes a t 240” C.
With liquid ammonium nitrate virtually all of the water
where p is the vapor pressure in millimeters, P the baro- vapor resulting from the decomposition was retained by
metric pressure in millimeters, u the volume in liters of the system, part by the condensed ammonium nitrate, and
ammonia and nitric acid produced by the dissociation of the remainder by the solid carbon dioxide trap. Since this
ammonium nitrate, and V the total gas volume in liters. water vapor also made u p part of the gas volume above
A known volume of dry gas (air or nitrogen) was passed the ammonium nitrate and was not measured by the
slowly over the heated sample (10 to 30 grams) of C.P. wet-test meter, its volume was calculated from the amount
ammonium nitrate, then through a n air-cooled condensation of decomposed ammonium nitrate and used to correct the
trap filled with glass wool, and successively through a solid vapor pressure calculations. In addition, a method of
carbon dioxide trap and a wet-test meter. Experiments iteration was used to correct for the period of time the
were of 1 to 120 hours’ duration. The temperature of the sample was warming to temperature. The vapor pressures
furnace was controlled by means of a Thermocap relay. were calculated on the assumption that the ammonium
The sample temperature was separately measured by a nitrate dissociated completely to nitric acid and ammonia
VOL. 7 , No. 2, APRIL 1962 227
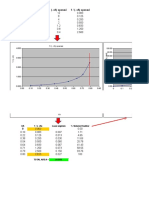
Table I. Vapor Pressure of Solid a n d Liquid
)
TEflPER4TURE
I
Ammonium Nitrate II
Temp., Vapor Pressure, No. of
State c. Mm. Hg Expts.
Solid 76 0.0024 14
98 0.0133 12
100 0.0154 13
109 0.0283 6
111 0.0368 4
123 0.0738 13
130 0.126 8
A
138 0.219 4
143 0.289 4
148 0.448 11
160 0.958 2 -0.5
165 1.17 2
Liquid 170 1.40 2 L LIQUID II
180 2.51 2
190 3.69 2
200 6.31 2
\
210 9.05 2 -1.5
220 12.3 2
230 22.1 2
240 33.4 0
on vaporizing and that only nitrous oxide and water were , , II
produced when the salt decomposed. 1.e 2.0 22 2.4 2.6
m
T
DISCUSSION OF RESULTS
Figure 1. Vapor pressure of ammonium
T h e vapor pressures averaged for the several experiments nitrate as a function
run at each temperature are given in Table I. I n Figure 1, of temperature
the points were drawn as two straight lines, one covering 0 Experimental data
the liquid phase and the other the three solid phases of Feick's data
ammonium nitrate covered by this study. T h e application T. O K.
of the method of least squares to the data yielded the
following equations:
from thermodynamic data on heats of dissociation supports
Solid. log p (mm.) = 10.708 - 4670/ T the assumption that the solid also vaporizes by dissocia-
tion into ammonia and nitric acid. A further confirmation
Liquid. log p (mm.) = 9.981 - 4360/ T of this mechanism was demonstrated by passing ammonia
with nitrogen through a sample of ammonium nitrate in
with an estimated standard deviation of 0.031 in each case. the apparatus described. When this was done, with both
Heats of vaporization calculated from the slopes are 42.7 solid and liquid ammonium nitrate, the weight loss per
and 39.9 kcal. per mole, respectively, for the solid and liter of nitrogen passed through the sample was reduced
the liquid, the latter being in agreement with Feick's work. to a fraction of its magnitude in the absence of ammonia.
These may be compared with the values for the heats of In several runs at 220" to 260" C., nitric acid vapor was
dissociation of solid and liquid ammonium nitrate cal- passed through liquid ammonium nitrate. Again vaporiza-
culated from the published heats of formation a t 25" C. tion was suppressed, judging by the weight of ammonium
for NHS(g) ( 4 ) , HN03(g) (2), and NH4N03(IV) ( 4 ) . nitrate recovered in the trap; however, nitric acid
From the foregoing and heat capacity and heat of transition accelerated the irreversible decomposition reaction.
data published by Kelley ( 3 ) , the following values were
calculated for the heats of dissociation:
LITERATURE CITED
42.1 kcal. per mole for solid at 115" C.
(1) Feick, G., J . Am. Chem. SOC.76, 5858 (1954).
39.0 kcal. per mole for liquid at 200" C. (2) Forsythe, W.R., Giauque, W.F., Ibid.,64, 48 (1942).
(3) Kelley, K.K., U. S. Bur. Mines, Bull. 584 (1960).
T h e above temperatures are the mid-points of the data (4) Natl. Bur. Standards, Circ. 500 (1952).
(5) Regnault, H.V., Ann. c h h . 15,129 (1845).
for the solid and liquid, respectively.
The agreement between experimental heats of vaporiza- RECEIVED
for review September 20, 1961. Accepted November 30,
tion calculated from vapor pressures and literature values 1961.
228 JOURNAL OF CHEMICAL AND ENGINEERING DATA
Anda mungkin juga menyukai
- Recommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsDari EverandRecommended Reference Materials for Realization of Physicochemical Properties: Pressure–Volume–Temperature RelationshipsE. F. G. HeringtonBelum ada peringkat
- Feick 1954Dokumen3 halamanFeick 1954magdy salehBelum ada peringkat
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringDari EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringBelum ada peringkat
- Correlating Vapor Pressures and Heats of Solution For The Ammonium Nitrate-Water System An Enthalpy-Concentration Diagram PDFDokumen5 halamanCorrelating Vapor Pressures and Heats of Solution For The Ammonium Nitrate-Water System An Enthalpy-Concentration Diagram PDFMagdy SalehBelum ada peringkat
- Mechanism, Kinetics, and Equilibrium Thermal Decomposition of Ammonium SulfateDokumen6 halamanMechanism, Kinetics, and Equilibrium Thermal Decomposition of Ammonium SulfateTyokBelum ada peringkat
- The Adsorption of Non-Polar Gases On Alkali Halide CrystalsDokumen19 halamanThe Adsorption of Non-Polar Gases On Alkali Halide CrystalsMGNMBelum ada peringkat
- Adiabatic Compressibility of Liquid AmmoniaDokumen3 halamanAdiabatic Compressibility of Liquid AmmoniaempanadaBelum ada peringkat
- Vapor Pressure and Evaporation Coefficient GlycerolDokumen4 halamanVapor Pressure and Evaporation Coefficient GlycerolCelrose FernandezBelum ada peringkat
- Solubility of Nitrogen in Liquid Ammonia at 25° From 25 To 1000 AtmospheresDokumen4 halamanSolubility of Nitrogen in Liquid Ammonia at 25° From 25 To 1000 Atmospheressoviet_880% (1)
- Sulphur VapoursDokumen12 halamanSulphur VapoursAnvay Choudhary100% (1)
- Menzies A.W.C., Lacoss D.A. - Allotropy of Liquid Benzene (1931)Dokumen4 halamanMenzies A.W.C., Lacoss D.A. - Allotropy of Liquid Benzene (1931)Seif SkiBelum ada peringkat
- A Comprehensive Analysis of Direct Contact Condensation of Saturated Steam On Subcooled Liquid JetsDokumen16 halamanA Comprehensive Analysis of Direct Contact Condensation of Saturated Steam On Subcooled Liquid JetsLuisBelum ada peringkat
- Jcpsa6 9 12 859 1Dokumen5 halamanJcpsa6 9 12 859 1Abiyyi SufyanBelum ada peringkat
- 1-4-4 - System PropertiesDokumen35 halaman1-4-4 - System PropertiesykthamykBelum ada peringkat
- Westrum1960 CP HacoonaDokumen2 halamanWestrum1960 CP HacoonaRiza Shinta RBelum ada peringkat
- Detonation Characteristics Hydrogen-Oxygen Mixtures High Initial PressuresDokumen5 halamanDetonation Characteristics Hydrogen-Oxygen Mixtures High Initial PressuresGustavo Gabriel JimenezBelum ada peringkat
- Mesmer 1963Dokumen3 halamanMesmer 1963Victor FerreiraBelum ada peringkat
- Benzoic Acid Vapor PressureDokumen5 halamanBenzoic Acid Vapor PressureRajeshBelum ada peringkat
- Chapter 10Dokumen22 halamanChapter 10احمد الدلالBelum ada peringkat
- RS1067 - Initial Results For Reflux Condensation of Hydrocarbon Vapour MixturesDokumen8 halamanRS1067 - Initial Results For Reflux Condensation of Hydrocarbon Vapour MixturesIan MannBelum ada peringkat
- A. Gordon: S. Shankman atDokumen4 halamanA. Gordon: S. Shankman atbuhalnitaBelum ada peringkat
- Bab 1 Gas Ideal Dan NyataDokumen64 halamanBab 1 Gas Ideal Dan NyataVincent PradjinataBelum ada peringkat
- The Influence On Gas Chromatography Analysis Caused by The Joule-Thomson Cooling Effect During The Natural Gas SamplingDokumen5 halamanThe Influence On Gas Chromatography Analysis Caused by The Joule-Thomson Cooling Effect During The Natural Gas SamplingWALDO DAVID SILVA SANCHEZBelum ada peringkat
- Evaporation of TitaniumDokumen11 halamanEvaporation of Titaniumnandza99Belum ada peringkat
- Isobaric Vapor Liquid Equilibria of The Water 2-Propanol System at 30, 60, and 100 KpaDokumen4 halamanIsobaric Vapor Liquid Equilibria of The Water 2-Propanol System at 30, 60, and 100 KpaRafael HenriqueBelum ada peringkat
- Effects of Particle Size, Heating Rate and Pressure On Measurement of Pyrolysis Kinetics by Thermogravimetric AnalysisDokumen6 halamanEffects of Particle Size, Heating Rate and Pressure On Measurement of Pyrolysis Kinetics by Thermogravimetric Analysisboniucira cantikBelum ada peringkat
- CP Na5p3o10Dokumen6 halamanCP Na5p3o10agnarindra01_8550147Belum ada peringkat
- A Nomograph For Correction of Boiling Points: Kent State University, KentDokumen2 halamanA Nomograph For Correction of Boiling Points: Kent State University, Kentthrowaway456456Belum ada peringkat
- Thermophysical Properties of Liquid Aluminum: 3036-VOLUME 48A, JUNE 2017 Metallurgical and Materials Transactions ADokumen10 halamanThermophysical Properties of Liquid Aluminum: 3036-VOLUME 48A, JUNE 2017 Metallurgical and Materials Transactions AEduardo Fernandez SanchezBelum ada peringkat
- Bab I Gas Ideal Dan Nyata 2009 - 2010Dokumen64 halamanBab I Gas Ideal Dan Nyata 2009 - 2010Kezia IreneBelum ada peringkat
- My Diffusion of A Gas ReportDokumen22 halamanMy Diffusion of A Gas ReportEmonbeifo Efosasere100% (3)
- Effect Heat Transfer Roughness On Convective in Commercial PipesDokumen7 halamanEffect Heat Transfer Roughness On Convective in Commercial PipesGustavo Gabriel JimenezBelum ada peringkat
- Surface Tension and Molar Surface Free Energy and Entropy of Water To - 27.2Dokumen4 halamanSurface Tension and Molar Surface Free Energy and Entropy of Water To - 27.2ANGELICA ALEJANDRA MORENO CONTEREASBelum ada peringkat
- The Joule-Thomson Effect Air. Frederick: G. in On ToDokumen19 halamanThe Joule-Thomson Effect Air. Frederick: G. in On ToirvinBelum ada peringkat
- Recent Fumarole Measurement in The Karapiti AreaDokumen6 halamanRecent Fumarole Measurement in The Karapiti AreaCodey DuBelum ada peringkat
- 2550 Temperatura PDFDokumen2 halaman2550 Temperatura PDFPenelope MeloBelum ada peringkat
- Ozone Decomposition KineticsDokumen5 halamanOzone Decomposition KineticsJESUS PLAZAS SALDAÑABelum ada peringkat
- CO2 Data Density (Kennedy, G.C.) - 422 - OCR PDFDokumen18 halamanCO2 Data Density (Kennedy, G.C.) - 422 - OCR PDFshaonaaBelum ada peringkat
- Scanlan 1970Dokumen18 halamanScanlan 1970Vilas AndhaleBelum ada peringkat
- Henry's Law Constant For The Ozone-WaterDokumen8 halamanHenry's Law Constant For The Ozone-WaterJESUS PLAZAS SALDAÑABelum ada peringkat
- On A Theory of The Van Der Waals Adsorption of Gases PDFDokumen10 halamanOn A Theory of The Van Der Waals Adsorption of Gases PDFJefersonCorreiaBelum ada peringkat
- Chlorine The Heat Capacity Vapor Pressure Heats ofDokumen5 halamanChlorine The Heat Capacity Vapor Pressure Heats ofHusain MochammadBelum ada peringkat
- Abstracts of The Papers Presented at The Thirteenth National Vacuum SymposiumDokumen1 halamanAbstracts of The Papers Presented at The Thirteenth National Vacuum SymposiumgfpeezyBelum ada peringkat
- Intrinsic and Global Reaction Rate of Methanol Dehydration Over 7-A1203 PelletsDokumen6 halamanIntrinsic and Global Reaction Rate of Methanol Dehydration Over 7-A1203 PelletsHectorBelum ada peringkat
- Determining Molar Volume and Absolute ZeroDokumen4 halamanDetermining Molar Volume and Absolute ZeroJeanine Bianca LastinoBelum ada peringkat
- 6 Phase Equilibria in Hydrocarbon Systems. Volumetric and Phase Behavior of The Methane-n-Heptane SystemDokumen14 halaman6 Phase Equilibria in Hydrocarbon Systems. Volumetric and Phase Behavior of The Methane-n-Heptane SystemRoy Royer Solorzano DuranBelum ada peringkat
- Thermodynamic Functions Methane: I I I IdDokumen2 halamanThermodynamic Functions Methane: I I I Idg_marianiBelum ada peringkat
- Informe 8Dokumen4 halamanInforme 8Miguel MorenoBelum ada peringkat
- Measure thermal conductivity of liquids using transient hot wire methodDokumen9 halamanMeasure thermal conductivity of liquids using transient hot wire methodhiddenkey1Belum ada peringkat
- Catalytic Decomposition of Nitric Oxide Over Platinum-Nickel CatalystDokumen6 halamanCatalytic Decomposition of Nitric Oxide Over Platinum-Nickel CatalystDeiber SernaBelum ada peringkat
- 1977 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of IsopentaneDokumen7 halaman1977 - Das - Reed - Eubank - PVT Surface and Thermodynamic Properties of IsopentaneAlexanderBelum ada peringkat
- Isothermal Sorption Characteristics of T PDFDokumen8 halamanIsothermal Sorption Characteristics of T PDFDestria FiryalBelum ada peringkat
- Tans - Aqueous Ammonium Sulfate - 1958Dokumen2 halamanTans - Aqueous Ammonium Sulfate - 1958Yulia KurniawatiBelum ada peringkat
- Explosions of Syngas/CO Mixtures in Oxygen-Enriched AirDokumen8 halamanExplosions of Syngas/CO Mixtures in Oxygen-Enriched AirEduardoBelum ada peringkat
- Ammonia: Latent Heat of OFDokumen34 halamanAmmonia: Latent Heat of OFCastoriadisBelum ada peringkat
- The Heat Capacities of Titanium Dioxide From The Thermodynamic Properties of Titanium DioxideDokumen3 halamanThe Heat Capacities of Titanium Dioxide From The Thermodynamic Properties of Titanium DioxideNaufal AdityasBelum ada peringkat
- NH In-Tube Condensation Heat Transfer and Pressure Drop in A Smooth TubeDokumen9 halamanNH In-Tube Condensation Heat Transfer and Pressure Drop in A Smooth TubealagaruBelum ada peringkat
- Achenbach 68Dokumen15 halamanAchenbach 68emilienbruand0Belum ada peringkat
- Problem Sheet 1 Basics and TemperatureDokumen4 halamanProblem Sheet 1 Basics and TemperatureS DBelum ada peringkat
- The solubility of nitrobenzene in light and heavy waterDokumen2 halamanThe solubility of nitrobenzene in light and heavy waterMuhammad Fiqih AlayubiBelum ada peringkat
- United States Patent (19) : Oftring Et AlDokumen12 halamanUnited States Patent (19) : Oftring Et AljerryBelum ada peringkat
- Cell Ula Se Filter Paper ActivityDokumen1 halamanCell Ula Se Filter Paper ActivitySiddiq SallehBelum ada peringkat
- Sssss XC SSCDokumen1 halamanSssss XC SSCjerryBelum ada peringkat
- How To Write The Perfect Blog Post - Social Triggers: Yes, Send Me Ebook. To Share This Graphc With Your Blog ReadersDokumen1 halamanHow To Write The Perfect Blog Post - Social Triggers: Yes, Send Me Ebook. To Share This Graphc With Your Blog ReadersjerryBelum ada peringkat
- Fuel From The SunDokumen3 halamanFuel From The SunjerryBelum ada peringkat
- How To Write The Perfect Blog Post - Social TriggersDokumen1 halamanHow To Write The Perfect Blog Post - Social TriggersjerryBelum ada peringkat
- How To Write The Perfect Blog Post - Social Triggers: Yes, Send Me Ebook. To Share This Graphc With Your Blog ReadersDokumen1 halamanHow To Write The Perfect Blog Post - Social Triggers: Yes, Send Me Ebook. To Share This Graphc With Your Blog ReadersjerryBelum ada peringkat
- How To Write The Perfect Blog Post - Social TriggersDokumen1 halamanHow To Write The Perfect Blog Post - Social TriggersjerryBelum ada peringkat
- Problem v-1 SD v-3Dokumen6 halamanProblem v-1 SD v-3jerryBelum ada peringkat
- CompetitionDokumen1 halamanCompetitionjerryBelum ada peringkat
- Literatur SkoogDokumen5 halamanLiteratur SkoogjerryBelum ada peringkat
- Aminpro FK TestDokumen9 halamanAminpro FK TestpeilinlanBelum ada peringkat
- Chapter 14 Modern SpectrosDokumen24 halamanChapter 14 Modern SpectrosChicken ChickenBelum ada peringkat
- E-CAPS-28 - For CoE (XI) - Chemistry - (Que. - Answer Key)Dokumen3 halamanE-CAPS-28 - For CoE (XI) - Chemistry - (Que. - Answer Key)darling deanBelum ada peringkat
- Lilin Downhole MotorDokumen35 halamanLilin Downhole MotorIAN.SEMUT100% (2)
- Single Disc Clutch DesignDokumen32 halamanSingle Disc Clutch DesignWeins GemerlapBelum ada peringkat
- P 211enDokumen26 halamanP 211enRadhakrishnan BalasubramanianBelum ada peringkat
- AP PHYSICS B 1988 MC + AnswersDokumen17 halamanAP PHYSICS B 1988 MC + AnswersbastardBelum ada peringkat
- Physics SL Paper 3 TZ2Dokumen20 halamanPhysics SL Paper 3 TZ2Dongjean SeoBelum ada peringkat
- Why Do We Study Physics - Socratic PDFDokumen1 halamanWhy Do We Study Physics - Socratic PDFMon LuffyBelum ada peringkat
- Signature RedactedDokumen49 halamanSignature RedactedG Pavan KumarBelum ada peringkat
- Basic Simulation LabDokumen69 halamanBasic Simulation LabkamalahasanmBelum ada peringkat
- 02 Jaulas de Agujas PDFDokumen52 halaman02 Jaulas de Agujas PDFRodrigo Schaider Dos SantosBelum ada peringkat
- Design and Manufacturing of Automatic Gear Shifter For BicycleDokumen10 halamanDesign and Manufacturing of Automatic Gear Shifter For BicycleMannam RujendraBelum ada peringkat
- ELIMINATION REACTIONS: AN OVERVIEWDokumen19 halamanELIMINATION REACTIONS: AN OVERVIEWSyuhadah NoordinBelum ada peringkat
- Noise Margin Definition ExplainedDokumen10 halamanNoise Margin Definition ExplainedAnil BhardwajBelum ada peringkat
- Docking With ArgusLabDokumen24 halamanDocking With ArgusLabDesmond MacLeod Carey100% (1)
- Carrefour-SA Shopping Center TurkeyDokumen2 halamanCarrefour-SA Shopping Center TurkeyVineet JogalekarBelum ada peringkat
- WRC 538 PDFDokumen27 halamanWRC 538 PDFsoojin gu25% (4)
- TCL Air Conditioner Service ManualDokumen138 halamanTCL Air Conditioner Service ManualFabian EtcheniqueBelum ada peringkat
- Stress Analysis of Flat Plates With Attached NozzlesDokumen125 halamanStress Analysis of Flat Plates With Attached NozzlesZarra FaktBelum ada peringkat
- Revised Design Report of Jetty 06.04.2014Dokumen10 halamanRevised Design Report of Jetty 06.04.2014Priodeep Chowdhury100% (2)
- A Study of Manufacturing of Steam TurbinesDokumen40 halamanA Study of Manufacturing of Steam TurbinesSaketh Varma MudunuriBelum ada peringkat
- 400 KV Tender Docs PDFDokumen356 halaman400 KV Tender Docs PDFtanujaayerBelum ada peringkat
- Is.1875 1992Dokumen14 halamanIs.1875 1992Sadashiva sahooBelum ada peringkat
- Transmision de Potencia NewDokumen12 halamanTransmision de Potencia NewGustavo ArmellaBelum ada peringkat
- Overview Aerodynamics 2017Dokumen10 halamanOverview Aerodynamics 2017marcoBelum ada peringkat
- Assg 03 1Dokumen7 halamanAssg 03 1Abdul ShakoorBelum ada peringkat
- Wiring DiagramDokumen24 halamanWiring DiagramReji Raju0% (1)
- Curriculum-Of Mathematics Government College Women University, SialkotDokumen119 halamanCurriculum-Of Mathematics Government College Women University, SialkotHuzaifa GurmaniBelum ada peringkat
- Sully: The Untold Story Behind the Miracle on the HudsonDari EverandSully: The Untold Story Behind the Miracle on the HudsonPenilaian: 4 dari 5 bintang4/5 (103)
- The Fabric of Civilization: How Textiles Made the WorldDari EverandThe Fabric of Civilization: How Textiles Made the WorldPenilaian: 4.5 dari 5 bintang4.5/5 (57)
- Packing for Mars: The Curious Science of Life in the VoidDari EverandPacking for Mars: The Curious Science of Life in the VoidPenilaian: 4 dari 5 bintang4/5 (1395)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaDari EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaBelum ada peringkat
- The Weather Machine: A Journey Inside the ForecastDari EverandThe Weather Machine: A Journey Inside the ForecastPenilaian: 3.5 dari 5 bintang3.5/5 (31)
- Highest Duty: My Search for What Really MattersDari EverandHighest Duty: My Search for What Really MattersBelum ada peringkat
- Hero Found: The Greatest POW Escape of the Vietnam WarDari EverandHero Found: The Greatest POW Escape of the Vietnam WarPenilaian: 4 dari 5 bintang4/5 (19)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestDari EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestPenilaian: 4 dari 5 bintang4/5 (28)
- Transformed: Moving to the Product Operating ModelDari EverandTransformed: Moving to the Product Operating ModelPenilaian: 4 dari 5 bintang4/5 (1)
- The End of Craving: Recovering the Lost Wisdom of Eating WellDari EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellPenilaian: 4.5 dari 5 bintang4.5/5 (80)
- 35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Dari Everand35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Penilaian: 4 dari 5 bintang4/5 (21)
- A Place of My Own: The Architecture of DaydreamsDari EverandA Place of My Own: The Architecture of DaydreamsPenilaian: 4 dari 5 bintang4/5 (241)
- The Future of Geography: How the Competition in Space Will Change Our WorldDari EverandThe Future of Geography: How the Competition in Space Will Change Our WorldPenilaian: 4.5 dari 5 bintang4.5/5 (4)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureDari EverandDirt to Soil: One Family’s Journey into Regenerative AgriculturePenilaian: 5 dari 5 bintang5/5 (124)
- Pale Blue Dot: A Vision of the Human Future in SpaceDari EverandPale Blue Dot: A Vision of the Human Future in SpacePenilaian: 4.5 dari 5 bintang4.5/5 (586)
- Across the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsDari EverandAcross the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsBelum ada peringkat
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseDari EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElsePenilaian: 3.5 dari 5 bintang3.5/5 (12)
- Recording Unhinged: Creative and Unconventional Music Recording TechniquesDari EverandRecording Unhinged: Creative and Unconventional Music Recording TechniquesBelum ada peringkat
- Einstein's Fridge: How the Difference Between Hot and Cold Explains the UniverseDari EverandEinstein's Fridge: How the Difference Between Hot and Cold Explains the UniversePenilaian: 4.5 dari 5 bintang4.5/5 (50)
- Reality+: Virtual Worlds and the Problems of PhilosophyDari EverandReality+: Virtual Worlds and the Problems of PhilosophyPenilaian: 4 dari 5 bintang4/5 (24)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationDari EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationPenilaian: 4.5 dari 5 bintang4.5/5 (46)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterDari EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterPenilaian: 5 dari 5 bintang5/5 (3)
- Grunt: The Curious Science of Humans at WarDari EverandGrunt: The Curious Science of Humans at WarPenilaian: 4 dari 5 bintang4/5 (429)
- Fallout: The Hiroshima Cover-up and the Reporter Who Revealed It to the WorldDari EverandFallout: The Hiroshima Cover-up and the Reporter Who Revealed It to the WorldPenilaian: 4.5 dari 5 bintang4.5/5 (82)
- The Path Between the Seas: The Creation of the Panama Canal, 1870-1914Dari EverandThe Path Between the Seas: The Creation of the Panama Canal, 1870-1914Penilaian: 4.5 dari 5 bintang4.5/5 (124)