Assignment 5: Optimization: (PO3, CO2)
Diunggah oleh
MohamedFittriJudul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Assignment 5: Optimization: (PO3, CO2)
Diunggah oleh
MohamedFittriHak Cipta:
Format Tersedia
Assignment 5: Optimization
(PO3, CO2)
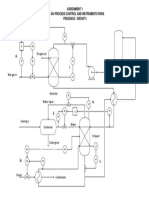
Figure 1 shows a section of process for converting toluene to benzene in a hydro alkylation reactor.
The main reaction is: C7 H8 H 2 C6 H 6 CH 4
The side reaction that produces biphenyl is: 2C6 H 6 C12 H10 H 2
Figure 1: Process for production of benzene.
The condition for the feed and two recycles streams are given in Table 1.
Component Feed (lbmol/hr) Recycle (lbmol/hr) Gas Recycle (lbmol/hr)
Hydrogen 2045.9
Methane 3020.8
Benzene 3.4 42.8
Toluene 274.2 82.5 5.3
Biphenyl 1.0
Temperature (0F) 75 250 121
Pressure (psi) 569 569 569
The pressure and temperature at various block is specified in Table 2.
Location Pressure (psi) Temperature (0F)
HeatX output for Furnace inlet 569 1000
Furnace outlet 494 1200
Reactor outlet 494 1268
Pump outlet 494
Heater outlet 480 100
Flash 480 100
Conversion of toluene is 75%.
2%of benzene present after the first reaction occurs is converted to biphenyl.
The overall heat transfer in HeatX is 60 Btu/(hr-ft2-0F).
The SPLIT block has flow split fraction 0.8 of PROD stream.
The property method is PENG-ROB
Question 1:
1. Specify the material balances of the PROD stream and cost utility of the plant.
2. Optimize the flash temperature 0F in order to maximize the PROFIT of the plant.
PROFIT = P_PROD*PRODUCT - C_QHEATX*QHEATX
Where, P_PROD is the price of product (=RM 1.2 lbmol-1 hr), C_QHEATX is the cost of heat duty
in the HX ( = RM 10-6Btu-1hr). PRODUCT is the mole flow BENZENE in the PROD steam (lbmol/hr).
QHEATX is calculated exchanger heat duty in HX block (Btu/hr).
Determine the optimum flash temperature, PRODUCT and QHEATX as long as the maximum
PROFIT obtained.
3. Compare the utility cost before and after optimization task. Discuss the result obtained.
Anda mungkin juga menyukai
- Agru Catalogue PDFDokumen505 halamanAgru Catalogue PDFAhmed HussienBelum ada peringkat
- Ferrous and Non Ferrous Materials - Dr. ChalimbaDokumen61 halamanFerrous and Non Ferrous Materials - Dr. ChalimbaTadala Angella GomondaBelum ada peringkat
- Distillation Column PipingDokumen57 halamanDistillation Column PipingKaran Singh92% (13)
- Production of MTBE Using Reactive DistilDokumen4 halamanProduction of MTBE Using Reactive DistilIndraBelum ada peringkat
- UntitledDokumen12 halamanUntitledapi-256504985Belum ada peringkat
- SPE Papers Well DeliverabilityDokumen279 halamanSPE Papers Well DeliverabilitySyed Ahmed FlareBelum ada peringkat
- Stoichiometry Notes KEYDokumen17 halamanStoichiometry Notes KEYOnofre Algara Jr.Belum ada peringkat
- Hydrogenation of Fatty Acid Methyl Esters To FattyDokumen9 halamanHydrogenation of Fatty Acid Methyl Esters To FattyYulius Harmawan Setya PratamaBelum ada peringkat
- CHAPTER 3 (v3) - ETHYLENE BASED PRODUCTIONDokumen46 halamanCHAPTER 3 (v3) - ETHYLENE BASED PRODUCTIONAleeya KamalBelum ada peringkat
- Statistics for Process Control Engineers: A Practical ApproachDari EverandStatistics for Process Control Engineers: A Practical ApproachBelum ada peringkat
- Allyl Chloride Production A Case Study in Debottlenecking Retrofitting and DesignDokumen7 halamanAllyl Chloride Production A Case Study in Debottlenecking Retrofitting and DesignPaola PorrasBelum ada peringkat
- Chemical Kinetics On Thermal Decompositions of CumeneDokumen8 halamanChemical Kinetics On Thermal Decompositions of CumeneMario Alonso Velasquez FlorezBelum ada peringkat
- Che 456 Spring 2011 Major 2 Styrene Production BackgroundDokumen6 halamanChe 456 Spring 2011 Major 2 Styrene Production Backgroundyamel huaira taipeBelum ada peringkat
- 4 ContentDokumen25 halaman4 ContentMohamedFittriBelum ada peringkat
- Production of N Octane From Ethylene and I ButaneDokumen2 halamanProduction of N Octane From Ethylene and I ButaneRamyaBelum ada peringkat
- International Thermodynamic Tables of the Fluid State: Propylene (Propene)Dari EverandInternational Thermodynamic Tables of the Fluid State: Propylene (Propene)Belum ada peringkat
- Elc 590 PersuasiveDokumen4 halamanElc 590 PersuasiveMohamedFittriBelum ada peringkat
- FullDokumen33 halamanFullEja RotiKeju100% (2)
- Mini Project Full PDFDokumen37 halamanMini Project Full PDFMohamad El KheirBelum ada peringkat
- School of Chemical Engineering - 20Dokumen372 halamanSchool of Chemical Engineering - 20biroutiBelum ada peringkat
- Project Report FINALDokumen38 halamanProject Report FINALSagar PhullBelum ada peringkat
- Butene-1: Trans-2-Butene, Isobutylene, and ButadieneDokumen1 halamanButene-1: Trans-2-Butene, Isobutylene, and ButadieneYESIKBMARTIN100% (1)
- Styrene From Ethane and BenzeneDokumen6 halamanStyrene From Ethane and BenzeneAmy Puah100% (2)
- MEK in School SecondDokumen13 halamanMEK in School Secondifiok100% (1)
- Production of Methyl Ethyl KetoneDokumen89 halamanProduction of Methyl Ethyl KetonePablo HernandezBelum ada peringkat
- Mtbe 3 - DP 2Dokumen303 halamanMtbe 3 - DP 2Faiz ZainiBelum ada peringkat
- Production of Phenol and Bisphenol A ReportDokumen47 halamanProduction of Phenol and Bisphenol A ReportvpsrpuchBelum ada peringkat
- Synthesis of Epichlorohydrin Kinetic PDFDokumen6 halamanSynthesis of Epichlorohydrin Kinetic PDFTaylor PennaBelum ada peringkat
- Plant LocationDokumen8 halamanPlant LocationEeHuey ChooBelum ada peringkat
- Salman PDFDokumen263 halamanSalman PDFRoger FernandezBelum ada peringkat
- FORMALDEHYDE Cost EstimationDokumen5 halamanFORMALDEHYDE Cost EstimationPradeep Munna100% (2)
- Lab 4 SimulationDokumen8 halamanLab 4 SimulationaziziBelum ada peringkat
- Formic Acid TechnologyDokumen3 halamanFormic Acid Technologyatharnadim_osBelum ada peringkat
- Chemical Modification of Natural Rubber Under Supercritical CarbonDokumen8 halamanChemical Modification of Natural Rubber Under Supercritical CarbonKristina HuffmanBelum ada peringkat
- Overall Flowsheet Simulation Benzene Cyclohexane TW6Dokumen7 halamanOverall Flowsheet Simulation Benzene Cyclohexane TW6Mitesh ParmarBelum ada peringkat
- 1ethanol To Ethylene B1 - ProcessdesignDokumen7 halaman1ethanol To Ethylene B1 - ProcessdesignAdi PutraBelum ada peringkat
- Ethylene Oxide AppDokumen2 halamanEthylene Oxide AppSyifa AnggrainiBelum ada peringkat
- A Project Report Submitted By: in Partial Fulfilment For The Award of The DegreeDokumen91 halamanA Project Report Submitted By: in Partial Fulfilment For The Award of The DegreeHari BharathiBelum ada peringkat
- Chapter 1 Feasibility StudyDokumen102 halamanChapter 1 Feasibility Studyolescoot67% (3)
- Pichia FermentationDokumen11 halamanPichia FermentationmicromanpBelum ada peringkat
- Ethylbenzene ProductionDokumen30 halamanEthylbenzene ProductionUum LukmanBelum ada peringkat
- DSTWU - A Shortcut Distillation Model in Aspen Plus V8.0Dokumen11 halamanDSTWU - A Shortcut Distillation Model in Aspen Plus V8.0JúpiterBelum ada peringkat
- Progress in Synthesis of Ethylene Glycol Through C1 ChemicalDokumen10 halamanProgress in Synthesis of Ethylene Glycol Through C1 ChemicalFelipe A. Peña RincónBelum ada peringkat
- Jurnal Reaktor Metatesis Propilen PDFDokumen8 halamanJurnal Reaktor Metatesis Propilen PDFAnonymous 8UdbKWu2Belum ada peringkat
- Malaysian Institute's Glycerol Production DesignDokumen56 halamanMalaysian Institute's Glycerol Production DesignNUR AKMAL HISHAMBelum ada peringkat
- Aspen Plus Simulation of Polyethylene GasificationDokumen17 halamanAspen Plus Simulation of Polyethylene Gasificationkishna009Belum ada peringkat
- Production of Acrylonitrile by Ammoxidation of PropyleneDokumen33 halamanProduction of Acrylonitrile by Ammoxidation of PropyleneJ José B VelasquezBelum ada peringkat
- CumeneDokumen21 halamanCumeneDiv SavaliyaBelum ada peringkat
- Cost AnalysisDokumen24 halamanCost Analysisabdaziz_salamBelum ada peringkat
- Brandstaedter Willi MichaelDokumen202 halamanBrandstaedter Willi MichaelApril JuneBelum ada peringkat
- FYP ProposalDokumen11 halamanFYP ProposalArslan SamBelum ada peringkat
- Methyl Tertiary Butyl Ether (MTBE) Full ReportDokumen369 halamanMethyl Tertiary Butyl Ether (MTBE) Full Reportnasnazir100% (1)
- Feasibility Study of Ethylene Oxide ProductionDokumen3 halamanFeasibility Study of Ethylene Oxide ProductionIntratec SolutionsBelum ada peringkat
- Christian Andersson Thesis-Succinic PDFDokumen142 halamanChristian Andersson Thesis-Succinic PDFprivaz81Belum ada peringkat
- Kuwait University Chemical Engineering Plant Design Hysys ReportDokumen20 halamanKuwait University Chemical Engineering Plant Design Hysys ReportCrazy HelloBelum ada peringkat
- Lab Report Effect of Residence Time On TDokumen26 halamanLab Report Effect of Residence Time On TMuhammad IqmalBelum ada peringkat
- 64788Dokumen35 halaman64788ghatak2100% (1)
- Production of Aniline by Direct AminationDokumen29 halamanProduction of Aniline by Direct AminationSatyshikh SrivBelum ada peringkat
- Mononitration of TolueneDokumen4 halamanMononitration of TolueneNur Syafiqah Izzuddin100% (1)
- Ilovepdf MergedDokumen39 halamanIlovepdf MergedmoheedBelum ada peringkat
- Preparation of Catalysts II: Scientific Bases for the Preparation of Heterogeneous CatalystsDari EverandPreparation of Catalysts II: Scientific Bases for the Preparation of Heterogeneous CatalystsBelum ada peringkat
- Counter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsDari EverandCounter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsBelum ada peringkat
- CR4-Results and DiscussionDokumen8 halamanCR4-Results and DiscussionAbdulbari UshBelum ada peringkat
- Cev440 - Chemical Reaction Engineering: Sms/elementary - ReactionsDokumen2 halamanCev440 - Chemical Reaction Engineering: Sms/elementary - ReactionsMohamedFittriBelum ada peringkat
- Cev440 - Chemical Reaction EngineeringDokumen2 halamanCev440 - Chemical Reaction EngineeringMohamedFittriBelum ada peringkat
- 5 6233135072879312915Dokumen3 halaman5 6233135072879312915MohamedFittriBelum ada peringkat
- 5 6233135072879312915Dokumen3 halaman5 6233135072879312915MohamedFittriBelum ada peringkat
- 5 6233135072879312915Dokumen3 halaman5 6233135072879312915MohamedFittriBelum ada peringkat
- Chapter 5 - Mass BalanceDokumen21 halamanChapter 5 - Mass BalanceMohamedFittriBelum ada peringkat
- Size Calculation:: T L G GDokumen2 halamanSize Calculation:: T L G GMohamedFittriBelum ada peringkat
- Size Enlargement Through GranulationDokumen1 halamanSize Enlargement Through GranulationMohamedFittriBelum ada peringkat
- Assignment NumecDokumen4 halamanAssignment NumecMohamedFittriBelum ada peringkat
- RESULTSDokumen3 halamanRESULTSMohamedFittriBelum ada peringkat
- AssigmntDokumen11 halamanAssigmntMohamedFittriBelum ada peringkat
- 1 IntroductionDokumen2 halaman1 IntroductionMohamedFittriBelum ada peringkat
- Biological Wastewater Treatment Case StudyDokumen1 halamanBiological Wastewater Treatment Case StudyMohamedFittriBelum ada peringkat
- Assignment 1 Cev 544 Process Control and Instrumentations PEH2246C2 / GROUP 5Dokumen1 halamanAssignment 1 Cev 544 Process Control and Instrumentations PEH2246C2 / GROUP 5MohamedFittriBelum ada peringkat
- Chapter 2.1 Definitions and Classifications: Notes On This ChapterDokumen3 halamanChapter 2.1 Definitions and Classifications: Notes On This ChapterMohamedFittriBelum ada peringkat
- ObjectivesDokumen2 halamanObjectivesMohamedFittriBelum ada peringkat
- Chapter 2Dokumen62 halamanChapter 2nidBelum ada peringkat
- Assignment 5Dokumen13 halamanAssignment 5MohamedFittri100% (1)
- Asthma, Airway Symptoms and Rhinitis in Office Workers in Malaysia - Associations With House Dust Mite (HDM) Allergy, Cat Allergy and Levels of House Dust Mite Allergens in Office Dust PDFDokumen14 halamanAsthma, Airway Symptoms and Rhinitis in Office Workers in Malaysia - Associations With House Dust Mite (HDM) Allergy, Cat Allergy and Levels of House Dust Mite Allergens in Office Dust PDFMohamedFittriBelum ada peringkat
- 1 Physico ChemicalWasteWaterTreatmentTechnologiesAnOverviewDokumen13 halaman1 Physico ChemicalWasteWaterTreatmentTechnologiesAnOverviewMohamedFittriBelum ada peringkat
- Exercise 1Dokumen1 halamanExercise 1MohamedFittriBelum ada peringkat
- Allergic RhintisDokumen10 halamanAllergic RhintisMohamedFittriBelum ada peringkat
- Chapter 2.1 Definitions and Classifications: Notes On This ChapterDokumen3 halamanChapter 2.1 Definitions and Classifications: Notes On This ChapterMohamedFittriBelum ada peringkat
- Assignment 2Dokumen6 halamanAssignment 2MohamedFittriBelum ada peringkat
- A2 Assignment Distill RedfracDokumen1 halamanA2 Assignment Distill RedfracMohamedFittriBelum ada peringkat
- Test2 SolutionDokumen5 halamanTest2 SolutionMohamedFittriBelum ada peringkat
- Concentration Units in Environmental MeasurementsDokumen8 halamanConcentration Units in Environmental MeasurementsMohamedFittriBelum ada peringkat
- Investigations On Fouling Rate in Convective BundlesDokumen11 halamanInvestigations On Fouling Rate in Convective BundlesAnitha Kumari SivathanuBelum ada peringkat
- BASF Ultramid BrochureDokumen68 halamanBASF Ultramid BrochureshaeyhameedBelum ada peringkat
- Pipe Support PDFDokumen111 halamanPipe Support PDFm2110Belum ada peringkat
- Weld Design-GrDokumen58 halamanWeld Design-Grvenky100% (1)
- Admixtures and Shotcrete DurabilityDokumen7 halamanAdmixtures and Shotcrete DurabilityMulyawan WIdiasmanBelum ada peringkat
- Cutting Tool Tech and Tool Life CalcDokumen25 halamanCutting Tool Tech and Tool Life CalcPrashant ChouhanBelum ada peringkat
- FAME - Automated Fatty Acid Derivatization & GC - MS AnalysisDokumen3 halamanFAME - Automated Fatty Acid Derivatization & GC - MS AnalysisHushla ShudriBelum ada peringkat
- Chapter 17Dokumen97 halamanChapter 17Marco KrugerBelum ada peringkat
- Ch. 13 Carbonyl (1) Answers: Organic Chem II-1Dokumen38 halamanCh. 13 Carbonyl (1) Answers: Organic Chem II-1Nguyễn A.ThưBelum ada peringkat
- CT TPDokumen5 halamanCT TPcesarchiletBelum ada peringkat
- M.E.Forge Tech: Customer:M/s L & T Valves LimitedDokumen1 halamanM.E.Forge Tech: Customer:M/s L & T Valves LimitedK.s. Raghavendra KumarBelum ada peringkat
- Characterization of InclusionsDokumen8 halamanCharacterization of Inclusionsmahdisajjadi100% (1)
- L-5 Secondary TreatmentDokumen53 halamanL-5 Secondary Treatmentzubair k peerzadeBelum ada peringkat
- Equipment for Drilling Fluid Laboratory TestingDokumen14 halamanEquipment for Drilling Fluid Laboratory TestingAlok SinghBelum ada peringkat
- Khaled El Deeb Aquence 866 Process - ManualDokumen39 halamanKhaled El Deeb Aquence 866 Process - ManualNew Wrld100% (1)
- Packaging Materials and Articles For Food: Legislation and Codes of Good Manufacturing PracticeDokumen10 halamanPackaging Materials and Articles For Food: Legislation and Codes of Good Manufacturing PracticeMilanVukicBelum ada peringkat
- Palm Oil Fiber ConcreteDokumen9 halamanPalm Oil Fiber ConcreteGladys Eras ValladolidBelum ada peringkat
- 9h47.02 CD Emerald LyseDokumen9 halaman9h47.02 CD Emerald LyseanggitasaputriBelum ada peringkat
- Epoxy resin free flow grout for heavy duty applicationsDokumen4 halamanEpoxy resin free flow grout for heavy duty applicationsFeri Oktara IrawanBelum ada peringkat
- Elisa: Enzyme-Linked Immunosorbent AssayDokumen12 halamanElisa: Enzyme-Linked Immunosorbent AssayAmitBelum ada peringkat
- GDokumen3 halamanGjeas grejoy andrewsBelum ada peringkat
- B42 - Midterm10w CH 15-16-17-1Dokumen7 halamanB42 - Midterm10w CH 15-16-17-1Siao Ryan YangBelum ada peringkat
- Origins of Rheology: A Brief Look at the Evolution of the Study of Material FlowDokumen9 halamanOrigins of Rheology: A Brief Look at the Evolution of the Study of Material FlowAmlan PalBelum ada peringkat
- J Parenter Enteral Nutr - 2015 - Frank - Thiamin in Clinical PracticeDokumen18 halamanJ Parenter Enteral Nutr - 2015 - Frank - Thiamin in Clinical Practicejuhh tavaresBelum ada peringkat
- 005-1-Vocabulary Qs 2PsgsDokumen2 halaman005-1-Vocabulary Qs 2PsgsAlondra RezaBelum ada peringkat