Novel Antisense Technologies To Treat Filoviruses (Ebola and Marburg) - Marc N.
Diunggah oleh
MicroposterDeskripsi Asli:
Judul Asli
Hak Cipta
Format Tersedia
Bagikan dokumen Ini
Apakah menurut Anda dokumen ini bermanfaat?
Apakah konten ini tidak pantas?
Laporkan Dokumen IniHak Cipta:
Format Tersedia
Novel Antisense Technologies To Treat Filoviruses (Ebola and Marburg) - Marc N.
Diunggah oleh
MicroposterHak Cipta:
Format Tersedia
Efficacy of Novel Anti-sense Treatment for Filovirus Infections
Marc Nakashima | BIOL113 | DUE 11.05.10
INTRODUCTION
DATA
Popularized by Richard Preston’s non-fiction novel The Hot Zone, Ebola and Marburg viruses are highly pathogenic and lethal to humans and non-human
primates. Five species of Ebola have been discovered; of these five, Zaire Ebola (ZEBOV) has the highest case-fatality rate, at 90 percent. Marburg virus (MARV), a FIG1. Antisense therapy significantly mitigates VHF in non-
less deadly cousin of Ebola, has a case-fatality rate of 23-25 percent. Initial diagnosis of both diseases is difficult, because early symptoms are similar to other human primates infected with Zaire-Ebola. a) Kaplan-Meier
infections. The cause of death is by systemic organ failure and viral hemorrhagic fever (VHF); patients often “bleed out” from natural openings or cuts on the survival analysis of AVI-6002-treated monkeys. Five out of
body.[1] To date, there is no known cure for either disease. eight (62.5%) AVI-6002-treated monkeys survived ZEBOV
infection. Negative control died day 7. b-d) Multiple-dose
Both Ebola and Marburg belong to a family of single-stranded, negative-sense RNA viruses known as Filoviridiae, or filoviruses.[2] The RNA basis for gene post-exposure efficacy assessment of AVI-6002 for
storage and expression makes for an excellent drug target; inhibition of viral translation via novel anti-sense technology is a recent attempt at a cure, discussed treatment of ZEBOV infection. b) Kaplan-Meier analysis
below. The antisense molecule used is a positively charged phosphorodiamidate morpholino oligomer (PMOplus). Administration of equivalent concentrations shows higher survival rate with increased dosage.
of PMOplus molecules specific to ZEBOV viral protein 24 and 35 transcripts (eVP24 and eVP35, respectively) have demonstrated great success in mice and guinea Statistically significant differences (*P < 0.05) between
pigs (≥90% mice; 83% guinea pig survival rate).[3] Furthermore, survival rate was independent of time of treatment, i.e. pre-exposure and post-exposure means of AVI-6002 treatments and the negative control
AVI-6003 treatment are indicated. Negative controls did not
administration of the PMOs had no significant effect on survival. The combination therapy of eVP24 and eVP35 specific PMOplus molecules was designated as survive the experiment. c) Mean platelet count shows
AVI-6002. The aim of the following experiment with ZEBOV is to test the efficacy of AVI-6002 in a non-human primate. Based on the success of this experiment, a steady decline, then increase in surviving subjects receiving
similar cocktail of equivalent concentrations of PMOplus molecules specific to MARV nucleoprotein and MARV VP24 was prepared, designated as AVI-6003. The AVI-6002. d) Mean peripheral blood lymphocyte count
efficacy of AVI-6003 was also tested in a non-human primate. shows steady decline, then increase in surviving subjects
receiving AVI-6002. e) Mean AST concentration reaches a
variable maxima corresponding to dosage gradient. f)
RESEARCH QUESTION: Does a cure exist for fatal filoviral infections, such as Ebola and Marburg virus? Plasma viremia assessed by quantitative real-time PCR
shows viremia increase corresponding to dosage gradient.
METHODS
ZEBOV Experiment 1 (FIG1a) FIG2. Antisense therapy significantly mitigates VHF in non-
1) Infect rhesus monkeys (n = 9) with a lethal dose of ZEBOV (~1 000 ZEBOV-Kikwit plaque-forming units) by intramuscular injection. human primates infected with Marburg virus. a) Combined
survival and viremia plots of AVI-6003-treated monkeys. All
2) Deliver 40 mg per kg body weight AVI-6002 by intraperitoneal and subcutaneous injection 30-60 min. post-exposure to experimental group (n = 8). Four AVI-6003-treated monkeys, regardless of means of
monkeys receive treatment for 10 d and the remaining four receive treatment for 14 d. Negative control (n = 1) receives no treatment. treatment, survived MARV infection. No controls survived.
3) Observe mortality over time (t = 30 d) and analyze by Kaplan-Meier estimation. b-d) Multiple-dose post-exposure efficacy assessment of
AVI-6003 for treatment of MARV infection. b) Kaplan-Meier
ZEBOV Experiment 2 (FIG1b-f) analysis shows higher survival rate with increased dosage.
Statistically significant differences (*P < 0.05) between
1) Test efficacy of AVI-6002 dosage on rhesus monkeys, post-exposure to ZEBOV. AVI-6002 administered to experimental group at four dose levels: 40 (6002-40; means of AVI-6003 treatments and the negative control AVI-
n = 5), 28 (6002-28; n = 5), 16 (6002-16; n = 5), or 4 (6002-4; n = 5) mg per kg body weight. Negative control: Four monkeys received 40 mg per kg body 6002 treatment are indicated. Negative controls did not
weight AVI-6003-40, and one monkey PBS. All treatments administered by daily intravenous injection through day 14 after infection. survive the experiment. c) Mean platelet count shows
2) Observe monkeys and monitor vital signs (platelet count, lymphocyte count, AST concentration, viremia concentration) over time (t = 28-30 d) steady decline, then increase in surviving subjects receiving
AVI-6003. d) Mean peripheral blood lymphocyte count
shows steady decline, then increase in surviving subjects
MARV Experiment 1 (FIG2a) receiving AVI-6003. e) Mean AST concentration reaches a
1) Infect cynomolgus monkeys with a lethal dose of MARV (~1 000 MARV-Musoke plaque-forming units) by subcutaneous injection. variable maxima corresponding to dosage gradient. f)
2) Deliver AVI-6003 30-60 min. post-exposure to experimental group at doses of 40 (n -= 4) or 30 (n = 3) mg per kg body weight via intraperitoneal and Plasma viremia assessed by quantitative real-time PCR
subcutaneous injection or at 40 mg per kg body weight by subcutaneous (n = 3) or intravenous (n = 3) injection. Negative controls received no treatment. shows viremia increase corresponding to dosage gradient.
3) Observe mortality and viremia over time.
MARV Experiment 2 (FIG2b-f)
RESULTS/DISCUSSION
1) Test efficacy of AVI-6003 dosage on cynomolgus monkeys, post-exposure to MARV. AVI-6003 administered to experimental group at three dose levels: 30
Protective efficacy gradient observed against ZEBOV; 60% survival rate among recipients of either 28 or 40 mg per kg body weight AVI-6002, 20%
(6003-30; n = 5), 15 (6003-15; n = 5), or 7.5 (6003-7.5; n = 5) mg per kg body weight. Negative control: Four monkeys received 30 mg per kg body weight AVI-
survival among 16 mg per kg body weight recipients, and no survivors among 4 mg per kg body weight recipients suggests direct correlation with
6002-30, and one monkey PBS.
dosage and protection (FIG1b).
2) Observe monkeys and monitor vital signs (platelet count, lymphocyte count, AST concentration, viremia concentration) over time (t = 28-30 d)
Thrombocytopenia (FIG1c) and lymphocytopenia (FIG1d) unaffected by treatment among all treatment groups through day 8; increase in platelet
and lymphocyte count suggests disease resolution.
KEY TERMS Relatively low AST concentration among AVI-6002-28 and AVI-6002-40 recipients suggests mitigation of liver damage by ZEBOV.
Negative-sense RNA virus: An RNA virus that must use an RNA polymerase to convert its RNA into positive-sense RNA, which can be read as mRNA and translated by host ribosomes
Antisense therapy: The use of molecules complementary to nucleic acids that modify or block gene expression via irreversible binding or competitive inhibition. At the peak of plasma viremia (day 8) the mean viral load in AVI-6002-40 recipients was suppressed approx. 100 times that of AVI-6003 recipients
Morpholino oligomer: An antisense molecule designed to block small (~25 base pair) regions of RNA. (analysis not shown).
Kaplan-Meier survival analysis: An estimate of the survival function from life-time data. Viremia in monkeys that survived to day 8 generally correlates with survival, suggesting that suppression of viral replication may be a crucial
Intramuscular (IM) injection: Injection into a muscle.
Intraperitoneal injection: Injection into the body cavity. mechanism of AVI-6002-mediated protection against ZEBOV.
Intravenous (IV) injection: Injection into a vein.
Subcutaneous (subcut) injection: Injection just beneath the surface of the skin. Protective efficacy gradient observed against MARV; 100% survival rate among recipients of 30 mg per kg body weight AVI-6003 and 60% survival
Aspartate aminotransferase (AST): An enzyme found primarily in the liver, and in lesser quantities in the brain, heart, kidneys, and skeletal muscle. Elevated levels of AST is an indication of severe damage to these
organs. among 7.5 mg per kg body weight and 15 mg per kg body weight recipients suggests direct correlation with dosage and protection (FIG2b).
Platelet/thrombocyte: Anucleate cells which circulate in the mammalian bloodstream and are responsible for hemostasis by providing clotting and growth factors. Thrombocytopenia (FIG2c) and lymphocytopenia (FIG2d) unaffected by treatment among all treatment groups.
Thrombocytopenia: A medical condition where platelet count falls below 50 000 per microliter. Low platelet concentration increases the risk of uncontrolled bleeding. Significant reductions in circulating concentrations of AST relative to control treatments (FIG2e).
Lymphocyte: Cells involved in the immune system. Includes T cells, B cells, and natural killer (NK) cells.
Lymphocytopenia: A medical condition where lymphocyte count is abnormally low. Analysis of peak viremia in individual monkeys suggests AVI-6003 mediated protection by reducing viral burden (FIG2f).
Viremia: A medical condition where viruses have entered the bloodstream.
CONCLUSIONS
PMOplus antisense therapy is an effective treatment for lethal Ebola and Marburg
filoviruses in non-human primates
Protective gradient correlates with dosage of PMOplus drugs
Treatment may be given by different delivery routes with no significant effect on
efficacy
Future studies needed to assess window for effective intervention in non-human
primates
Clinical studies for AVI-6002 and AVI-6003 recently approved by US Food and Drug
Administration
REFERENCES
Ebola virus (SEM) [4] [1] Viral Hemorrhagic Fevers. CDC Special Pathogens Branch http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/vhf.htm
[2] Filoviruses. CDC Special Pathogens Branch http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/filoviruses.htm
[3] Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S. “Advanced antisense therapies for postexposure
Marburg virus (SEM 10^6 X; negative stain) [3] protection against lethal filovirus infections.” Nat Med. 2010 Sep;16(9):991-4. Epub 2010 Aug 22.
Phosphorodiamidate morpholino oligomer (PMO) bound to RNA [6] [4] Marburg Hemorrhagic Fever. CDC Special Pathogens Branch http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/marburg/qa.htm#marburgem
[5] Ebola Hemorrhagic Fever. CDC Special Pathogens Branch http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/ebola/qa.htm
[6] Morpholino. Wikipedia. http://en.wikipedia.org/wiki/Morpholino
Anda mungkin juga menyukai
- Pathogens of The Vagina-CitationsDokumen1 halamanPathogens of The Vagina-CitationsMicroposterBelum ada peringkat
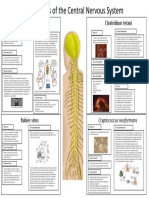
- Jesse Wackerbarth - CNS Pathogens RevisedDokumen1 halamanJesse Wackerbarth - CNS Pathogens RevisedMicroposterBelum ada peringkat
- Pathogens of The Vagina-Annie Espinosa - This Is The Revised VersionDokumen1 halamanPathogens of The Vagina-Annie Espinosa - This Is The Revised VersionMicroposterBelum ada peringkat
- Infections of The Liver Revised - Nick GriffinDokumen1 halamanInfections of The Liver Revised - Nick GriffinMicroposterBelum ada peringkat
- Eye Infections by Allison BakerDokumen1 halamanEye Infections by Allison BakerMicroposterBelum ada peringkat
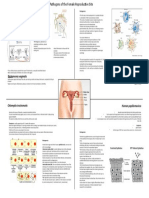
- Pathogens of The Female Reproductive Site, Erika HuertaDokumen1 halamanPathogens of The Female Reproductive Site, Erika HuertaMicroposterBelum ada peringkat
- Pathogens in The Lungs-Jeffrey DelgadilloDokumen1 halamanPathogens in The Lungs-Jeffrey DelgadilloMicroposterBelum ada peringkat
- CNS Pathogens-Jesse WackerbarthDokumen1 halamanCNS Pathogens-Jesse WackerbarthMicroposterBelum ada peringkat
- Savannah Whitington: Your Brain On DrugsDokumen1 halamanSavannah Whitington: Your Brain On DrugsMicroposterBelum ada peringkat
- Emily Scroggs Microorganisms Affecting The KidneyDokumen1 halamanEmily Scroggs Microorganisms Affecting The KidneyMicroposterBelum ada peringkat
- The Urogenital Tract-Maija SwansonDokumen1 halamanThe Urogenital Tract-Maija Swansonmswanson5975Belum ada peringkat
- Pathogens of The Lungs - Michelle CumbaaDokumen1 halamanPathogens of The Lungs - Michelle CumbaaMicroposterBelum ada peringkat
- Wylie, ClareDokumen1 halamanWylie, ClareMicroposterBelum ada peringkat
- Pathogens of The Gut - Christine ProchnowDokumen1 halamanPathogens of The Gut - Christine ProchnowMicroposterBelum ada peringkat
- Liver PathogensDokumen1 halamanLiver PathogensMicroposterBelum ada peringkat
- Lung Infections - Jessica de AndaDokumen1 halamanLung Infections - Jessica de AndaMicroposterBelum ada peringkat
- Gaby Saenz - The MeningesDokumen1 halamanGaby Saenz - The MeningesMicroposterBelum ada peringkat
- Pathogens of The Female Reproductive System - Leah NechamkinDokumen1 halamanPathogens of The Female Reproductive System - Leah NechamkinMicroposterBelum ada peringkat
- Pathogen (Poster Med Micro)Dokumen1 halamanPathogen (Poster Med Micro)joejackson6Belum ada peringkat
- EarInfections FatimaKhalidDokumen1 halamanEarInfections FatimaKhalidMicroposterBelum ada peringkat
- Zach Lee - Mouth MicrobesDokumen1 halamanZach Lee - Mouth MicrobesMicroposterBelum ada peringkat
- BlaC Genes and The Battle Against B-Lactamse in MDA TuberculosisDokumen1 halamanBlaC Genes and The Battle Against B-Lactamse in MDA TuberculosisMicroposterBelum ada peringkat
- Maladies of The Heart - Milan KantariaDokumen1 halamanMaladies of The Heart - Milan KantariaMicroposterBelum ada peringkat
- Pathogens of The Vagina-Annie EspinosaDokumen1 halamanPathogens of The Vagina-Annie Espinosaannie_espinosa_2Belum ada peringkat
- Courtney Chinn - Skin InfectionsDokumen1 halamanCourtney Chinn - Skin InfectionsMicroposterBelum ada peringkat
- Shoe Dog: A Memoir by the Creator of NikeDari EverandShoe Dog: A Memoir by the Creator of NikePenilaian: 4.5 dari 5 bintang4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDari EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifePenilaian: 4 dari 5 bintang4/5 (5795)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDari EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RacePenilaian: 4 dari 5 bintang4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)Dari EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Penilaian: 4 dari 5 bintang4/5 (98)
- Grit: The Power of Passion and PerseveranceDari EverandGrit: The Power of Passion and PerseverancePenilaian: 4 dari 5 bintang4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingDari EverandThe Little Book of Hygge: Danish Secrets to Happy LivingPenilaian: 3.5 dari 5 bintang3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerDari EverandThe Emperor of All Maladies: A Biography of CancerPenilaian: 4.5 dari 5 bintang4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDari EverandNever Split the Difference: Negotiating As If Your Life Depended On ItPenilaian: 4.5 dari 5 bintang4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDari EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyPenilaian: 3.5 dari 5 bintang3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealDari EverandOn Fire: The (Burning) Case for a Green New DealPenilaian: 4 dari 5 bintang4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDari EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FuturePenilaian: 4.5 dari 5 bintang4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDari EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryPenilaian: 3.5 dari 5 bintang3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDari EverandTeam of Rivals: The Political Genius of Abraham LincolnPenilaian: 4.5 dari 5 bintang4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDari EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaPenilaian: 4.5 dari 5 bintang4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDari EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersPenilaian: 4.5 dari 5 bintang4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaDari EverandThe Unwinding: An Inner History of the New AmericaPenilaian: 4 dari 5 bintang4/5 (45)
- Rise of ISIS: A Threat We Can't IgnoreDari EverandRise of ISIS: A Threat We Can't IgnorePenilaian: 3.5 dari 5 bintang3.5/5 (137)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDari EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You ArePenilaian: 4 dari 5 bintang4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Dari EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Penilaian: 4.5 dari 5 bintang4.5/5 (121)
- Her Body and Other Parties: StoriesDari EverandHer Body and Other Parties: StoriesPenilaian: 4 dari 5 bintang4/5 (821)
- Case Report of Misdiagnosis of Avian Colibacillosis in Laying BirdsDokumen6 halamanCase Report of Misdiagnosis of Avian Colibacillosis in Laying Birdsdokter fatmawatiBelum ada peringkat
- Rheumatic Heart DiseaseDokumen3 halamanRheumatic Heart Diseaseel_sapunganBelum ada peringkat
- Lecture Note On Renal Diseases For Medical Students - GNDokumen10 halamanLecture Note On Renal Diseases For Medical Students - GNEsayas KebedeBelum ada peringkat
- Bodies in PathologyDokumen2 halamanBodies in PathologySreekanth KrishnamurthyBelum ada peringkat
- Lab Report Pathogen Microbe 7 (SPUTUM)Dokumen5 halamanLab Report Pathogen Microbe 7 (SPUTUM)Syazmin KhairuddinBelum ada peringkat
- Bee Propolis HandbookDokumen23 halamanBee Propolis HandbookSabdono100% (1)
- AidsDokumen22 halamanAidsVicky Singh100% (2)
- PathologyDokumen48 halamanPathologyAjay DivvelaBelum ada peringkat
- Sterilization by Physical MethodsDokumen64 halamanSterilization by Physical Methodstummalapalli venkateswara rao100% (1)
- Microblot Array - 2023 - A4 - ANDokumen28 halamanMicroblot Array - 2023 - A4 - ANhazem alzed100% (1)
- 1 1 6 A FinaldiagnosisDokumen2 halaman1 1 6 A Finaldiagnosisapi-327503253Belum ada peringkat
- History of VirologyDokumen20 halamanHistory of VirologyshiyasBelum ada peringkat
- CURS - ENGLEZA - Boli Infectioase PDFDokumen246 halamanCURS - ENGLEZA - Boli Infectioase PDFLesan IulianaBelum ada peringkat
- Biosafety and Bioethics of Biotechnology ABS-832: DR Attya Bhatti Assistant Professor Head of Department Asab-NustDokumen25 halamanBiosafety and Bioethics of Biotechnology ABS-832: DR Attya Bhatti Assistant Professor Head of Department Asab-NustVoltha HerryBelum ada peringkat
- Chemotherapeutic AgentsDokumen53 halamanChemotherapeutic AgentsGrape JuiceBelum ada peringkat
- HPV PowerpointDokumen11 halamanHPV PowerpointharyatikennitaBelum ada peringkat
- Vriddhi KshayaDokumen37 halamanVriddhi KshayaVenkatesan VidhyaBelum ada peringkat
- USMLE Biostats GuideDokumen45 halamanUSMLE Biostats GuideMohammad ObaidullahBelum ada peringkat
- Tda 2Dokumen4 halamanTda 2lottieBelum ada peringkat
- Peritonsillar Abscess in Emergency MedicineDokumen14 halamanPeritonsillar Abscess in Emergency Medicinerissa neBelum ada peringkat
- Do Eosinophils Have A Role in The Severity of Babesia Annae Infection 2005 Veterinary ParasitologDokumen2 halamanDo Eosinophils Have A Role in The Severity of Babesia Annae Infection 2005 Veterinary ParasitologGabriela Victoria MartinescuBelum ada peringkat
- 1 s2.0 S1201971210000354 Main PDFDokumen189 halaman1 s2.0 S1201971210000354 Main PDFSABA YOUNASBelum ada peringkat
- SN4 Question Paper Sum 2006Dokumen16 halamanSN4 Question Paper Sum 2006EmmaBelum ada peringkat
- PCAP GuidelinesDokumen20 halamanPCAP GuidelinesApril Rae BarairoBelum ada peringkat
- AVN HCCC ReplyDokumen27 halamanAVN HCCC Replyd-fbuser-31847459Belum ada peringkat
- Cough, Hemoptysis, Dyspnea, WheezingDokumen43 halamanCough, Hemoptysis, Dyspnea, WheezingMelissa SalayogBelum ada peringkat
- Chart Pox VirusesDokumen1 halamanChart Pox Virusesshiner99Belum ada peringkat
- Switch Antibiotic IV To OralDokumen2 halamanSwitch Antibiotic IV To OralRidwan Ibnu BahariBelum ada peringkat
- 929044522XDokumen286 halaman929044522XKonstantinVoroninBelum ada peringkat
- Guia Ingles - Grado 11Dokumen8 halamanGuia Ingles - Grado 11Juan CuervoBelum ada peringkat